Abstract
Apart from being applied as an energy carrier, hydrogen is in increasing demand as a commodity. Currently, the majority of hydrogen (H2) is produced from fossil fuels, but from an environmental perspective, sustainable H2 production should be considered. One of the possible ways of hydrogen production is through fermentation, in particular, at elevated temperature, i.e. thermophilic biohydrogen production. This short review recapitulates the current status in thermophilic biohydrogen production through fermentation of commercially viable substrates produced from readily available renewable resources, such as agricultural residues. The route to commercially viable biohydrogen production is a multidisciplinary enterprise. Microbiological studies have pointed out certain desirable physiological characteristics in H2-producing microorganisms. More process-oriented research has identified best applicable reactor types and cultivation conditions. Techno-economic and life cycle analyses have identified key process bottlenecks with respect to economic feasibility and its environmental impact. The review has further identified current limitations and gaps in the knowledge, and also deliberates directions for future research and development of thermophilic biohydrogen production.
Keywords: Thermophilic, Biohydrogen, Agricultural residues, Techno-economic analysis, LCA
Introduction
Our heavy dependency on fossil energy sources has created societal problems related to its inherent environmental pollution. It urges for alternative, clean and renewable sources, fuelling huge research interests among scientists, albeit without any economic success so far. The utopian world of energy sufficiency, without any hazardous emissions, is thought to be plausible by many, if only renewable hydrogen (H2) could replace fossil-based energy carriers (Schrope 2001). As early as 1874, Jules Verne fancied the concept of ‘Hydrogen economy’ in his book The Mysterious Island (Hoffmann 2001). Indeed, as a fuel, H2 has many desirable properties, among others, rapid burning speed, higher energy yield, low minimum ignition point and very high octane number (Ingersoll 1996; Mu et al. 2006; Balat and Kirtay 2010; Luque et al. 2011). However, introducing H2 into the society faces several key technical barriers including storage, delivery and its end-user applications (Balat and Kirtay 2010).
By far, petroleum refineries are the largest producers (non-merchant) and consumers of H2 (Freedonia 2010). However, the increasing demand of low sulphur and clean-burning fossil fuels can be a driver for renewable (merchant) H2 applied in petroleum refineries (Freedonia 2010). Moreover, other applications of H2 as a non-fuel commodity in chemical manufacturing, glass making, heat treatment of metals and hydrogenation of processed foods will also contribute to higher near-future demands for renewable (merchant) H2. In a recent study, it has been estimated that the cost price of H2 as a non-fuel is about 1–2 €/kg H2 as based on estimated oil prices in 2020 (Mansilla et al. 2012). Hence, it has become of the utmost importance to develop an efficient method to produce H2 from renewable feedstocks. This review attempts to bring together the current status of thermophilic fermentative H2 production as one of those methods. We also propose a list of preferred properties (A to I) that an ideal H2-producing microorganism should possess. These properties are discussed throughout the review.
Microorganisms for thermophilic fermentative hydrogen production
Pure culture studies
Higher temperatures (≥60 °C) are energetically more favourable for biological H2 production (Stams 1994), enabling thermophiles to reach higher H2 yields than mesophiles (Schönheit and Schäfer 1995, property A). As a consequence, thermophiles produce fewer by-products, i.e. especially acetic acid as its generation is accompanied with formation of ATP (Kengen et al. 2009). Moreover, strictly anaerobic thermophilic conditions seem to restrict contamination by hydrogenotrophic methanogens. In general, thermophilic H2 producers have also higher H2 tolerances; however, the latter varies depending on the sugar(s) present in the feedstock (Willquist et al. 2011). Even so, a drawback of thermophiles is their relatively low volumetric productivity, as their tendency to grow in lower cell densities in suspension cultures than mesophiles (Chou et al. 2008). The highest biological H2 productivities ever reported have been for mesophilic cultures (Das 2009), but their accompanying low H2 yields remain a critical problem.
Thermophilic H2 producers are found within both the bacterial and the archaeal domain, and several of them have been characterized with their genome annotated (see list in VanFossen et al. 2008). Most of them are able to hydrolyse various polysaccharides (Blumer-Schuette et al. 2008) and can ferment the released hexoses and pentoses to H2 with yields close to the theoretical maximum (or Thauer limit, 4 mol H2/mol hexose; Thauer et al. 1977). Recent reviews list many of those microorganisms involved (Kengen et al. 2009; van Niel et al. 2011), so here, only a selection of the best performers is given (Tables 1 and 2). Most of these organisms were isolated from extremely hot and reducing conditions (Fiala and Stetter 1986; Huber et al. 1986; Rainey et al. 1994; Xue et al. 2001; Mäkinen et al. 2009), under which they produce reduced metabolic end products, including H2, as an electron sink for reducing equivalents (Thauer et al. 1977).
Table 1.
Overview of thermophilic hydrogen producing microorganisms (continued from Kengen et al. 2009)
| Organism | Domain | T opt (°C) | Cultivation | Substrate | Y H2 mmol/mmol C6 | References |
|---|---|---|---|---|---|---|
| Thermobrachium celere | Bacteria | 67 | Batch | Glucose | 3.36 | (Ciranna et al. 2011) |
| Clostridium stercorarium DSM 2910 | Bacteria | 58 | Continuous | Lactose | 1.57 | (Collet et al. 2004) |
| Thermovorax subterraneus | Bacteria | 70 | Batch | Glucose | 1.4 | (Mäkinen et al. 2009) |
Table 2.
Metabolic features of thermophilic hydrogen producers (modified and continued from Chou et al. 2008)
| Organism | Fermentability of feedstocks/polymers | CCR | Auxotrophy to amino acids | Electron carriers | Hydrogenasea | Reductant sink | References |
|---|---|---|---|---|---|---|---|
| Clostridia (Cl. thermocellum) | Starch, cellulose, lignocellulose | Yes | No | NADH, ferredoxin | Uptake, Fe-only, FNOR | Alcohol, organic acids, lactate | Johnson et al. (1981), Desvaux (2006) |
| Thermococcales (Pyroccus furiosus) | Maltose, cellobiose, β-glucans, starch | No | Yes | Ferredoxin | MBH, NiFe-only, FNOR | Alanine, ethanol | Hoaki et al. (1994), Maeder et al. (1999), Silva et al. (2000), Robb et al. (2001) |
| Thermotogales (T. maritima/T. neapolitana) | Cellulose, xylan, starch, cellobiose, lignocellulose | Yes | No | NADH, ferredoxin | Fe-only, NMOR, FNOR | Lactate, alanine | Schönheit and Schäfer (1995), Vargas and Noll (1996), Rinker and Kelly (2000), Bonch-Osmolovskaya (2001) |
| Caldicellulosiruptor (C. saccharolyticus) | Cellulose (avicel, amorp.), xylan, pectin, α-glucan, β-glucan, lignocellulose, guargum | No | No | NADH, ferredoxin | Fe-only, NiFe-only | Lactate, ethanol | Rainey et al. (1994), de Vrije et al. (2007), van de Werken et al. (2008), Ivanova et al. (2008), Willquist and van Niel (2012) |
| Thermoanaerobacter (T. tengcongensis MB4) | Starch, sucrose, glycerol | Yes | Yes | NADH, Ferredoxin | Fe-only, NiFe-only | Ethanol | Xue et al. (2001), Warner and Lolkema (2003), Soboh et al. (2004) |
CCR carbon catabolite repression
aTypes of hydrogenases—uptake, NiFe type hydrogen uptake hydrogenase, FNOR (ferredoxin:NAD(P)H oxidoreductase), Fe-only, Fe-only evolution hydrogenase, NiFe-only, NiFe-only evolution hydrogenase, NMOR (NADH:methylviologen oxidoreductase) and MBH (membrane-bound hydrogenase)
Under stressful conditions of high H2 partial pressures and/or high medium osmolality, organisms tend to shift their metabolism to other reduced end products such as lactate, ethanol and alanine (Table 2; Kengen et al. 2009; Willquist et al. 2009, 2011), which in turn affect the H2 yield negatively. Although, when purified, these by-products have a reasonable market value for a variety of purposes, but their concentration in the effluent will be too low for an economically viable downstream process. Alternatively, microorganisms can be engineered to produce more valuable product under stress (property G). On the other hand, the by-products can be fed to a complementary process, which can further produce valuable products including H2 and methane (Hallenbeck and Ghosh 2009).
