Abstract
Objective
To evaluate trochlear morphology as a potential risk factor for patellofemoral osteoarthritis, determined by morphological and quantitative measurements of cartilage degeneration using 3T magnetic resonance imaging (MRI) of the knee.
Materials and Methods
MR images of right knees of 304 randomly selected subjects, aged 45–60 years, from the Osteoarthritis Initiative (OAI) progression cohort were screened for trochlear dysplasia, defined by an abnormal trochlear depth. Out of 304 subjects, n=85 demonstrated a shallow trochlea (depth ≤3mm; 28%). In these, and also in a random sample of controls with normal trochlear depth (n=50), the facetal ratio and the sulcus angle were calculated and knee structural abnormalities were assessed by using a modified Whole-Organ-MR-Imaging Score (WORMS). Cartilage segmentation was performed and T2 relaxation times and patellar cartilage volume were determined. ANOVA and multivariate regression models were used for statistical analysis of the association of MRI structural measures and trochlear morphology.
Results
Knees with a shallow trochlea showed higher patellofemoral degeneration (WORMS mean ±standard deviation, 11.2±0.5 versus 5.7±0.6; Multivariate regression, P<0.001) and lower patellar cartilage volume than controls (900±664mm3 versus 1671±671mm3; P<0.001). Knees with an abnormal medial-to-lateral facetal ratio (<0.4) showed increased patellofemoral WORMS scores (12.3±0.9 versus 8.3±0.5; P<0.001). Knees with an abnormal sulcus angle (>170°) also showed increased WORMS scores (12.2±1.1 versus 8.6±0.6; P=0.003). T2 values at the patella were significantly lower in the dysplasia group with a shallow trochlea. However, significance was lost after adjustment for cartilage volume (P=0.673).
Conclusion
Trochlear dysplasia, defined by a shallow trochlea, was associated with higher WORMS scores and lower cartilage volume, indicating more advanced osteoarthritis at the patellofemoral joint.
Keywords: Patellofemoral joint, Magnetic Resonance Imaging, Osteoarthritis, Cartilage
Introduction
Patellofemoral joint disorders represent a common cause of anterior knee pain [1, 2]. As the largest sesamoid bone of the body, the patella functions to magnify the moment arm of the patellar tendon; thereby increasing the effectiveness of extensor musculature [3]. Between 30 and 90 degrees of flexion, the patella is engaged with the trochlea. The morphology of the trochlea, in addition to several other static and dynamic stabilizers, helps to keep the patella stable during this engagement.
Trochlear dysplasia is a geometric abnormality in the shape and depth of the trochlear groove. It can be assessed with axial [4] and lateral knee radiographs [5], computed tomography or MRI of the knee. On MRI, Pfirrmann et al. proposed that a trochlear depth of 3 mm or less or a facet asymmetry defined by an abnormal medial-to-lateral facetal ratio of less than 0.4 have good sensitivity and specificity for trochlear dysplasia [6]. On plain radiographs, a sulcus angle >150° indicates trochlear dysplasia. However, two-dimensional imaging underestimates the angle and shows substantial differences of about 20° to more accurate MR measurements [7–10].
A dysplastic trochlea can lead to abnormal patellar tracking, chronic patellar dislocation and to an abnormal distribution of loading, which increase the risk for osteoarthritis (OA) [11, 12]. Most studies have used plain radiographs to evaluate trochlear dysplasia and OA. There is little information about trochlear dysplasia and its association with degenerative changes at the patellofemoral joint when evaluated with 3T MRI studies, which are particularly sensitive to detecting early degenerative biochemical intrasubstance changes. T2 relaxation time measurements can be used as a biomarker to non-invasively detect and quantify intrasubstance degeneration, more specifically collagen disruption and water content changes.
The Osteoarthritis Initiative (OAI) was launched by the NIH and is a longitudinal, observational multi-center study that includes nearly 5000 participants. One of the primary goals of the study is to better understand the evolution of knee OA and associated factors (http://www.oai.ucsf.edu/) [13].
The purpose of the present study was to determine whether knees with an abnormal trochlear depth, an abnormal medial-to-lateral trochlear facetal ratio or an abnormal sulcus angle have a higher prevalence of MRI findings of OA, as measured by semi-quantitative morphological joint scores, cartilage volume and T2 relaxation time.
Materials and Methods
Subjects
From the Progression cohort of the OAI, 304 subjects aged 45 to 60 were randomly selected and measurements of trochlear dysplasia on MRIs of their right knee were performed. The progression cohort is characterized by the presence of symptomatic knee OA. A shallow trochlea was identified in 85 subjects. A control group of 50 right knees without shallow trochlea was randomly selected from the remaining subjects with normal trochlear depth. An abnormal medial-to-lateral facetal ratio was found in 30/ 135 subjects. An abnormal sulcus angle was found in 22/ 135 subjects. There was no significant difference regarding age, BMI, gender, Physical Activity Scale for the Elderly (PASE) [14], and knee-bending activities between the subjects with a shallow trochlea (n=85) versus controls (n=50; P>0.05 for all comparisons; Table 1). There was no significant difference between subjects with an abnormal facetal ratio (n=30) and controls (n=105; gender, P=0.686; BMI, P=0.327; age, P=0.973; knee-bending, P=0.462; PASE, P=0.641). Also, there was no significant difference between subjects with an abnormal sulcus angle (n=22) and controls (n=113; gender, P=0.772; BMI, P=0.113; age, P=0.486; knee-bending, P=0.364; PASE, P=0.199).
Table 1.