Defined and undefined culture studies
As an alternative to pure cultures, enrichment cultures can be employed for H2 production. Such enrichment cultures are usually obtained from methanogenic anaerobic digesters. In some cases, household or municipal waste is also used for enriching hydrogenogenic microorganisms (Table 3). Advantages of enriched consortia are their (1) higher robustness to fluctuations in the fermentation process, (2) proneness to form biofilms and (3) are able to tackle more different substrates, thus improving conversion efficiencies (Brenner et al. 2008). In addition, at industrial scale, sterilization of feedstocks is not cost-effective; so during the fermentation, consortia can offer resilience to any contamination. On the other hand, consequences are that methods are required to suppress methanogenic activities (O-Thong et al. 2008a, b), and all studies with enrichments showing best performances have H2 yields hardly exceeding 2.5 mol H2/mol hexose (first four rows in Table 3). The latter could be inherent to the history of the inoculum containing also non-hydrogen-producing microorganisms (Chaganti et al. 2012; Kargi et al. 2012). This might not be an issue for biowaste streams as feedstock, but it is of importance when considering more costly energy crops.
Table 3.
Selection of thermophilic H2 production using mixed/pure culture in various reactor types and/or industrial media. First four are best cases using model substrates
| Reactor type | Conditions | Feedstock/substrate | Organism/source of inoculum for mixed culture | Enrichment with complex substrate | H2 yield | Q H2 (mM/h) | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Method of cultivation | HRT (h) | T (°C) | pH (C/nC) | ml-H2/gVS | mol/C6 mol | ||||||
| CSTR | Continuous | 2.86 | 72 | 6.7 (C) | Glucose | Caldicellulosiruptor saccharolyticus | Yeast extract | ND | 3.0 | 12.4 | de Vrije et al. (2007) |
| CSTR + carrier | Continuous | 3 | 58 | 6 (C) | Glucose | Geothermal spring, Hveravellir, Iceland | Yeast extract | ND | 1.54 | 45.80 | Koskinen et al. (2008) |
| Gas lift fermentor | Continuous | 5 | 85 | 6 (C) | Pyruvate | Thermococcus kodakaraensis KOD1 | Peptone | ND | 2.18† | 9.46 | Kanai et al. (2005) |
| UASB | Continuous | 0.75 | 60 | 5 (C) | Sucrose | Thermoanaerobacterium thermosaccharolyticum PSU-2 | Peptone | ND | 1.3 | 152 | O-Thong et al. (2008a, b) |
| UASB | Continuous | 24 | 70 | 5.1 (nC) | Wheat straw hydrolysate | ND | Yeast extract | 89.00 | ND | 1.52 | Kongjan et al. (2010a, b) |
| CSTR | Continuous | 24 | 70 | 7 (nC) | Pig slurry | Raw pig slurry | – | ND | ND | ~4.6 | Kotsopoulos et al. (2009) |
| CSTR | Continuous | 24 | 55 | 5.25 (nC) | Rapeseed straw stillage from ethanol plant | Thermophilic anaerobic digested manure from biogas plant | – | 40.00 | ND | 6.04 | Luo et al. (2011) |
| CSTR | Continuous | 12 | 60 | 6.8 (C) | Sugar factory waste water | Sludge compost | – | ND | 2.5 | 8.30 | Ueno et al. (1996) |
| CSTR | Continuous | 4 | 60 | 5.5 (C) | Tofu waste water + glucose | Hydrogenogenic labscale CSTR | – | ND | 2.3 | 20.70 | Kim and Lee (2010) |
| biofilm | Continuous | 3 | 55 | 5 (C) | Sucrose | Hydrogenogenic labscale CSTR | Yeast extract | ND | 1.59 | 4.66 | Keskin et al. (2011) |
| Anaerobic filter | Continuous | 24 | 70 | 5.4 (nC) | Wheat straw hydrolysate | Enriched hydrogenogenic culture in CSTR | Yeast extract | ND | ND | 0.85 | Kongjan et al. (2010a, b) |
| Membrane bioreactor | Continuous | 4 | 60 | 5.5 (C) | Tofu waste water | Municipal sewage | – | ND | 1.45 | 34.25 | Kim et al. (2011a, b) |
| Upflow anaerobic | Continuous | 2 | 55 | 5.5 (C) | Rice winery wastewater | Municipal sewage | – | ND | 1.9 | 3.81 | Yu et al. (2002) |
| Semi-continuous | Continuous | 16 | 60 | 5.5 (nC) | Cassava stillage | UASB treating cassava stillage | – | 56.70 | ND | 6.21 | Luo et al. (2010) |
| UASB | Continuous | 24 | 55 | nd | De-sugared molasses | Anaerobic digested manure | Yeast extract | 159.60 | ND | 7.76 | Kongjan et al. (2011) |
| EGSB | Continuous | 6 | 70 | nd | Glucose, arabinose | Household solid waste | – | ND | ND | 4.66 | Abreu et al. (2010) |
| ASBR | Batch | 96 | 60 | 5.5 (C) | palm oil mill effluent (POME) | Palm oil mill wastewater treatment plant | Peptone | ND | 2.60 | 1.08 | O-Thong et al. (2008a, b) |
| ASBR | Batch | 48 | 60 | 5.5 (C) | POME | Palm oil mill wastewater treatment plant | Peptone | ND | ND | 16.90 | Prasertsan et al. (2009) |
EGSB expanded granular sludge blanket, C pH controlled, nC pH not controlled, ND not determined, † estimated
Besides pure culture and undefined cultures, an interesting third option is to design cocktails of H2-producing strains that either possess complementary sugar preferences or display synergies, thereby increasing the H2 yield and conversion efficiencies. In this respect, only few studies have been carried out so far for thermophilic H2 production. Liu et al. (2008) investigated a natural co-culture of Clostridium thermocellum and Thermoanaerobacterium thermosaccharolyticum isolated from decomposing wheat straw. In batch fermentations on cellulose, the co-culture reached higher H2 yields (1.8 mol H2/mol glucose) than Clostridium thermocellum alone (0.8 mol H2/mol glucose) mainly due to higher conversion efficiency. C. thermocellum hydrolyzed the cellulose but was not able to consume all glucose and cellobiose. T. thermosaccharolyticus thus fermented part of the sugar and possibly the lactate produced by the other partner (Liu et al. 2008). In another study, synergies were found between two Caldicellulosiruptor species each originating from a different habitat (Zeidan et al. 2010). The synergy could be based on the excretion of one or several compounds by one of the species, Caldicellulosiruptor saccharolyticus that stimulated both the growth rate and biomass yield of the second species Caldicellulosiruptor kristjanssonii. This co-culture revealed a remarkable stability in continuous culture even when sharing one carbon and energy source in the medium. Moreover, this co-culture possessed better H2 yields (3.7 mol H2/mol glucose) than either species alone (3.5 mol H2/mol glucose) under the same conditions (Zeidan et al. 2010). As has been reviewed recently (Ren et al. 2011), other defined co-culture studies have been carried out focusing on improved thermophilic cellulose hydrolysation, in which synergistic mechanisms play a major role.
Enzymes of thermophilic hydrogen production
Hydrogenases
Evolution of H2 through reduction of a proton is carried out by metalloenzymes, i.e. hydrogenases, which differ with respect to their size, structure, electron donors, and metal ions present in their active site (Meyer 2007; Vignais and Billoud 2007). Hydrogenases are known to be very sensitive to oxygen (O2) (Vignais and Billoud 2007); even 1 % of O2 can completely inhibit their H2-forming capacity but not H2 oxidation (Lukey et al. 2011). Hallenbeck and Gosh (2009) and Hallenbeck et al. (2012) argue that, for a variety of reasons, a limited amount of aerobic respiration along with fermentation may help achieve H2 yields near the absolute maximum (12 mol/mol of hexose) through complete conversion of glucose to CO2 via the TCA cycle. A recent study revealed successful engineering of an O2-tolerant [NiFe] hydrogenase, through site-directed mutagenesis (Lukey et al. 2011). In addition, native O2-tolerant hydrogenases have been found in Ralstonia eutropha H16 (Burgdorf et al. 2005) and Aquifex aeolicus (Guiral et al. 2006). Such O2-tolerant hydrogenases could be instrumental for performing micro-aerobic fermentations (property I). However, further research is needed to assess the validity of this hypothesis.
Hydrogenases use NADH or reduced ferredoxin (Fdred) as electron donors, which are formed in the catabolism of organic substrates (Kengen et al. 2009). Under standard conditions, the mid-point redox potential for redox couples, NAD+/NADH and oxidized ferredoxin (Fdox)/Fdred is −320 and −398 mV, respectively (Thauer et al. 1977), which clearly indicates that Fd-dependent H2 production is thermodynamically more favourable (property C). Alternatively, other relatively uncommon hydrogenases, such as ferredoxin:NAD(P)H oxidoreductase (FNOR, or electron-bifurcating hydrogenases) and membrane-bound hydrogenases (MBH), can also be desired for an ideal H2 producer. FNOR produces H2 using both NADH and Fdred simultaneously, by coupling unfavourable oxidation of NADH with exergonic oxidation of Fdred (Schut and Adams 2009), whereas MBH conserves valuable energy by coupling H2 evolution to ATP synthesis via proton translocation (Sapra et al. 2003).