Epidemiological data of study cohorts. The control cohort was randomly selected.
| Individuals with shallow trochlea | Control | P-Value | |
|---|---|---|---|
| Number of subjects (n) | 85 | 50 | |
| Age (years) | 64.0 (SD: ±9.2) | 64.2 (SD: ±9.8) | 0.875 |
| Gender (female, %) | 81 % | 80% | 0.869 |
| body mass index (kg/m2) | 28.3 (SD: ±4.1) | 28.2 (SD: ±4.6) | 0.862 |
| PASE (absolute score) | 140.6 (SD: ±70.7) | 141.8 (SD ±71.7) | 0.929 |
| Knee bending actividies (% positive) | 72.9 % | 77.4 % | 0.510 |
| Kellgren-Lawrence grade (0 : 1 : 2 : 3 : 4) | 2 : 3 : 45 : 35 : 0 | 6 : 8 : 26 : 10 : 0 | <0.001 |
The study protocol, amendments and informed consent documentation were reviewed and approved by the local institutional review boards of the participating OAI sites. Datasets 0.C.1 and 0.E.1 used in the preparation of this article were obtained from the OAI public website (http://www.oai.ucsf.edu/). Exclusion criteria for the OAI were rheumatoid arthritis, bilateral severe knee joint space narrowing, contraindications or inability for MRI, and poor MR quality.
Bilateral knee radiographs
Bilateral standing posterior anterior (PA) “fixed flexion” plain radiographs of the knee were obtained in a plexiglass positioning frame (SynaFlexerTM; CCBR-Synarc, San Francisco, California) with 20–30° flexion and 10° internal rotation of bilateral feet. Kellgren-Lawrence (KL) scores were assessed in our institution (T.M.L., 22 years of experience).
MR imaging
MRI examinations were obtained with dedicated 3T MRI systems (Trio, Siemens, Erlangen, Germany). A standard knee coil was used. The following sequences of the right knee were analyzed in this study: (1) coronal 2D intermediate-weighted (IW) fast spin-echo (FSE) sequence (TE / TR = 29 / 3850 ms, field of view (FOV) = 14 cm, slice thickness = 3 mm, in-plane spatial resolution = 0.365 × 0.456 mm2, flip angle = 180, bandwidth = 352 Hz / pixel), (2) sagittal 3D dual-echo steady-state (DESS) sequence with water excitation (WE) and coronal and axial reformations (TE / TR = 4.7 / 16.3 ms, field of view (FOV) = 14 cm, slice thickness = 0.7 mm, in-plane spatial resolution = 0.365 × 0.456 mm2, flip angle = 25, bandwidth = 185 Hz / pixel), (3) sagittal 2D IW fat-suppressed (fs) FSE sequence (TE / TR = 30 / 3200 ms, field of view (FOV) = 16 cm, slice thickness = 3 mm, in-plane spatial resolution = 0.357 × 0.511 mm2, flip angle = 180, bandwidth = 248 Hz / pixel) and (4) a sagittal 2D multislice multiecho (MSME) spin echo (SE) sequence (TR = 2700 ms, seven TEs = 10 ms, 20 ms, 30 ms, 40 ms, 50 ms, 60 ms, 70 ms, field of view (FOV) = 12 cm, slice thickness = 3 mm with 0.5mm gap, in-plane spatial resolution = 0.313 × 0.446 mm2, bandwidth = 250 Hz / pixel), which was used to obtain quantitative T2 relaxation time measurements (21).
Trochlear Morphology
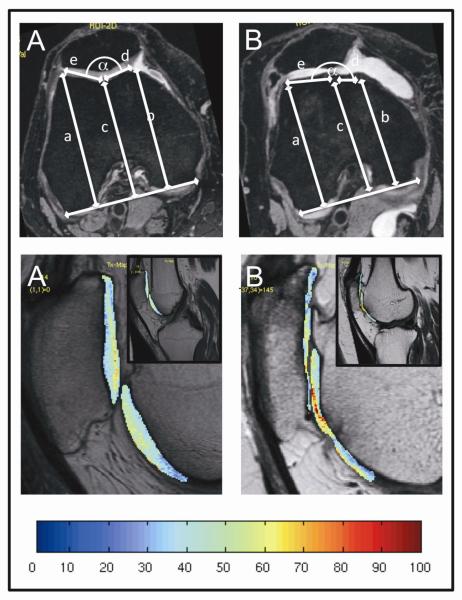
Trochlear measurements were performed in rights knees using the axial reconstructed image from the 3D dual-echo in steady state (DESS) sequence with selective water excitation (WE) in all right knees. At the axial slice 30 mm proximal to the knee joint line [6], the following trochlear measurements were obtained (Figure 1; upper row): (i) the maximal anteroposterior distance from the medial femoral condyle to the line paralleling the posterior aspects of both femoral condyles (distance a), (ii) the maximal anteroposterior distance from the lateral femoral condyle to the line paralleling the posterior aspects of both femoral condyles (distance b), (iii) the minimal anteroposterior distance from the deepest point in the trochlear groove to the line paralleling the posterior aspects of both femoral condyles (distance c), (iv) the length of the medial (distance d) and lateral (distance e) facets of the patella. The trochlear depth was calculated as [(a + b) / 2] – c. A trochlear depth of 30 mm or less was considered shallow. The facetal ratio was calculated as d / e. A facetal ratio of 0.4 or less was considered abnormal. The sulcus angle (α) was calculated as 180° – asin ((a–c)/e) – asin ((b–c)/d). A sulcus angle above 170° was considered as abnormal.
Figure 1.
Upper row: Measurements in the axial 3D DESS sequence with selective water excitation, 30 mm proximal of the joint line in a normal (A) and abnormal (B) patellofemoral joint with trochlear dysplasia. Lower row: Corresponding T2 color maps of the patellofemoral joint overlaid with the first-echo images of MSME sequences of the same subjects. Blue color indicates low cartilage T2, while red color indicates high cartilage T2. Severe loss of the superficial cartilage and only a thin remaining profound cartilage layer results in low T2 values.
Semi-quantitative, morphological image analyses
MR images of the right knee were reviewed on picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA). Two musculoskeletal radiologists (S.C.T., T.M.L.) separately scored knees using the UCSF modified whole-organ magnetic resonance imaging score (WORMS) [15–17]; if scores were not identical, consensus readings by both radiologists were performed. For the patellofemoral joint, the following joint structures were evaluated: (i) cartilage, (ii) bone marrow edema pattern, (iii) subchondral cysts and (iv) the patellar tendon. For the femorotibial joint compartment, (iv) collateral ligaments and (v) meniscus abnormalities were evaluated additionally to (i) to (iii). Cartilage was scored as follows: 0=normal; 1=increased signal; 2=partial-thickness defect <1 cm; 2.5=full-thickness defect <1 cm; 3=multiple areas of partial-thickness defects, or >1 cm but <75% of the region; 4=≥75% of the region; 5=multiple areas of full- thickness loss or a >1 cm but <75% of the region; 6=≥75% of the region. Subchondral bone marrow edema pattern (BME) was graded as follows: 0=none; 1=<0.5cm; 2=0.5 to 2.0 cm; 3=>2.0cm. Subarticular cysts were graded as follows: 0=none; 1=<0.5cm; 2=0.5 to 2.0 cm; 3=>2.0cm. The patellar tendon and the collateral ligaments were graded as either normal or abnormal. Meniscus changes were graded in six regions (medial and lateral: anterior, body, posterior) by the following: 0=normal; 1=intra-substance abnormalities; 2=non-displaced tear; 3=complex tear; 4=maceration. For the entire medial and lateral meniscus, grading was defined by the following: 0 if all compartments were graded as 0, 1 if one or more compartments were graded as 1, 2 if one compartment was graded as 2, 3 if more than one compartment was graded as 2, 4 if one or more compartments were graded as 3, 5 if one compartment was graded as 4, 6 if more than one compartment was graded as 4. Maximum WORMS scores are summarized in Table 2.
Table 2.
Calculation of total WORMS score for the patellofemoral joint and the medial and lateral femorotibial joint.
| Patellofemoral total WORMS | Femorotibial total WORMS | |||
|---|---|---|---|---|
| Max. score | Max. score | |||
| Cartilage | Patella | 6 | Femoral Compartment | 6 |
| Trochlea | 6 | Tibial Compartment | 6 | |
| BME | Patella | 3 | Femoral Compartment | 3 |
| Trochlea | 3 | Tibial Compartment | 3 | |
| Cysts | Patella | 3 | Femoral Compartment | 3 |
| Trochlea | 3 | Tibial Compartment | 3 | |
| Tendon | Patella | 1 | Collateral tendon | 1 |
| Meniscus | Total Meniscus score | 6 | ||
| Total score | 25 | 31 | ||
T2 relaxation time measurements and patellar cartilage volume measurements
Segmentation of the cartilage at the patella and trochlea, the medial and lateral femoral condyle and medial and lateral tibia plateau, was performed by one investigator (S.C.T.) and supervised by a musculoskeletal radiologist (T.M.L.) to generate T2 maps from the sagittal 2D MSME SE sequences of the right knee. Images were transferred to a remote SUN/SPARC workstation (Sun Microsystems, Mountain View, CA, USA) and analyzed with software developed at our institution using an Interactive Display Language (IDL; Research Systems, Boulder, CO, USA) environment. An IDL routine was used to simplify the manual drawing of splines delineating cartilage areas and to calculate the mean T2 values from the regions of interest created in the T2 maps. T2 values were calculated as global values (mean of all compartments), and for each individual compartment. Using the same software, segmentation for patellar cartilage volume measurements was performed by one radiologist (P.M.J.) and supervised by a musculoskeletal radiologist (T.M.L.). Absolute cartilage volume (mm3) was calculated for the patellar compartment from the regions of interest created in the maps.
Reproducibility measurements
Reproducibility for the semi-quantitative analyses of the WORMS score for each compartment from our group was reported previously [18]. Inter-observer agreement for T2 measurements in our group was described previously with an inter-reader reproducibility error for mean T2 of 1.57 % or 0.53 ms [19]. Mean intra-reader reproducibility for T2 measurements was 1.66 % or 0.55 ms.
Statistical Analysis
All statistical analyses were performed with JMP software Version 9 (SAS Institute, Cary, NC). The level of significance for all calculations was defined as p<0.05. For covariates, mean values are reported with ± standard deviation (SD). KL-scores were considered as ordinate variables. T-tests were used to detect differences between the groups. Multivariate regression analyses were performed to analyze the association of either trochlear abnormality (abnormal trochlear depth, abnormal facetal ratio and abnormal sulcus angle) with WORMS scores and T2 relaxation time. Mean values are reported with ± Standard Error of the Mean (SEM), if not otherwise stated. Cartilage volume was included in the multivariate regression model to account for possible associations between cartilage volume and T2 relaxation time. As previously suspected [13, 20], T2 values may reach a ceiling or even decrease with increasing cartilage loss. Such an association would affect T2 values found with OA, which are typically higher than normal (not lower) due to increased water content and collagen fibrillation. Total WORMS scores and scores for cartilage and meniscus were approximately normally distributed and considered as linear values in this model. BME and subchondral cysts were analyzed as dichotomous variables (absence versus presence of abnormalities, defined as score >0) using a logistic regression, since these variables were not normally distributed. All regression models were adjusted for age, gender and OA risk factors, including previous injury or surgery at the knee, family history of joint replacement, presence of Herbeden's nodes and BMI.
Results
Study cohort
Out of 304 individuals from the OAI progression cohort, 85 had a shallow trochlear groove (trochlear depth ≤3 mm) in the right knee with a mean trochlear depth of 2.0 ± 1.0 mm (mean ± standard deviation (SD)). In the control knees, the mean trochlear depth was 4.4 ±1.0 mm. Twenty-six of 85 knees with a shallow trochlea and 4 of 50 control knees had a medial-to-lateral trochlear facetal ratio of less than 0.4. The 30 knees with a low trochlea facetal ratio had an average ratio of 0.35 ±0.04, while the subjects with a normal trochlea facetal ratio (n=105) had a mean ratio of 0.57 ±0.11. The mean length ± SD of the medial and lateral facet in the entire cohort were 11.4 ±3.0mm and 22.1 ±2.5mm, respectively. In the subcohort with an abnormal facetal ratio means of 7.9 ±1.0mm and 22.8 ±2.6mm were detected; in the subcohort with a normal facetal ratio means of 12.4 ±2.5mm and 21.9 ±2.4mm were found. The mean sulcus angle in the entire cohort was 162 ±10°. Twenty-two of the knees with a shallow trochlea and no control knees had an abnormal sulcus angle of >170°. The 22 knees with a high sulcus angle had an average angle of 177 ±8°, while the subjects with a normal sulcus angle (n=113) had an average angle of 159 ±8°. A significant difference in sulcus angle was found between the group with a shallow trochlea and the control group (167 ±8° versus 152 ±6°; P<0.001).