Redox enzymes
The central carbon metabolism of thermophilic H2 producers has diverse metabolic pathways to reduce electron carriers, i.e. Fd or NAD+. Bacterial H2 producers oxidize glyceraldehyde-3-phosphate (GAP) via GAP dehydrogenase generating one ATP and one NADH in the reaction. However, re-oxidation of the latter to H2 is inherent to a thermodynamic constraint and thus instead it might easily be oxidized to undesired electron sinks, such as lactate and/or ethanol (for details, see Bielen et al. 2013). In contrast, archaeal H2 producers have a unique enzyme, GAP oxidoreductase (GAPOR), which oxidizes GAP generating one Fdred but no ATP. Thus, introduction of GAPOR in bacterial H2 producers may redirect more pyruvate flux towards acetate generating the required ATP and will consequently improve H2 yields. In addition, a host of other redox enzymes may also be involved in oxidation of substrates other than conventional sugars such as glycerol or rhamnose.
Similarly, at the pyruvate node, most of the distinguished thermophilic H2 producers possess pyruvate:ferredoxin oxidoreductase (PFOR), which oxidizes pyruvate to generate Fdred (Carere et al. 2012). In contrast, most mesophilic H2 producers possess pyruvate:formate lyase (PFL) which generates formate (Carere et al. 2012). Some mesophilic organisms containing PFL also possess FHL to oxidize formate to CO2 and H2. Nevertheless, PFOR remains a better enzyme for oxidation of pyruvate, contributing to higher H2 yields in thermophilic H2 producers.
Reactors and culture conditions applied for thermophilic biohydrogen production
Conventional and advanced bioreactors
A majority of the research on thermophilic H2 production is directed to determining physiological characteristics of the microorganisms involved (Kengen et al. 2009). This requires well-controlled laboratory conditions; hence, the continuous stirred tank reactor (CSTR) combined with sparging gas (usually N2) is the obvious choice. However, as a system cultivating cells in suspension, the CSTR does not allow biomass retention thus restricting the extent of substrate conversion and also limiting the hydraulic retention time (HRT), making low productivities inherent to this system. In recent years, there have seen several investigations of advanced bioreactor systems that allow biomass retention and low HRT, of which a selection based on best performance is presented in Table 3 (for a more extensive list of reactor studies, see Ren et al. 2011). The CSTR clearly has an upper productivity limit of about 20 mmol H2/L/h, which can be further improved to about threefold if cells are immobilized on a carrier (Koskinen et al. 2008; Table 3). Yet, to make the process economically feasible, the productivity should be at least an order of magnitude higher. Interestingly, a comparative study between an upflow anaerobic sludge blanket reactor (UASB) and CSTR revealed that in the former biomass retention of a pure culture of a thermophile can increase productivity by nearly 15-folds (O-Thong et al. 2008a, b; Table 3). However, it must be noted that, since H2 production is a growth-dependent phenomenon for most of the thermophiles (Schröder et al. 1994; Schönheit and Schäfer 1995; van Niel et al. 2003), the strategy of biomass retention usually results in lower H2 yields (Table 3) compared to suspension cultures in CSTR (Table 1; Kengen et al. 2009). Nevertheless, biomass retention is required to achieve high conversion efficiencies. Although not studied in depth yet, other promising reactor configurations are based on the trickle bed reactor (van Groenestijn et al. 2002; Oh et al. 2004) and membrane bioreactor (Kim et al. 2011a, b). Like with the UASB, these reactor systems can operate without a sparging gas, which will significantly simplify the process and reduce operation costs. Instead, back-mixing is a promising alternative to sparging gas, for which the recycle ratio will be a crucial parameter to optimize H2 production (Fontes Lima and Zaiat 2012).
Culture conditions
So far, the best H2 production performances have been observed at neutral to slightly acidic pH, but in the presence of pH control (Table 3). Generation of volatile fatty acids as by-products decreases the pH of the fermentation medium, which can be corrected with an alkaline agent. However, the latter causes significant environmental impact (Ochs et al. 2010) and also restricts water recirculation due to accumulation of salts. Moreover, addition of caustic agents like sodium hydroxide incurs significant costs (Ljunggren et al. 2011a, b). When using expensive feedstocks such as lignocellulosic hydrolysates, it might require pH control to keep up better H2 yields and productivities. In that case, it will remain a challenge to find cheap caustic agents and how to deal with the environmental burden of the fermentation effluent. Most of the cultures performed without any pH control have been achieved with undefined consortia, wherein thermophilic acetoclastic methanogens may have helped in maintaining the pH in a suitable range by consuming acetic acid. In some cases, the feed were supplemented with cheap caustic agents, such as sodium bicarbonate or urea (O-Thong et al. 2008a, b; Kongjan and Angelidaki 2010; Kongjan et al. 2010a, b; Abreu et al. 2012). Alternatively, performing fermentations at slightly acidic pH (~pH 6) may also ensure that lesser amounts of alkaline agent are added to the medium. To increase the productivity further, the substrate concentration in the reactor can be increased. However, this will require a H2 producer that can withstand osmotic pressure exerted by high substrate/product concentrations (property H). In addition, various different modes of reactor operation—batch, continuous, semi-continuous and anaerobic sequential batch reactor (ASBR)—have been evaluated (Table 3) to increase H2 productivities, of which the continuous mode of operation in particular has been the preferred choice (Table 3).
Complex media
Complex substrates, such as yeast extract or peptone, are regularly used as nutritional supplements to aid the growth of microorganisms at lab scale. Apart from providing amino acids, these media supplements provide buffering capacity, reducing agents and chelators for metal ions. So far, most of the physiological studies on thermophilic H2 producers have been performed containing such complex substrates (Kengen et al. 2009; van Niel et al. 2011). Moreover, some of the applied studies for more practical evaluation of biohydrogen production have also been performed using such substrates (Table 3). However, use of yeast extract and/or peptone can incur significant production cost in any industrial process (Ljunggren and Zacchi 2010). Hence, an organism with the ability to synthesize all the amino acids will allow omission of complex substrates from the medium and thus help reduce the costs (property D).
Appropriate feedstock
Over the years, a variety of readily available feedstocks including industrial and municipal waste streams, glycerol from biodiesel production and various lignocellulosic materials have been evaluated for H2 production (Table 3) with reasonable success. Lignocellulosic materials generally consist of a range of crop residues, dedicated energy crops, saw dust, forest residues and solid animal waste. For a more extensive list of industrial substrates used for biological H2 production, see van Niel et al. (2011). It is often difficult to estimate the extent of future usage of these feedstocks due to their heterogeneous nature, uncertainties in their availability and sustainable recoverability, and their competing traditional applications (Gregg and Smith 2010; Rosillo-Calle and Woods 2012). Nevertheless, crop residues are estimated to be about 1010 tons/year globally (Lal 2005) and hence are increasingly considered as a potential feedstock for biological H2 production.
Lignocellulosic feedstocks largely contain lignin, hemicellulose and cellulose, albeit in diverse fractions depending on the nature of the feedstock (Sun and Cheng 2002). Various physico-chemical methods are available to separate lignin from hemicellulose and cellulose. Solubilized polymers of hemicellulose and cellulose are further hydrolysed to mono- or disaccharides depending on the enzymes used for hydrolysis (Sun and Cheng 2002). Application of thermophilic, hydrolytic enzymes will allow integration of hydrolysis and fermentation together in a single step, i.e. simultaneous saccharification and fermentation (SSF). Caldicellulosiruptor saccharolyticus, Clostridium thermocellum and Thermotogales are known to secrete hydrolytic enzymes required for hydrolysis of pretreated lignocellulosic materials into monosaccharides (Table 2; property E), which, therefore, become ideal candidates for such a consolidated process. Alternatively, hydrolytic enzymes produced by these organisms during fermentations can be separated from the effluent and used for the hydrolysis, minimizing the cost for enzymes. However, the cost of separation and re-usability of ‘spent’ effluent remains to be studied to conclude its feasibility.
A diverse fraction of monosaccharides, such as glucose, xylose, arabinose, mannose, galactose and uronic acid, are obtained upon hydrolysis of pretreated lignocellulosic materials (Maris et al. 2006). Organisms having a diverse catabolic range for sugars will strengthen the robustness of the process and allows flexibility in the choice of feedstock. This will be of particular importance considering the seasonal and unpredictable availability of agricultural residues. Moreover, co-utilization of sugars present in the hydrolysate is very important for economically viable process. Thus, organisms having a natural ability to co-utilize the sugars will ideally be preferred (property F) over organisms unable to do so owing to ‘carbon catabolite repression’.
LCA/economical feasibility studies inherent on process development
So far, hardly any attempts have been made to evaluate the potential of any existing thermophilic biohydrogen production technology on a scale beyond that of laboratory studies. Nevertheless, a few techno-economic and life cycle analyses (LCA) have been performed using available literature to identify potential bottlenecks from environmental as well as techno-economical perspectives and steer the research towards pre-emptive measures.