Morphological patellofemoral abnormalities
The total WORMS score of the patellofemoral joint was 5.7 ±0.6 (mean ±SEM) in the control group (Figure 2). In individuals with a shallow trochlea it was significantly higher (11.2 ±0.5; P<0.001). The patellofemoral WORMS score was also significantly **higher in individuals with low trochlear facetal ratio or abnormal sulcus angle than in the corresponding control cohort (facetal ratio, 12.3 ±0.9 versus 8.3 ±0.5; P<0.001; sulcus angle, 12.2 ±1.1 versus 8.6 ±0.6; P=0.003; Figure 2).
Figure 2.
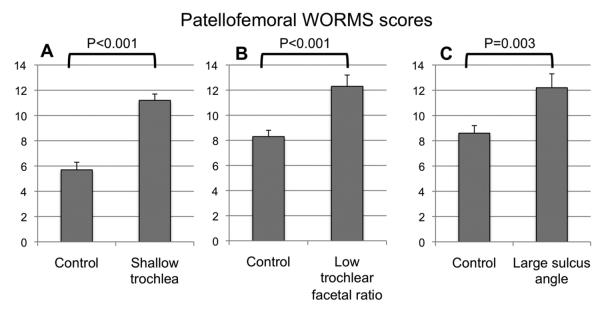
Total WORMS score of the patellofemoral joint in subjects with trochlear dysplasia versus control. Subjects presenting A: a shallow trochlea of ≤3mm, B: a small facetal ratio of ≤0.4 and C: a large sulcus angle of >170° presented more patellofemoral abnormalities than control subjects.
Considering the individual parameters of the patellofemoral WORMS score, subjects with trochlear dysplasia showed significantly more cartilage defects at the patellar cartilage as well as at the trochlear cartilage (P<0.001). Significantly higher scores for bone marrow edema pattern and subchondral cysts were found in individuals with a shallow trochlea in the trochlear and patellar compartments and in individuals with an abnormal sulcus angle in the patellar compartment (P<0.05). Subjects with a low trochlear facetal ratio had significantly more bone marrow abnormalities (Table 3).
Table 3.
Summary of mean WORMS sores regarding individual parameters and total score for the patellofemoral joint and the femorotibial joint, depending on trochlea depth, medial-to-lateral trochlea facetal ratio and sulcus angle.
| A: | Trochlear depth | Facetal ratio | Sulcus angle | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compartment | Parameter | Shallow n=85 | Normal n=50 | Difference (Lower 95% CI; Upper 95% CI) | Abnormal n=30 | Normal n=105 | Difference (Lower 95% CI; Upper 95% CI) | Abnormal n=22 | Normal n=113 | Difference (Lower 95% CI; Upper 95% CI) |
| Patellofemoral | Total WORMS | 11.2 ×0.5 | 5.7 ×0.6 | 5.5 (3.9; 7.0)* | 12.3 ×0.9 | 8.3 ×0.5 | 3.9 (1.9; 6.0)* | 12.2 ×1.1 | 8.6 ×0.6 | 3.7 (1.3; 6.1)* |
| Patella | Cartilage | 4.5 ×0.2 | 3.1 ×0.2 | 1.4 (0.9; 1.9)* | 4.9 ×0.3 | 3.7 ×0.2 | 1.2 (0.5; 1.8)* | 4.8 ×0.3 | 3.8 ×0.2 | 1.1 (0.4; 1.8)* |
| Trochlea | Cartilage | 3.5 ×0.2 | 1.6 ×0.3 | 1.9 (1.2; 2.5)* | 4.0 ×0.2 | 2.5 ×0.2 | 1.5 (0.7; 2.3)* | 3.7 ×0.5 | 2.6 ×0.2 | 1.1 (0.1; 2.0)* |
| Medial femorotibial | Total WORMS | 7.4 ×0.7 | 5.0 ×0.9 | 2.5 (0.4; 4.5)* | 6.1 ×1.1 | 6.6 ×0.6 | 0.4 (−2.0; 2.9) | 8.0 ×1.3 | 6.2 ×0.7 | 1.7 (−1.1; 4.5) |
| Meniscus | 2.2 ×0.2 | 1.9 ×0.3 | 0.4 (−0.4; 1.1) | 1.5 ×0.4 | 2.3 ×0.2 | 0.8 (−0.1; 1.7) | 1.9 ×0.5 | 2.2 ×0.3 | 0.4 (−0.7; 1.4) | |
| Cartilage (Sum) | 4.0 ×0.3 | 2.6 ×0.4 | 1.4 (0.4; 2.5)* | 3.7 ×0.6 | 3.4 ×0.3 | 0.3 (−1.0; 1.5) | 4.3 ×0.7 | 3.3 ×0.4 | 0.9 (−0.5; 2.4) | |
| Lateral femorotibial | Total WORMS | 6.4 ×0.6 | 4.8 ×0.8 | 1.7 (−0.4; 3.7) | 6.3 ×1.1 | 5.7 ×0.6 | 0.6 (−1.8; 3.1) | 8.2 ×1.3 | 5.4 ×0.7 | 3.0 (0.4; 5.7)* |
| Meniscus | 2.2 ×0.2 | 1.6 ×0.3 | 0.6 (−0.1; 1.3) | 2.2 ×0.4 | 1.9 ×0.2 | 0.3 (−0.6; 1.2) | 2.9 ×0.4 | 1.8 ×0.2 | 1.2 (0.3; 2.2)* | |
| Cartilage (Sum) | 3.7 ×0.4 | 2.9 ×0.5 | 0.8 (−0.4; 2.0) | 3.6 ×0.6 | 3.3 ×0.3 | 0.3 (−1.1; 1.7) | 4.5 ×0.7 | 3.2 ×0.4 | 1.5 (−0.1; 3.0) | |
| B: | Trochlear depth | Facetal ratio | Sulcus angle | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compartment | Parameter | Shallow n=85 | Normal n=50 | Odds ratio (Lower 95% CI; Upper 95% CI) | Abnormal n=30 | Normal n=105 | Odds ratio (Lower 95% CI Upper 95% CI) | Abnormal n=22 | Normal n=113 | Odds ratio (Lower 95% CI; Upper 95% CI) |