Life cycle analysis
LCA involves assessment of environmental impact of different stages of a product's life cycle typically from cradle-to-grave. Ochs et al. (2010) performed a LCA evaluation (cradle-to-gate) of a proposed plant for thermophilic production of biohydrogen using potato steam peels under the assumption of a complete substrate oxidation to produce only CO2 and sewage as by-products.1 The study revealed that, during thermophilic fermentation, process inputs such as phosphates and alkali produced using fossil fuels are the most potential contributors to high environmental impact.2 Moreover, as discussed earlier, the presence of excessive salts in the growth medium can restrict the recirculation of process water, which can add to the environmental impact. Hence, measures are needed to be taken to minimize the usage of phosphate buffers in the growth medium as well as evaluating strategies for minimizing addition of alkali agents during fermentations. In addition, to minimize environmental impact, a complementary process capable of converting the generated by-products present in the effluent, thereby reducing the chemical oxygen demand, is an absolute requirement. A recent study reports about 93 % reduction in COD after converting the effluent to methane via anaerobic digestion (Willquist et al. 2012).
Techno-economic evaluation
Techno-economic analysis assesses the technical feasibility of the different parts involved in the process and also the effect of different parameters on the cost of production with the help of computer programs such as Aspen Plus (Aspen Technology, Burlington, USA). Recent technological advancements allow heat recovery in the fermentation step. Such being the case, when compared to mesophilic fermentation, additional heat demand required in thermophilic fermentation did not incur significantly higher costs (Ljunggren and Zacchi 2010). On the other hand, the production cost is largely influenced by (1) the cost of media ingredients and (2) low substrate (sugar) concentrations (Ljunggren and Zacchi 2010). As discussed above, yeast extract is the most expensive component of the medium and is not needed by the H2 producers having the ability to synthesize most of the growth factors present in the yeast extract. Secondly, low substrate concentrations in the medium will require larger reactors along with larger facility and consequently will demand more water and energy. Increasing the substrate concentration may not be a quick and easy solution, as it increases the osmolality of the medium causing undesirable effects on microbial biomass and H2 yields (Ljunggren et al. 2011a, b).
Other aspects
Given that H2 needs a unique and costly distribution infrastructure, a decentralized model of production can be imagined for a biomass-dependent thermophilic H2 process. A decentralized production will also benefit from a locally available market. Alternatively, thermophilic biohydrogen can be produced in an add-on plant to another industrial process. For example, by-products and waste heat of a sugar factory can be used for the production of biohydrogen (Markowski et al. 2010).
Challenges and outlook
Up till now, the physiology of thermophilic H2 producers has been studied to a reasonable depth for a few thermophilic H2 producers only (Verhaart et al. 2010; Willquist et al. 2010; van Niel et al. 2011). Insight into the preferences and the physiological boundaries of H2 producers will facilitate reactor design and optimal operation conditions. Genetic engineering is an important tool to obtain required knowledge in the most convenient way. In spite of the availability of vectors for genetic modification, no major breakthrough has been reported for the genetic modification of distinguished thermophilic H2 producers (Desai et al. 2004; Tyurin et al. 2004; Waege et al. 2010; Han et al. 2012; Chung et al. 2013). Indeed, challenges for performing proper modifications are related mainly to the practical hurdles inherent to these organisms, such as strict anaerobic nature limiting their ability to grow on solid media, scarcity of selection markers and unique defence mechanisms restricting transformation with foreign DNA (Noll and Vargas 1997; Thomas and Nielsen 2005; Chung et al. 2012). Knowledge obtained here gives feedback to (1) the essential composition of the feedstocks, (2) under what conditions the reactor should be operating and (3) what kind of by-products can be expected. Next to wet experiments, in silico experiments using genome-scale metabolic models will facilitate obtaining this knowledge and, moreover, identifying new metabolic engineering strategies. These metabolic models are now becoming available for several of the thermophilic H2 producers (Zhang et al. 2009; Roberts et al. 2010; Munro et al. 2011; Zeidan 2011; Nogales et al. 2012).
One such new strategy could be to engineer H2-producing pathways in thermophiles to increase H2 yields beyond the current limit of 4 to up to 8 mol H2/mole hexose, such as the oxidative pentose phosphate pathway (OPPP) (Hallenbeck and Benemann 2002; de Vrije et al. 2007). In simulations with a metabolic model of Thermotoga maritima this is possible, provided that the NADPH produced in the OPPP is either reoxidized with an introduced NADPH-NADH transhydrogenase or a NADPH-Fd reductase (Nogales et al. 2012). The outcome of significantly improved H2 yields in the models was based on optimizing for H2 production. However, it is expected that in reality most organisms will naturally choose for optimizing their growth rate, which then results in a marginal improvement of H2 yields. Indeed, introducing non-native redox pathways hardly improved H2 yields (Kim et al. 2011a, b). This is mainly attributed to thermodynamic barriers, which is not covered by the current genome-wide metabolic models, showing, as yet, the limited power of these models.
The majority of H2 producers have been isolated from environments low in free carbohydrates and usually possess variety of hydrolases to breakdown polysaccharides. Mono- and disaccharides are released slowly, being often the rate-determining step of growth. As a consequence, the organisms are exposed only to low sugar concentrations and thus relatively sugar-sensitive strains are selected (Willquist et al. 2010). However, when applied in bioreactors, growth of these organisms is influenced strongly by osmotic pressure exerted by high sugar concentrations (Ljunggren et al. 2011a, b). For industrial application, this is a negative characteristic, for which solutions have to be found. Among several options, SSF is probably the most practical one. Indeed, a positive effect of SSF for H2 production has been described recently (Quéméneur et al. 2012), but more studies are necessary to evaluate whether it is applicable for thermophilic H2 production. Alternatively, osmotolerant strains can be used (property H), which can be obtained by either genetic transformation or evolutionary adaptation. This will allow high substrate loading rates, thus lower the water demand, and it might keep any contamination further at bay.
So far, reactor design for thermophilic H2 production is still in the lab-scale phase. The trials show low to high volumetric productivities, but usually are combined with low H2 yields (Oh et al. 2004). Owing to the trade-off between H2 productivity and yield, a choice should be made between higher productivity and higher yield to make the process most profitable. Generally, for a process using inexpensive raw materials, H2 productivity can be given more importance than H2 yields, and vice versa for processes utilizing rather expensive raw materials. The level of productivity normally depends on the use of pure cultures or undefined consortia, type of reactor (configuration), efficiency of H2 removal and type of feedstock. For economic reasons, volumetric productivities need to be high, making the choice of bioreactor type crucial. At best, the reactor should allow for short HRT, high biomass retention times and fast removal of H2, hence trickling filters and UASB-type reactors are among the best choices (van Niel et al. 2011). These reactors are designed for long biomass retention times and H2 can be removed effectively through optimal back mixing or sparging gas. However, the latter complicates gas handling and downstream processing and thus adds to extra cost. To ensure high hydrogen yields, thermophiles possessing an acceptable high hydrogen tolerance should be applied in these reactors, e.g. up to 60 kPa (Willquist et al. 2011). A reactor coupled to a selective membrane allowing for in situ removal of H2 is another promising concept as it has seen to increase volumetric productivities (Lee et al. 2007). H2 can be separated effectively with dense ceramic membranes (Lu et al. 2007) and substantial experience how to overcome limitations with these kinds of membranes, such as bio-fouling and energy demand, has been gained in wastewater treatment (Judd 2008). Therefore, ceramic membrane-based bioreactors for thermophilic H2 production could have promising potential.