| Patella | BME | 65/85 | 20/50 | 5.2 (1.4; 11.8)* | 24/30 | 61/105 | 3.0 (1.2; 8.8)* | 19/22 | 66/113 | 5.7 (1.7; 26.4)* |
| Cyst | 17/85 | 0/50 | − (6.5; -)* | 4/30 | 13/105 | 1.0 (0.2; 3.3) | 6/22 | 11/113 | 4.1 (1.2; 13.9)* | |
| Trochlea | BME | 46/85 | 11/50 | 4–4 (2.0; 5.7)* | 20/30 | 37/105 | 3.7 (1.6; 9.1)* | 14/22 | 43/113 | 2.5 (1.0; 6.9) |
| Cyst | 19/85 | 0/50 | − (7.1; -)* | 8/30 | 11/105 | 2.9 (1.0; 8.3)* | 6/22 | 13/113 | 2.5 (0.8; 7.8) | |
| Medial femorotibial | Medial BME | 27/85 | 9/50 | 2.2 (1.0; 0.9) | 11/30 | 25/105 | 2.1 (0.8; 5.2) | 10/22 | 26/113 | 3.2 (1.1; 9.4)* |
| Medial Cyst | 5/85 | 1/50 | − (0.4; -) | 0/30 | 6/105 | 0.0 (0; 5.8) | 3/22 | 3/113 | 17.4 (2.1; 187.8)* | |
| Lateral femorotibial | Lateral BME | 12/85 | 6/50 | 1.2 (0.4; 3.8) | 4/30 | 14/105 | 1.1 (0.3; 3.4) | 5/22 | 13/113 | 2.9 (0.8; 10.1) |
| Lateral Cyst | 4/85 | 2/50 | 1.3 (0.2;9.8) | 2/30 | 4/105 | 0.0 (0; 3.5) | 1/22 | 5/113 | 1.9 (0.1; 15.2) | |
BME=bone marrow edema pattern; Multivariate regression analysis
P<0.05
A: Adjusted1 means × SEM and differences are presented for numeric outcomes.
B: The ratio n (abnormal scores)/ n (total) is presented for dichotomous variables (ratios are not adjusted; P-values, odds ratios and confidence intervals are adjusted1).
All regression models were adjusted for age, gender and OA risk factors, including previous injury or surgery at the knee, family history of joint replacement, presence of Herbeden's nodes and BMI.
Morphological femorotibial abnormalities
Only trochlear depth was associated with an increased WORMS score at the medial tibiofemoral compartment (5.0 ±0.8 for subjects with a deep trochlea versus 7.4 ±0.7 for subjects with a shallow trochlea; P=0.003; Table 3). Subjects with large sulcus angles had significantly higher WORMS scores at the lateral tibiofemoral compartment compared to controls (8.2 ±1.3 versus 5.4 ±0.7; P=0.026). Neither trochlear depth (medial meniscus, P=0.400; lateral meniscus, P=0.110) nor trochlear facetal ratio (P=0.073; P=0.532) was significantly associated with meniscus abnormalities. The mean WORMS score for the medial meniscus was 2.2 ±0.2 for the cohort with abnormal trochlear depth and 1.9 ±0.3 for controls (P=0.400). Subjects with an abnormal sulcus angle had more severe lateral meniscus lesions than corresponding controls (2.9 ±0.4 versus 1.8 ±0.2; P=0.012). For the medial femorotibial compartment, subjects with a shallow trochlea had significantly increased maximum cartilage scores (4.0 ±0.3 versus 2.6 ±0.4; P=0.010).
T2 relaxation time measurements
Global T2 relaxation time of the entire knee was not significantly associated with trochlear depth, trochlear facetal ratio or sulcus angle (P=0.442, P=0.903 and 0.541; Table 4). For individuals with abnormal trochlear depth, the mean global T2 value was 44.4 ±0.3 ms, while the control group had a mean global value of 44.9 ±0.4 ms. When femoropatellar compartments were analyzed separately, individuals with a shallow trochlea had significantly lower T2 values than controls at the patellar compartment (40.9 ±0.5 ms versus 42.7 ±0.6 ms; P=0.037). Patellar T2 values did not show a significant difference in individuals with abnormal trochlear facetal ratio compared to controls (40.8 ±0.9 ms versus 41.8 ±0.4 ms; P=0.310) or in individuals with an abnormal sulcus angle compared to controls (41.5 ±1.0 ms versus 41.7 ±0.5 ms; P=0.464).
Table 4.