Conclusions
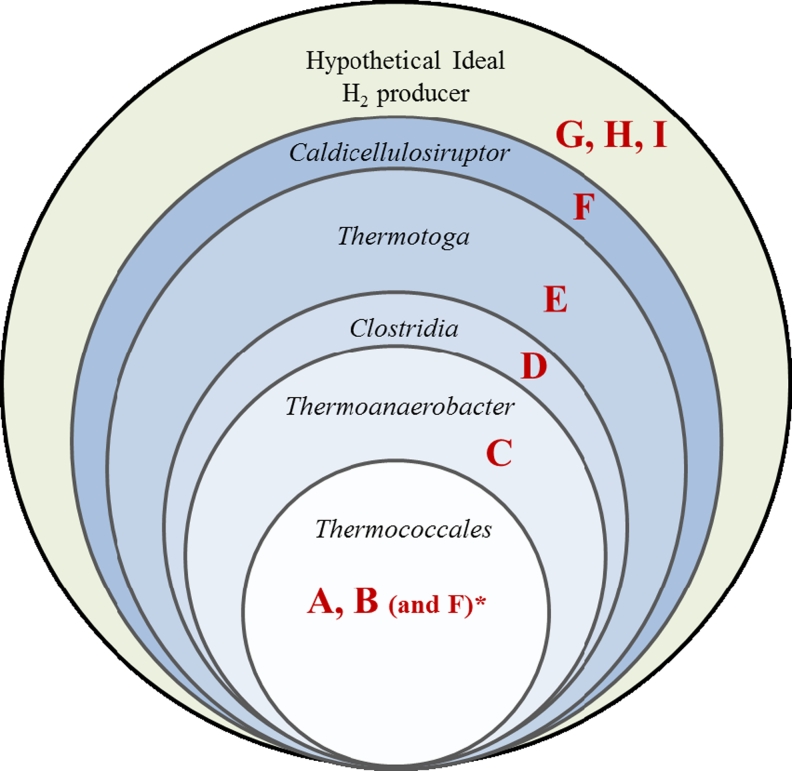
The last few decades of research on thermophilic biohydrogen producers have given plentiful insights into their physiology and intricate metabolism. While microbial physiologists will continue to explore the unknowns and/or continue to modify the known organisms in search of ideal H2-producing microorganism, we propose a combination of features that such an ideal H2 producer may possess: (A) thermophilic, (B) has specific vectors/tools designed for genetic modification(s), (C) possesses Fd-dependent hydrogenases, (D) is not auxotrophic to any amino acids, (E) has ability to degrade a wide range of biomass, (F) can metabolize multiple sugars simultaneously (absence of carbon catabolite repression), (G) when under stress shifts metabolism to useful by-products, (H) is tolerant to high osmotic stress exerted by high substrate/by-product concentrations and (I) is oxygen-tolerant. Amongst the distinguished H2 producers, organisms belonging to the genera Caldicellulosiruptor and Thermotoga come closest to being ideal H2 producers (Fig. 1). Alternatively, a consortium of known microorganisms might be designed possessing together all the features listed above.
Fig. 1.
A Venn diagram displaying comparison between distinguished H2 producers with respect to desirable properties an ideal H2 producer may possess. A, thermophilic; B, has specific vectors/tools designed for genetic modification(s); C, possesses Fd-dependent hydrogenases; D, is not auxotrophic to any amino acids; E, has ability to degrade a wide range of biomass; F, can metabolize multiple sugars simultaneously (absence of carbon catabolite repression); G, when under stress shifts metabolism to useful by-products; H, is tolerant to high osmotic stress exerted by high substrate/by-product concentrations and I, is oxygen-tolerant. (Asterisk, note: property F is also present in Thermococcales and is indeed absent from other genera as depicted)
Process engineers will keep on testing more types of feedstocks and reactor configurations to enhance productivities and yields, whereas systems analysts and economists continue scrutinizing new available published data to merit processes on their environmental impact and cost-effectiveness. However, the majority of the current challenges can only be overcome through intensive cross-disciplinary collaboration, thus ensuring essential synergies for the development of a commercial thermophilic H2 production process. And probably the economic feasibility outcomes may very well indicate that thermophilic biological H2 production best fits into a biorefinery process (Willquist et al. 2012).
Acknowledgments
This work was financially supported by the Swedish Research Council (VR).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Authors assumed a complementary step of photo-fermentation for further oxidation of DF by-products.
Environmental impact for pretreatment of a biomass will vary depending on the nature of biomass and the method of pretreatment used. Hence, the pretreatment phase has been omitted from the discussion.
References
- Abreu AA, Alves JI, Pereira MA, Karakashev D, Alves MM, Angelidaki I. Engineered heat treated methanogenic granules: a promising biotechnological approach for extreme thermophilic biohydrogen production. Biores Technol. 2010;101(24):9577–9586. doi: 10.1016/j.biortech.2010.07.070. [DOI] [PubMed] [Google Scholar]
- Abreu A, Karakashev D, Angelidaki I, Sousa D, Alves M. Biohydrogen production from arabinose and glucose using extreme thermophilic anaerobic mixed cultures. Biotechnol Biofuels. 2012;5(1):6. doi: 10.1186/1754-6834-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balat M, Kirtay E. Major technical barriers to a “hydrogen economy”. Energy Sources Part A Recover Utilization Environ Effects. 2010;32(9):863–876. doi: 10.1080/15567030802606293. [DOI] [Google Scholar]
- Bielen A, Verhaart M, van der Oost J, Kengen S. Biohydrogen production by the thermophilic bacterium Caldicellulosiruptor saccharolyticus: current status and perspectives. Life. 2013;3(1):52–85. doi: 10.3390/life3010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumer-Schuette SE, Kataeva I, Westpheling J, Adams MWW, Kelly RM. Extremely thermophilic microorganisms for biomass conversion: status and prospects. Curr Opin Biotechnol. 2008;19(3):210–217. doi: 10.1016/j.copbio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bonch-Osmolovskaya E (2001) Thermotogales. eLS, John Wiley & Sons, Ltd
- Brenner K, You L, Arnold FH. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 2008;26(9):483–489. doi: 10.1016/j.tibtech.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Burgdorf T, Lenz O, Buhrke T, van der Linden E, Jones AK, Albracht SPJ, Friedrich B. [NiFe]-hydrogenases of Ralstonia eutropha H16: modular enzymes for oxygen-tolerant biological hydrogen oxidation. J Mol Microbiol Biotechnol. 2005;10(2–4):181–196. doi: 10.1159/000091564. [DOI] [PubMed] [Google Scholar]
- Carere C, Rydzak T, Verbeke T, Cicek N, Levin D, Sparling R. Linking genome content to biofuel production yields: a meta-analysis of major catabolic pathways among select H2 and ethanol-producing bacteria. BMC Microbiol. 2012;12(1):295. doi: 10.1186/1471-2180-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti SR, Lalman JA, Heath DD. 16S rRNA gene based analysis of the microbial diversity and hydrogen production in three mixed anaerobic cultures. Int J Hydrogen Energy. 2012;37(11):9002–9017. doi: 10.1016/j.ijhydene.2012.02.146. [DOI] [Google Scholar]
- Chou C-J, Jenney FE, Jr, Adams MWW, Kelly RM. Hydrogenesis in hyperthermophilic microorganisms: implications for biofuels. Metabol Eng. 2008;10(6):394–404. doi: 10.1016/j.ymben.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Chung D, Farkas J, Huddleston JR, Olivar E, Westpheling J. Methylation by a unique α-class N4-cytosine methyltransferase is required for DNA transformation of Caldicellulosiruptor bescii DSM6725. PLoS One. 2012;7(8):e43844. doi: 10.1371/journal.pone.0043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D, Cha M, Farkas J, Westpheling J. Construction of a stable replicating shuttle vector for Caldicellulosiruptor species: use for extending genetic methodologies to other members of this genus. PLoS One. 2013;8(5):e62881. doi: 10.1371/journal.pone.0062881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciranna A, Santala V, Karp M. Biohydrogen production in alkalithermophilic conditions: Thermobrachium celere as a case study. Biores Technol. 2011;102(18):8714–8722. doi: 10.1016/j.biortech.2011.01.028. [DOI] [PubMed] [Google Scholar]
- Collet C, Adler N, Schwitzguébel J-P, Péringer P. Hydrogen production by Clostridium thermolacticum during continuous fermentation of lactose. Int J Hydrogen Energy. 2004;29(14):1479–1485. doi: 10.1016/j.ijhydene.2004.02.009. [DOI] [Google Scholar]
- Das D. Advances in biohydrogen production processes: an approach towards commercialization. Int J Hydrogen Energy. 2009;34(17):7349–7357. doi: 10.1016/j.ijhydene.2008.12.013. [DOI] [Google Scholar]
- de Vrije T, Mars AE, Budde MA, Lai MH, Dijkema C, de Waard P, Claassen PAM. Glycolytic pathway and hydrogen yield studies of the extreme thermophile Caldicellulosiruptor saccharolyticus. Appl Microbiol Biotechnol. 2007;74(6):1358–1367. doi: 10.1007/s00253-006-0783-x. [DOI] [PubMed] [Google Scholar]
- Desai SG, Guerinot ML, Lynd LR. Cloning ofl-lactate dehydrogenase and elimination of lactic acid production via gene knockout in Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl Microbiol Biotechnol. 2004;65(5):600–605. doi: 10.1007/s00253-004-1575-9. [DOI] [PubMed] [Google Scholar]
- Desvaux M. Unravelling carbon metabolism in anaerobic cellulolytic bacteria. Biotechnol Prog. 2006;22(5):1229–1238. doi: 10.1002/bp060016e. [DOI] [PubMed] [Google Scholar]
- Fiala G, Stetter KO. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100 °C. Arch Microbiol. 1986;145(1):56–61. doi: 10.1007/BF00413027. [DOI] [Google Scholar]
- Fontes Lima DM, Zaiat M. The influence of the degree of back-mixing on hydrogen production in an anaerobic fixed-bed reactor. Int J Hydrogen Energy. 2012;37(12):9630–9635. doi: 10.1016/j.ijhydene.2012.03.097. [DOI] [Google Scholar]
- Freedonia (2010) World hydrogen: industry study with forecasts for 2013 and 2018. Cleveland, OH, USA, The Freedonia Group: 336
- Gregg J, Smith S. Global and regional potential for bioenergy from agricultural and forestry residue biomass. Mitig Adapt Strateg Glob Change. 2010;15(3):241–262. doi: 10.1007/s11027-010-9215-4. [DOI] [Google Scholar]
- Guiral M, Tron P, Belle V, Aubert C, Léger C, Guigliarelli B, Giudici-Orticoni M-T. Hyperthermostable and oxygen resistant hydrogenases from a hyperthermophilic bacterium Aquifex aeolicus: Physicochemical properties. Int J Hydrogen Energy. 2006;31(11):1424–1431. doi: 10.1016/j.ijhydene.2006.06.007. [DOI] [Google Scholar]
- Hallenbeck PC, Benemann JR. Biological hydrogen production; fundamentals and limiting processes. Int J Hydrogen Energy. 2002;27(11–12):1185–1193. doi: 10.1016/S0360-3199(02)00131-3. [DOI] [Google Scholar]
- Hallenbeck PC, Ghosh D. Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol. 2009;27(5):287–297. doi: 10.1016/j.tibtech.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Hallenbeck PC, Abo-Hashesh M, Ghosh D. Strategies for improving biological hydrogen production. Biores Technol. 2012;110:1–9. doi: 10.1016/j.biortech.2012.01.103. [DOI] [PubMed] [Google Scholar]
- Han D, Norris SM, Xu Z. Construction and transformation of a Thermotoga–E. coli shuttle vector. BMC Biotechnol. 2012;12:2. doi: 10.1186/1472-6750-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoaki T, Nishijima M, Kato M, Adachi K, Mizobuchi S, Hanzawa N, Maruyama T. Growth requirements of hyperthermophilic sulfur-dependent heterotrophic archaea isolated from a shallow submarine geothermal system with reference to their essential amino acids. Appl Environ Microbiol. 1994;60(8):2898–2904. doi: 10.1128/aem.60.8.2898-2904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P. Tomorrow's energy: hydrogen, fuel cells, and the prospects for a cleaner planet. Cambridge: MIT; 2001. [Google Scholar]
- Huber R, Langworthy TA, König H, Thomm M, Woese CR, Sleytr UB, Stetter KO. Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90 °C. Arch Microbiol. 1986;144(4):324–333. doi: 10.1007/BF00409880. [DOI] [Google Scholar]
- Ingersoll JG. Natural gas vehicles. Lilburn: Fairmont; 1996. [Google Scholar]
- Ivanova G, Rákhely G, Kovács KL. Hydrogen production from biopolymers by Caldicellulosiruptor saccharolyticus and stabilization of the system by immobilization. Int J Hydrogen Energy. 2008;33(23):6953–6961. doi: 10.1016/j.ijhydene.2008.08.058. [DOI] [Google Scholar]
- Johnson EA, Madia A, Demain AL. Chemically defined minimal medium for growth of the anaerobic cellulolytic thermophile Clostridium thermocellum. Appl Environ Microbiol. 1981;41(4):1060–1062. doi: 10.1128/aem.41.4.1060-1062.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd S. The status of membrane bioreactor technology. Trends Biotechnol. 2008;26(2):109–116. doi: 10.1016/j.tibtech.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kanai T, Imanaka H, Nakajima A, Uwamori K, Omori Y, Fukui T, Atomi H, Imanaka T. Continuous hydrogen production by the hyperthermophilic archaeon, Thermococcus kodakaraensis KOD1. J Biotechnol. 2005;116(3):271–282. doi: 10.1016/j.jbiotec.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Kargi F, Eren NS, Ozmihci S. Bio-hydrogen production from cheese whey powder (CWP) solution: comparison of thermophilic and mesophilic dark fermentations. Int J Hydrogen Energy. 2012;37(10):8338–8342. doi: 10.1016/j.ijhydene.2012.02.162. [DOI] [Google Scholar]
- Kengen SWM, Goorissen HP, Verhaart M, Stams AJM, van Niel EWJ, Claassen PAM (2009) Biological hydrogen production by anaerobic microorganisms. In: Biofuels. Wiley, Oxford, pp. 197–221
- Keskin T, Giusti L, Azbar N. Continuous biohydrogen production in immobilized biofilm system versus suspended cell culture. Int J Hydrogen Energy. 2011;37:1418–1424. doi: 10.1016/j.ijhydene.2011.10.013. [DOI] [Google Scholar]
- Kim M-S, Lee D-Y. Fermentative hydrogen production from tofu-processing waste and anaerobic digester sludge using microbial consortium. Biores Technol. 2010;101(1, Supplement):S48–S52. doi: 10.1016/j.biortech.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Kim M-S, Lee D-Y, Kim D-H. Continuous hydrogen production from tofu processing waste using anaerobic mixed microflora under thermophilic conditions. Int J Hydrogen Energy. 2011;36(14):8712–8718. doi: 10.1016/j.ijhydene.2010.06.040. [DOI] [Google Scholar]
- Kim YM, Cho H-S, Jung GY, Park JM. Engineering the pentose phosphate pathway to improve hydrogen yield in recombinant Escherichia coli. Biotechnol Bioeng. 2011;108(12):2941–2946. doi: 10.1002/bit.23259. [DOI] [PubMed] [Google Scholar]
- Kongjan P, Angelidaki I. Extreme thermophilic biohydrogen production from wheat straw hydrolysate using mixed culture fermentation: effect of reactor configuration. Biores Technol. 2010;101(20):7789–7796. doi: 10.1016/j.biortech.2010.05.024. [DOI] [PubMed] [Google Scholar]
- Kongjan P, O-Thong S, Angelidaki I. Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Biores Technol. 2010;102(5):4028–4035. doi: 10.1016/j.biortech.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Kongjan P, O-Thong S, Kotay M, Min B, Angelidaki I. Biohydrogen production from wheat straw hydrolysate by dark fermentation using extreme thermophilic mixed culture. Biotechnol Bioeng. 2010;105(5):899–908. doi: 10.1002/bit.22616. [DOI] [PubMed] [Google Scholar]
- Kongjan P, O-Thong S, Angelidaki I. Biohydrogen production from desugared molasses (DM) using thermophilic mixed cultures immobilized on heat treated anaerobic sludge granules. Int J Hydrogen Energy. 2011;36(21):14261–14269. doi: 10.1016/j.ijhydene.2011.06.130. [DOI] [Google Scholar]
- Koskinen PEP, Lay C-H, Puhakka JA, Lin P-J, Wu S-Y, Örlygsson J, Lin C-Y. High-efficiency hydrogen production by an anaerobic, thermophilic enrichment culture from an Icelandic hot spring. Biotechnol Bioeng. 2008;101(4):665–678. doi: 10.1002/bit.21948. [DOI] [PubMed] [Google Scholar]
- Kotsopoulos TA, Fotidis IA, Tsolakis N, Martzopoulos GG. Biohydrogen production from pig slurry in a CSTR reactor system with mixed cultures under hyper-thermophilic temperature (70 °C) Biomass Bioenergy. 2009;33(9):1168–1174. doi: 10.1016/j.biombioe.2009.05.001. [DOI] [Google Scholar]
- Lal R. World crop residues production and implications of its use as a biofuel. Environ Int. 2005;31(4):575–584. doi: 10.1016/j.envint.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lee K-S, Lin P-J, Fangchiang K, Chang J-S. Continuous hydrogen production by anaerobic mixed microflora using a hollow-fiber microfiltration membrane bioreactor. Int J Hydrogen Energy. 2007;32(8):950–957. doi: 10.1016/j.ijhydene.2006.09.018. [DOI] [Google Scholar]
- Liu Y, Yu P, Song X, Qu Y. Hydrogen production from cellulose by co-culture of Clostridium thermocellum JN4 and Thermoanaerobacterium thermosaccharolyticum GD17. Int J Hydrogen Energy. 2008;33(12):2927–2933. doi: 10.1016/j.ijhydene.2008.04.004. [DOI] [Google Scholar]