Mean cartilage T2 relaxation time values (ms) for subjects with trochlear dysplasia.
| T2 | Trochlear depth | Facetal ratio | Sulcus angle | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compartment | Shallow n=85 | Normal n=50 | P | Difference (lower 95% CI; upper 95% CI) | Abnormal n=30 | Normal n=105 | P | Difference (lower 95% CI; upper 95% CI) | Abnormal n=22 | Normal n=113 | P | Difference (lower 95% CI; upper 95% CI) |
| Global | 44.4 ×0.3 | 44.9 ×0.4 | 0.442 | 0.5 (−0.6; 1.4) | 44.7 ×0.6 | 44.6 ×0.3 | 0.903 | 0.1 (−1.1; 1.4) | 44.5 ×0.7 | 44.6 ×0.3 | 0.541 | 0.4 (−1.0; 1.8) |
| Patella | 40.9 ×0.5 | 42.6 ×0.6 | 0.037* | 1.7 (0.2; 3.1) | 40.8 ×0.9 | 41.8 ×0.4 | 0.306 | 1.1 (−0.8; 2.9) | 41.5×1.0 | 41.7 ×0.5 | 0.464 | 0.8 (−1.3; 2.8) |
| Trochlea | 45.7 ×0.5 | 45.7 ×0.6 | 0.973 | 0.1 (−1.3; 1.5) | 45.9 ×0.4 | 44.9 ×0.8 | 0.251 | 1.0 (−0.7; 2.7) | 46.2 ×1.0 | 45.6 ×0.5 | 0.873 | 0.2 (−1.8; 2.1) |
| Medial femorotibial | 45.7 ×0.6 | 46.0 ×0.6 | 0.667 | 0.2 (−0.9; 1.4) | 46.2 ×0.8 | 45.8 ×0.5 | 0.430 | 0.4 (−0.9; 1.8) | 45.6 ×0.8 | 45.9 ×0.4 | 0.489 | 0.6 (−1.0; 2.2) |
| Lateral femorotibial | 44.4 ×0.6 | 44.4 ×0.7 | 0.901 | 0.0 (−1.2; 1.3) | 44.8 ×0.8 | 44.3 ×0.6 | 0.280 | 0.7 (−0.8; 2.1) | 44.1 ×0.8 | 44.4 ×0.4 | 0.604 | 0.5 (−1.3; 2.2) |
Subjects with an abnormal trochlear depth (shallow), medial-to-lateral trochlea facetal ratio or sulcus angle were compared to those subject with normal values.
P<0.05
Patella cartilage volume measurements
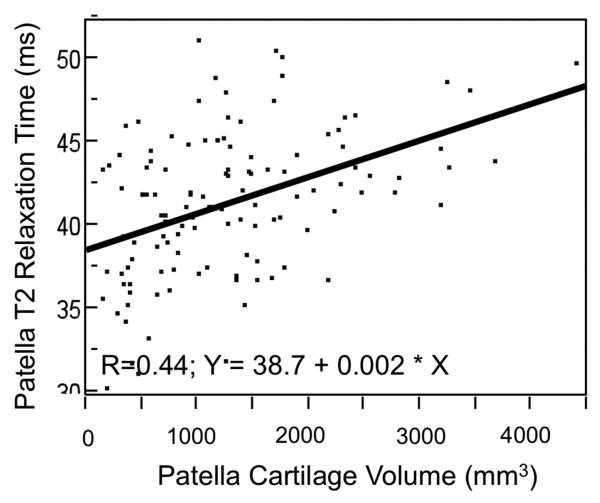
Given the unexpected low T2 values at the patella, the association of shallow trochlea and lower T2 values was investigated by including patellar cartilage volume in the multivariate models because there are hints that T2 values do not increase further or even decrease with advanced cartilage loss and OA. Increasing patellar T2 values correlated significantly with increasing cartilage volume (Figure 3; R=0.44; P<0.001). Including cartilage volume (mm3) in the regression model, it showed a significant relationship with patellar T2 values (P=0.003) and the influence of trochlear depth was eliminated (P=0.673). Low trochlear depth was further associated with a small cartilage volume at the patella (P<0.001; shallow trochlea, 900 ±72 mm3, control, 1671 ±95 mm3). Individuals with an abnormal facetal ratio also had smaller cartilage volume than individuals with normal facetal ratios, but the difference was not significant (1237 ±73 mm3 versus 1004 ±137 mm3; P=0.263). An abnormal sulcus angle was significantly associated with smaller patellar cartilage volume (795 ±158 mm3 versus 1259 ±94 mm3; P<0.001).
Figure 3.
Scatter plot with bivariate linear fit, visualizing the correlation of patellar cartilage T2 values with patellar cartilage volume. Smaller cartilage volumes were associated with lower T2 relaxation times.
Discussion
This cross-sectional study study examined the association of trochlear dysplasia, assessed by 3.0T MRI and defined as (i) a trochlear depth of 3 mm or less, (ii) a medial-to-lateral facet ratio of 0.4 or less and (iii) a sulcus angle of >170° with the presence and severity of MRI findings of knee OA. Patellofemoral WORMS scores were significantly higher in the group with a shallow trochlea and patellar cartilage volume was significantly lower in the group with a shallow trochlea, suggesting a strong association of trochlear dysplasia with more severe patellofemoral joint degeneration.
A dysplastic joint component can potentially lead to early degeneration and damage of the joint. At the knee, a dysplastic trochlea has been shown to contribute to patellar maltracking and recurrent dislocations [21]. Minor dislocations can cause instability and chronic stress on the cartilage, which may lead to early OA. This was previously shown by Dejour et al on knee radiographs and computed tomography. On a lateral knee radiograph, the “crossing sign” was described, a geometrical abnormality at the cranial portion of the trochlea that prevents proper engagement of the patella during the early phases of knee flexion [22]. However, two-dimensional imaging may lead to misinterpretation of the patellar morphology [7]. Therefore, 3.0T MRI was used in our study for a more detailed analysis of cartilage, tendon and bone marrow and found similar results; individuals with lower trochlear depth showed significantly increased patellofemoral degeneration. Pfirrmann et al previously demonstrated a correlation of a trochlear depth of ≤3 mm or a medial-to-lateral facetal ratio of ≤0.4, measured in MR images, with trochlear dysplasia, diagnosed in lateral knee radiographs [6]. Further, the parameter “sulcus angle” was determined due to its clinical relevance regarding the diagnosis of trochlear dysplasia and postoperative follow-up measurements [9, 23, 24]. A sulcus angle of >150° on plain radiographs was reported to indicate trochlear dysplasia [9]. However, radiographs underestimate this angle as compared to MR measurements [8]. The sulcus can be measured either from the subchondral bone or from the articular cartilage; both have been shown to be highly accurate [10]. Van Huyssteen et al described a mean bony sulcus angle of 168° as measured in MRI, while the mean cartilage sulcus angle was 187° [25]. Using the osseous surface as a reference, Toms et al reported that the sulcus angle was larger in patients with severe cartilage defects (mean = 173°) than in patients with normal cartilage (mean = 151°) in a young patient cohort (<40 years) [10]. Salzmann et al. found a mean sulcus angle of 164° in patients with type B dysplasia (144° in plain radiographs) and 168° in patients with type C dysplasia (146° in plain radiographs) [8]. Based on these findings, 170° was selected as a threshold for an abnormal bony sulcus angle in our MRI analyses, which was present in 22/ 135 subjects.