- Ljunggren M, Zacchi G. Techno-economic analysis of a two-step biological process producing hydrogen and methane. Biores Technol. 2010;101(20):7780–7788. doi: 10.1016/j.biortech.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Ljunggren M, Wallberg O, Zacchi G. Techno-economic comparison of a biological hydrogen process and a 2nd generation ethanol process using barley straw as feedstock. Biores Technol. 2011;102(20):9524–9531. doi: 10.1016/j.biortech.2011.06.096. [DOI] [PubMed] [Google Scholar]
- Ljunggren M, Willquist K, Zacchi G, van Niel E. A kinetic model for quantitative evaluation of the effect of H2 and osmolarity on hydrogen production by Caldicellulosiruptor saccharolyticus. Biotechnol Biofuels. 2011;4(1):31. doi: 10.1186/1754-6834-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu GQ, Diniz da Costa JC, Duke M, Giessler S, Socolow R, Williams RH, Kreutz T. Inorganic membranes for hydrogen production and purification: a critical review and perspective. J Coll Interface Sci. 2007;314(2):589–603. doi: 10.1016/j.jcis.2007.05.067. [DOI] [PubMed] [Google Scholar]
- Lukey MJ, Roessler MM, Parkin A, Evans RM, Davies RA, Lenz O, Friedrich B, Sargent F, Armstrong FA. Oxygen-tolerant [NiFe]-hydrogenases: the individual and collective importance of supernumerary cysteines at the proximal Fe-S cluster. J Am Chem Soc. 2011;133(42):16881–16892. doi: 10.1021/ja205393w. [DOI] [PubMed] [Google Scholar]
- Luo G, Xie L, Zou Z, Wang W, Zhou Q. Exploring optimal conditions for thermophilic fermentative hydrogen production from cassava stillage. Int J Hydrogen Energy. 2010;35(12):6161–6169. doi: 10.1016/j.ijhydene.2010.03.126. [DOI] [Google Scholar]
- Luo G, Xie L, Zhou Q, Angelidaki I. Enhancement of bioenergy production from organic wastes by two-stage anaerobic hydrogen and methane production process. Biores Technol. 2011;102(18):8700–8706. doi: 10.1016/j.biortech.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Luque R, Campelo J, Clark JH. Handbook of biofuels production : processes and technologies. Oxford: Woodhead; 2011. [Google Scholar]
- Maeder DL, Weiss RB, Dunn DM, Cherry JL, Gonzalez JM, DiRuggiero J, Robb FT. Divergence of the hyperthermophilic archaea Pyrococcus furiosus and P. horikoshii inferred from complete genomic sequences. Genetics. 1999;152(4):1299–1305. doi: 10.1093/genetics/152.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen A, Kaksonen A, Puhakka J. Thermovorax subterraneus, gen. nov., sp. nov., a thermophilic hydrogen-producing bacterium isolated from geothermally active underground mine. Extremophiles. 2009;13(3):505–510. doi: 10.1007/s00792-009-0235-5. [DOI] [PubMed] [Google Scholar]
- Mansilla C, Avril S, Imbach J, Le Duigou A. CO2-free hydrogen as a substitute to fossil fuels: what are the targets? Prospective assessment of the hydrogen market attractiveness. Int J Hydrogen Energy. 2012;37(12):9451–9458. doi: 10.1016/j.ijhydene.2012.03.149. [DOI] [Google Scholar]
- Maris AA, Abbott D, Bellissimi E, Brink J, Kuyper M, Luttik MH, Wisselink HW, Scheffers WA, Dijken J, Pronk J. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: current status. Antonie Van Leeuwenhoek. 2006;90(4):391–418. doi: 10.1007/s10482-006-9085-7. [DOI] [PubMed] [Google Scholar]
- Markowski M, Urbaniec K, Budek A, Trafczyński M, Wukovits W, Friedl A, Ljunggren M, Zacchi G. Estimation of energy demand of fermentation-based hydrogen production. J Clean Prod. 2010;18(Supplement 1(0)):S81–S87. doi: 10.1016/j.jclepro.2010.02.027. [DOI] [Google Scholar]
- Meyer J. [FeFe] hydrogenases and their evolution: a genomic perspective. Cell Mol Life Sci. 2007;64(9):1063–1084. doi: 10.1007/s00018-007-6477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Wang G, Yu H-Q. Kinetic modeling of batch hydrogen production process by mixed anaerobic cultures. Biores Technol. 2006;97(11):1302–1307. doi: 10.1016/j.biortech.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Munro SA, Zinder SH, Walker LP. ORIGINAL RESEARCH: comparative constraint-based model development for thermophilic hydrogen production. Ind Biotechnol. 2011;7(1):63–82. doi: 10.1089/ind.2011.7.063. [DOI] [Google Scholar]
- Nogales J, Gudmundsson S, Thiele I. An in silico re-design of the metabolism in Thermotoga maritima for increased biohydrogen production. Int J Hydrogen Energy. 2012;37(17):12205–12218. doi: 10.1016/j.ijhydene.2012.06.032. [DOI] [Google Scholar]
- Noll KM, Vargas M. Recent advances in genetic analyses of hyperthermophilic archaea and bacteria. Arch Microbiol. 1997;168(2):73–80. doi: 10.1007/s002030050472. [DOI] [PubMed] [Google Scholar]
- Ochs D, Wukovits W, Ahrer W. Life cycle inventory analysis of biological hydrogen production by thermophilic and photo fermentation of potato steam peels (PSP) J Cleaner Prod. 2010;18(Supplement 1(0)):S88–S94. doi: 10.1016/j.jclepro.2010.05.018. [DOI] [Google Scholar]
- Oh S-E, Iyer P, Bruns MA, Logan BE. Biological hydrogen production using a membrane bioreactor. Biotechnol Bioeng. 2004;87(1):119–127. doi: 10.1002/bit.20127. [DOI] [PubMed] [Google Scholar]
- O-Thong S, Prasertsan P, Intrasungkha N, Dhamwichukorn S, Birkeland N-K. Optimization of simultaneous thermophilic fermentative hydrogen production and COD reduction from palm oil mill effluent by Thermoanaerobacterium-rich sludge. Int J Hydrogen Energy. 2008;33(4):1221–1231. doi: 10.1016/j.ijhydene.2007.12.017. [DOI] [Google Scholar]
- O-Thong S, Prasertsan P, Karakashev D, Angelidaki I. High-rate continuous hydrogen production by Thermoanaerobacterium thermosaccharolyticum PSU-2 immobilized on heat-pretreated methanogenic granules. Int J Hydrogen Energy. 2008;33(22):6498–6508. doi: 10.1016/j.ijhydene.2008.07.060. [DOI] [Google Scholar]
- Prasertsan P, O-Thong S, Birkeland N-K. Optimization and microbial community analysis for production of biohydrogen from palm oil mill effluent by thermophilic fermentative process. Int J Hydrogen Energy. 2009;34(17):7448–7459. doi: 10.1016/j.ijhydene.2009.04.075. [DOI] [Google Scholar]
- Quéméneur M, Bittel M, Trably E, Dumas C, Fourage L, Ravot G, Steyer J-P, Carrère H. Effect of enzyme addition on fermentative hydrogen production from wheat straw. Int J Hydrogen Energy. 2012;37(14):10639–10647. doi: 10.1016/j.ijhydene.2012.04.083. [DOI] [Google Scholar]
- Rainey FA, Donnison AM, Janssen PH, Saul D, Rodrigo A, Bergquist PL, Daniel RM, Stackebrandt E, Morgan HW. Description of Caldicellulosiruptor saccharolyticus gen-nov, sp. nov. an obligately anaerobic, extremely thermophilic, cellulolytic bacterium. FEMS Microbiol Lett. 1994;120(3):263–266. doi: 10.1111/j.1574-6968.1994.tb07043.x. [DOI] [PubMed] [Google Scholar]
- Ren N, Guo W, Liu B, Cao G, Ding J. Biological hydrogen production by dark fermentation: challenges and prospects towards scaled-up production. Curr Opin Biotechnol. 2011;22(3):365–370. doi: 10.1016/j.copbio.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Rinker KD, Kelly RM. Effect of carbon and nitrogen sources on growth dynamics and exopolysaccharide production for the hyperthermophilic archaeon Thermococcus litoralis and bacterium Thermotoga maritima. Biotechnol Bioeng. 2000;69(5):537–547. doi: 10.1002/1097-0290(20000905)69:5<537::AID-BIT8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Robb FT, Maeder DL, Brown JR, DiRuggiero J, Stump MD, Yeh RK, Weiss RB, Dunn DM. Genomic sequence of hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Meth Enzymol. 2001;330:134–157. doi: 10.1016/S0076-6879(01)30372-5. [DOI] [PubMed] [Google Scholar]
- Roberts S, Gowen C, Brooks JP, Fong S. Genome-scale metabolic analysis of Clostridium thermocellum for bioethanol production. BMC Syst Biol. 2010;4(1):31. doi: 10.1186/1752-0509-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosillo-Calle F, Woods J. The biomass assessment handbook. London: Routledge; 2012. [Google Scholar]
- Sapra R, Bagramyan K, Adams MWW. A simple energy-conserving system: proton reduction coupled to proton translocation. PNAS. 2003;100(13):7545–7550. doi: 10.1073/pnas.1331436100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönheit P, Schäfer T. Metabolism of hyperthermophiles. World J Microbiol Biotechnol. 1995;11(1):26–57. doi: 10.1007/BF00339135. [DOI] [PubMed] [Google Scholar]
- Schröder C, Selig M, Schönheit P. Glucose fermentation to acetate, CO2 and H2 in the anaerobic hyperthermophilic eubacterium Thermotoga maritima: involvement of the Embden–Meyerhof pathway. Arch Microbiol. 1994;161(6):460–470. [Google Scholar]
- Schrope M. Which way to energy utopia? Nature. 2001;414(6865):682–684. doi: 10.1038/414682a. [DOI] [PubMed] [Google Scholar]
- Schut GJ, Adams MWW (2009) The iron-hydrogenase of Thermotoga maritima utilizes ferredoxin and NADH synergistically: a new perspective on anaerobic hydrogen production. J Bacteriol: JB.01582-01508 [DOI] [PMC free article] [PubMed]
- Silva PJ, van den Ban ECD, Wassink H, Haaker H, de Castro B, Robb FT, Hagen WR. Enzymes of hydrogen metabolism in Pyrococcus furiosus. Euro J Biochem. 2000;267(22):6541–6551. doi: 10.1046/j.1432-1327.2000.01745.x. [DOI] [PubMed] [Google Scholar]
- Soboh B, Linder D, Hedderich R. A multisubunit membrane-bound [NiFe] hydrogenase and an NADH-dependent Fe-only hydrogenase in the fermenting bacterium Thermoanaerobacter tengcongensis. Microbiology. 2004;150(7):2451–2463. doi: 10.1099/mic.0.27159-0. [DOI] [PubMed] [Google Scholar]
- Stams AJM. Metabolic interactions between anaerobic bacteria in methanogenic environments. Antonie Van Leeuwenhoek. 1994;66(1):271–294. doi: 10.1007/BF00871644. [DOI] [PubMed] [Google Scholar]
- Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Biores Technol. 2002;83(1):1–11. doi: 10.1016/S0960-8524(01)00212-7. [DOI] [PubMed] [Google Scholar]
- Thauer RK, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Microbiol Mol Biol Rev. 1977;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CM, Nielsen KM. Mechanisms of, and barriers to, horizontal gene transfer between bacteria. Nat Rev Micro. 2005;3(9):711–721. doi: 10.1038/nrmicro1234. [DOI] [PubMed] [Google Scholar]
- Tyurin MV, Desai SG, Lynd LR. Electrotransformation of Clostridium thermocellum. Appl Environ Microbiol. 2004;70(2):883–890. doi: 10.1128/AEM.70.2.883-890.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y, Otsuka S, Morimoto M. Hydrogen production from industrial wastewater by anaerobic microflora in chemostat culture. J Fermen Bioeng. 1996;82(2):194–197. doi: 10.1016/0922-338X(96)85050-1. [DOI] [Google Scholar]
- van de Werken HJG, Verhaart MRA, VanFossen AL, Willquist K, Lewis DL, Nichols JD, Goorissen HP, Mongodin EF, Nelson KE, van Niel EWJ, Stams AJM, Ward DE, de Vos WM, van der Oost J, Kelly RM, Kengen SWM. Hydrogenomics of the extremely thermophilic bacterium Caldicellulosiruptor saccharolyticus. Appl Environ Microbiol. 2008;74(21):6720–6729. doi: 10.1128/AEM.00968-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groenestijn JW, Hazewinkel JHO, Nienoord M, Bussmann PJT. Energy aspects of biological hydrogen production in high rate bioreactors operated in the thermophilic temperature range. Int J Hydrogen Energy. 2002;27(11–12):1141–1147. doi: 10.1016/S0360-3199(02)00096-4. [DOI] [Google Scholar]
- van Niel EWJ, Claassen PAM, Stams AJM. Substrate and product inhibition of hydrogen production by the extreme thermophile, Caldicellulosiruptor saccharolyticus. Biotechnol Bioeng. 2003;81(3):255–262. doi: 10.1002/bit.10463. [DOI] [PubMed] [Google Scholar]
- van Niel EWJ, Willquist K, Zeidan AA, de Vrije T, Mars AE, Claassen PAM. Hydrogen production by thermophilic fermentation. In: Azbar N, Levin DB, editors. State of the art and progress in production of biohydrogen. Sharjah: Bentham Science; 2011. pp. 137–159. [Google Scholar]
- VanFossen AL, Lewis DL, Nichols JD, Kelly RM. Polysaccharide degradation and synthesis by extremely thermophilic anaerobes. Ann N Y Acad Sci. 2008;1125(1):322–337. doi: 10.1196/annals.1419.017. [DOI] [PubMed] [Google Scholar]
- Vargas M, Noll KM. Catabolite repression in the hyperthermophilic bacterium Thermotoga neapolitana is independent of cAMP. Microbiology. 1996;142(1):139–144. doi: 10.1099/13500872-142-1-139. [DOI] [PubMed] [Google Scholar]
- Verhaart MR, Bielen AA, Oost J v d, Stams AJ, Kengen SW. Hydrogen production by hyperthermophilic and extremely thermophilic bacteria and archaea: mechanisms for reductant disposal. Environ Technol. 2010;31(8–9):993–1003. doi: 10.1080/09593331003710244. [DOI] [PubMed] [Google Scholar]
- Vignais PM, Billoud B. Occurrence, classification, and biological function of hydrogenases: an overview. Chem Rev. 2007;107(10):4206–4272. doi: 10.1021/cr050196r. [DOI] [PubMed] [Google Scholar]
- Waege I, Schmid G, Thumann S, Thomm M, Hausner W. Shuttle vector-based transformation system for Pyrococcus furiosus. Appl Environ Microbiol. 2010;76(10):3308–3313. doi: 10.1128/AEM.01951-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JB, Lolkema JS. CcpA-dependent carbon catabolite repression in bacteria. Microbiol Mol Biol Rev. 2003;67(4):475–490. doi: 10.1128/MMBR.67.4.475-490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willquist K, van Niel EWJ. Growth and hydrogen production characteristics of Caldicellulosiruptor saccharolyticus on chemically defined minimal media. Int J Hydrogen Energy. 2012;37(6):4925–4929. doi: 10.1016/j.ijhydene.2011.12.055. [DOI] [Google Scholar]
- Willquist K, Claassen PAM, van Niel EWJ. Evaluation of the influence of CO2 on hydrogen production in Caldicellulosiruptor saccharolyticus. Int J Hydrogen Energy. 2009;34:4718–4726. doi: 10.1016/j.ijhydene.2009.03.056. [DOI] [Google Scholar]
- Willquist K, Zeidan AA, van Niel EWJ. Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus: an efficient hydrogen cell factory. Microb Cell Fact. 2010;9(1):89. doi: 10.1186/1475-2859-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willquist K, Pawar SS, van Niel EWJ. Reassessment of hydrogen tolerance in Caldicellulosiruptor saccharolyticus. Microb Cell Fact. 2011;10(1):111. doi: 10.1186/1475-2859-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willquist K, Nkemka VN, Svensson H, Pawar S, Ljunggren M, Karlsson H, Murto M, Hulteberg C, van Niel EWJ, Liden G. Design of a novel biohythane process with high H2 and CH4 production rates. Int J Hydrogen Energy. 2012;37(23):17749–17762. doi: 10.1016/j.ijhydene.2012.08.092. [DOI] [Google Scholar]
- Xue Y, Xu Y, Liu Y, Ma Y, Zhou P. Thermoanaerobacter tengcongensis sp. nov., a novel anaerobic, saccharolytic, thermophilic bacterium isolated from a hot spring in Tengcong, China. Intl J Sys Evol Microbiol. 2001;51(4):1335–1341. doi: 10.1099/00207713-51-4-1335. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhu Z, Hu W, Zhang H. Hydrogen production from rice winery wastewater in an upflow anaerobic reactor by using mixed anaerobic cultures. Int J Hydrogen Energy. 2002;27(11–12):1359–1365. doi: 10.1016/S0360-3199(02)00073-3. [DOI] [Google Scholar]
- Zeidan AA (2011) Hydrogen production by Caldicellusiruptor species : the organism and the metabolism. PhD Thesis, Lund University, Sweden
- Zeidan A, Rådström P, van Niel E. Stable coexistence of two Caldicellulosiruptor species in a de novo constructed hydrogen-producing co-culture. Microb Cell Fact. 2010;9(1):102. doi: 10.1186/1475-2859-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Thiele I, Weekes D, Li Z, Jaroszewski L, Ginalski K, Deacon AM, Wooley J, Lesley SA, Wilson IA. Three-dimensional structural view of the central metabolic network of Thermotoga maritima. Science. 2009;325(5947):1544–1549. doi: 10.1126/science.1174671. [DOI] [PMC free article] [PubMed] [Google Scholar]