The ability to detect morphological trochlear abnormalities on an MRI study can potentially influence the management choices of the referring clinician. Individuals who underwent a Henri Dejour trochleoplasty for a dysplastic trochlea reported an improvement in symptoms [26].
Total patellofemoral WORMS score was increased in individuals with trochlear dysplasia. Once cartilage loss occurs, changes in MRI morphology are frequently seen [16]. WORMS values for cartilage lesions, bone marrow edema pattern, cysts and ligament abnormalities, were increased in the trochlear dysplasia group in this study.
Global T2 values, which are commonly used in the evaluation of early intrasubstance cartilage degeneration [27], were similar between the two groups. For patellar cartilage however, T2 values of individuals with low trochlear depth demonstrated significantly lower T2 values. These findings were unexpected, surprising and contradictory, since higher T2 values usually correlate with presence of OA [28]. On the contrary, the lower T2 values may be explained by greater cartilage loss in subjects with low trochlear depth, since significantly more cartilage abnormalities were detected in the trochlear dysplasia group. At least half of the individuals with low trochlear depth presented with full thickness cartilage loss at the patella (WORMS 5 or 6). Prior studies have shown that although T2 relaxation time is correlated to histological degeneration of cartilage and a good marker for early OA, it may not be suitable for analysis of advanced degenerative joint disease [29]. David-Vaudey et al noticed early stage OA was associated with increased T2, followed by slightly lower T2 values for more severe lesions. They explained these findings by the fact that changes in collagen fibril anisotropy, associated with an increased T2, precede changes in collagen content and a loss of water content, associated with slightly declining T2 [30]. Further, during OA progression, the region most heavily affected by cartilage loss is the superficial cartilage layer, which also happens to account for the highest T2 values [31]. With the additional risk factor of trochlear dysplasia, T2 values may not increase further with worsened cartilage degeneration, which is consistent with Crema et al [13]. The additional cartilage loss, especially the superficial layer, which usually incooperates high T2 values, may be responsible for the unexpected results. Therefore, cartilage volume at the patella was investigated and a significant association of lower cartilage volume with an abnormal trochlea depth was detected. Including cartilage volume in the multivariate regression model eliminated the significant influence of trochlea abnormalities on patella T2 values but instead a low cartilage volume was associated with lower T2 values. The additional cartilage loss likely accounts for the decrease in T2 values and is highlighting the careful interpretation of T2 relaxation time measurements in the context of morphological cartilage loss in subjects with advanced OA.
There are several limitations to this study. First, this study was cross-sectional and a longitudinal study is needed to better characterize the relationship between trochlear dysplasia and OA. In a cross-sectional study, effect cause has to be considered as an alternative possibility. Patellofemoral OA could potentially lead to secondary trochlear remodeling, resulting in abnormal trochlear depth, abnormal facetal ratio and abnormal sulcus angle. Individuals were recruited from the OAI progression cohort and all subjects already had OA, many of them already had severe OA changes at the patellofemoral joint, which made the interpretation of T2 relaxation time measurements challenging. Further research is needed to determine if irregular trochlear morphology is also found in normal participants or participants with early OA. Moreover, only the shallow trochlea (n=85) and control participants (n=50) were included in analyses for an abnormal facetal ratio; the studies knees may not be representative of all knees with abnormal facetal ratios or sulcus angles.
In summary, our study demonstrated that trochlear dysplasia, defined by a shallow trochlea, a low medial-to-lateral trochlear facetal ratio or an abnormal sulcus angle, was associated with MRI findings indicating patellofemoral OA, including higher WORMS scores and smaller patella cartilage volume. In conclusion, these findings demonstrate that detecting and especially monitoring morphological trochlear properties on 3.0T MRI may be clinically relevant to identify early OA patients and may be important for risk evaluation, treatment decisions and further follow-up of subjects at risk for patellofemoral OA.
Acknowledgements
This study was funded by NIH U01 AR059507 and P50 AR060752 as well as through the OAI, which is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
Footnotes
Conflict of interest disclosure No conflict of interest to declare for any of the authors.
References
- 1.Christian SR, Anderson MB, Workman R, Conway WF, Pope TL. Imaging of anterior knee pain. Clin Sports Med. 2006;25(4):681–702. doi: 10.1016/j.csm.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Hedayati B, Saifuddin A. Focal lesions of the patella. Skeletal Radiol. 2009;38(8):741–749. doi: 10.1007/s00256-009-0699-5. [DOI] [PubMed] [Google Scholar]
- 3.Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):235–240. doi: 10.1007/s00167-005-0683-0. [DOI] [PubMed] [Google Scholar]
- 4.Malghem J, Maldague B. Depth insufficiency of the proximal trochlear groove on lateral radiographs of the knee: relation to patellar dislocation. Radiology. 1989;170(2):507–510. doi: 10.1148/radiology.170.2.2911676. [DOI] [PubMed] [Google Scholar]
- 5.Grelsamer RP, Tedder JL. The lateral trochlear sign. Femoral trochlear dysplasia as seen on a lateral view roentgenograph. Clin Orthop Relat Res. 1992;(281):159–162. [PubMed] [Google Scholar]
- 6.Pfirrmann CW, Zanetti M, Romero J, Hodler J. Femoral trochlear dysplasia: MR findings. Radiology. 2000;216(3):858–864. doi: 10.1148/radiology.216.3.r00se38858. [DOI] [PubMed] [Google Scholar]
- 7.Koeter S, Bongers EM, de Rooij J, van Kampen A. Minimal rotation aberrations cause radiographic misdiagnosis of trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2006;14(8):713–717. doi: 10.1007/s00167-005-0031-4. [DOI] [PubMed] [Google Scholar]
- 8.Salzmann GM, Weber TS, Spang JT, Imhoff AB, Schottle PB. Comparison of native axial radiographs with axial MR imaging for determination of the trochlear morphology in patients with trochlear dysplasia. Arch Orthop Trauma Surg. 2010;130(3):335–340. doi: 10.1007/s00402-009-0912-y. [DOI] [PubMed] [Google Scholar]
- 9.Diederichs G, Issever AS, Scheffler S. MR imaging of patellar instability: injury patterns and assessment of risk factors. Radiographics. 2010;30(4):961–981. doi: 10.1148/rg.304095755. [DOI] [PubMed] [Google Scholar]
- 10.Toms AP, Cahir J, Swift L, Donell ST. Imaging the femoral sulcus with ultrasound, CT, and MRI: reliability and generalizability in patients with patellar instability. Skeletal Radiol. 2009;38(4):329–338. doi: 10.1007/s00256-008-0639-9. [DOI] [PubMed] [Google Scholar]
- 11.Fitoussi F, Akoure S, Chouteau Y, Bouger D. Hollow femoral trochlea and femoropatellar osteoarthritis. Rev Chir Orthop Reparatrice Appar Mot. 1994;80(6):520–524. [PubMed] [Google Scholar]
- 12.Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45–54. [PubMed] [Google Scholar]
- 13.Crema MD, Roemer FW, Marra MD, Burstein D, Gold GE, Eckstein F, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics. 2011;31(1):37–61. doi: 10.1148/rg.311105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 15.Peterfy CG, Guermazi A, Zaim S, Tirman PFJ, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects with higher physical activity levels have more severe focal knee lesions diagnosed with 3T MRI: analysis of a non-symptomatic cohort of the osteoarthritis initiative. Osteoarthritis Cartilage. 2010;18(6):776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum T, Stehling C, Joseph GB, Carballido-Gamio J, Schwaiger BJ, Muller-Hocker C, et al. Changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis and their association with focal knee lesions at baseline: Data from the osteoarthritis initiative. J Magn Reson Imaging. 2011 doi: 10.1002/jmri.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baum T, Joseph GB, Nardo L, Virayavanich W, Arulanandan A, Alizai H, et al. MRI-based knee cartilage T2 measurements and focal knee lesions correlate with BMI - 36 month follow-up data from the Osteoarthritis initiative. Arthritis Care Res (Hoboken) 2012 doi: 10.1002/acr.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19(8):984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jungmann P, Kraus M, Nardo L, Liebl H, Alizai H, Joseph G, et al. T2 relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur. Longitudinal data from the Osteoarthritis Initiative. J Magn Reson Imaging. 2013 doi: 10.1002/jmri.24137. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dandy DJ. Chronic patellofemoral instability. J Bone Joint Surg Br. 1996;78(2):328–335. [PubMed] [Google Scholar]
- 22.Dejour H, Walch G, Nove-Josserand L, Guier C. Factors of patellar instability: an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc. 1994;2(1):19–26. doi: 10.1007/BF01552649. [DOI] [PubMed] [Google Scholar]
- 23.Fucentese SF, Schottle PB, Pfirrmann CW, Romero J. CT changes after trochleoplasty for symptomatic trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc. 2007;15(2):168–174. doi: 10.1007/s00167-006-0140-8. [DOI] [PubMed] [Google Scholar]
- 24.Schottle PB, Fucentese SF, Pfirrmann C, Bereiter H, Romero J. Trochleaplasty for patellar instability due to trochlear dysplasia: A minimum 2-year clinical and radiological follow-up of 19 knees. Acta Orthop. 2005;76(5):693–698. doi: 10.1080/17453670510041781. [DOI] [PubMed] [Google Scholar]
- 25.van Huyssteen AL, Hendrix MR, Barnett AJ, Wakeley CJ, Eldridge JD. Cartilage-bone mismatch in the dysplastic trochlea. An MRI study. J Bone Joint Surg Br. 2006;88(5):688–691. doi: 10.1302/0301-620X.88B5.16866. [DOI] [PubMed] [Google Scholar]
- 26.Verdonk R, Jansegers E, Stuyts B. Trochleoplasty in dysplastic knee trochlea. Knee Surg Sports Traumatol Arthrosc. 2005;13(7):529–533. doi: 10.1007/s00167-004-0570-0. [DOI] [PubMed] [Google Scholar]
- 27.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan J, Pialat JB, Joseph T, Kuo D, Joseph GB, Nevitt MC, et al. Knee Cartilage T2 Characteristics and Evolution in Relation to Morphologic Abnormalities Detected at 3-T MR Imaging: A Longitudinal Study of the Normal Control Cohort from the Osteoarthritis Initiative. Radiology. 2011 doi: 10.1148/radiol.11102234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging. 2004;22(5):673–682. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 30.Akizuki S, Mow VC, Muller F, Pita JC, Howell DS. Tensile properties of human knee joint cartilage. II. Correlations between weight bearing and tissue pathology and the kinetics of swelling. J Orthop Res. 1987;5(2):173–186. doi: 10.1002/jor.1100050204. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Kuo D, Theologis A, Carballido-Gamio J, Stehling C, Link TM, et al. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2--initial experience with 1-year follow-up. Radiology. 2011;258(2):505–514. doi: 10.1148/radiol.10101006. [DOI] [PMC free article] [PubMed] [Google Scholar]