Abstract
A current threat to the marine ecosystem is the high level of solar ultraviolet radiation (UV). Large whales have recently been shown to suffer sun-induced skin damage from continuous UV exposure. Genotoxic consequences of such exposure remain unknown for these long-lived marine species, as does their capacity to counteract UV-induced insults. We show that UV exposure induces mitochondrial DNA damage in the skin of seasonally sympatric fin, sperm, and blue whales and that this damage accumulates with age. However, counteractive molecular mechanisms are markedly different between species. For example, sperm whales, a species that remains for long periods at the sea surface, activate genotoxic stress pathways in response to UV exposure whereas the paler blue whale relies on increased pigmentation as the season progresses. Our study also shows that whales can modulate their responses to fluctuating levels of UV, and that different evolutionary constraints may have shaped their response strategies.
Although the Montreal Protocol, (which in 1987 banned the use of ozone depleting substances) has been central to decelerating the loss of ozone1, substances released during the nineties continue to destroy the ozone today1,2. The resulting large amount of solar ultraviolet (UV) radiation reaching the biosphere represents a significant threat to our ecosystems and there is now evidence that, as is the case in humans3, wild species such as amphibians, fish, and invertebrates suffer from sun-induced skin damage4,5,6,7. Until recently, effects of UV on large marine mammals, which due to their life history and physiological constraints are unable to avoid continuous exposure to the sun, have been ignored8. Nevertheless, due to their long life expectancy and their extended distribution that spans all oceanic latitudes, cetaceans could reflect UV variation across large spatial and temporal scales and thus be considered as “UV-barometers of the ocean”. Although it has been demonstrated recently that UV-induced acute damage is widespread and significant in whales8, nothing is known about the genetic nature of this damage, or the strategies used by these animals to resolve or counteract UV-induced lesions. Following initial observations which revealed that having fewer melanocytes led to more UV-related skin lesions and fewer apoptotic cells amongst whale species, it was suggested that darker pigmentation confers cellular protection from sun irradiation and plays a role in the elimination of potentially precancerous cells in whales8. Whether such a role has an effect at the molecular level remains unknown. Considering that UV-induced DNA mutations can contribute to the development of skin cancer in humans9, it is relevant to record the occurrence and magnitude of UV-induced DNA damage in whales. One particularly successful biomarker of UV-exposure in human skin is mitochondrial DNA (mtDNA)10. Because mtDNA has a higher rate of mutation than nuclear DNA and a reduced capacity to repair damage10, UV-induced mtDNA lesions accumulate throughout the life of an individual and thus offer a reliable biomarker for cumulative exposure to UV10,11. Mitochondria are the predominant site for the formation of cellular reactive oxygen species, which tend to be exacerbated following UV exposure in human skin causing mtDNA damage in the form of strand breaks and deletions11. Furthermore, accumulated mtDNA damage has been associated with human skin cancer11. We sought to test two hypotheses: first, that whales accumulate mtDNA damage in their skin as they age, and second, that pigmentation confers protection against UV-induced mtDNA damage. We screened mtDNA from skin of three whale species (fin, sperm, and blue whales) searching for UV-induced damage using quantitative long-PCR assays12 and investigated their association with minimum age and with “individual pigmentation indices” including melanocyte abundance and melanin density. We next sought to investigate the capacity of whales to respond to UV and study interspecies differences in the mechanisms used in this process. For this, we tested three hypotheses: first, that UV-induced damage leads to changes in the expression of genes involved in genotoxic stress pathways; second, that species with different skin colour and sea surface behaviour (time spent at the sea surface) use different cellular mechanisms to protect themselves from UV exposure; and finally, that whales modulate UV-counteractive molecular mechanisms in response to the seasonal increase in UV levels, as is known to occur in humans13. To test these hypotheses, we used quantitative PCR assays to monitor the expression of carefully selected genes14, HSP70, encoding heat shock protein 70, an indicator of cell stress15,16, tumour protein P53 (P53), a central transcriptional factor activated by stressors such as UV and involved in cell cycle arrest, DNA repair and apoptosis17,18, and KIN17 (KIN), a cell cycle control protein up-regulated by UV19,20 and constructed linear mixed effect models14,21. To fully explore the role that cetacean skin pigmentation has in shaping protection against UV exposure, we also measured transcription levels of the tyrosinase gene (TYR), a key player of melanogenesis22,23.
Results
Do whales accumulate mtDNA damage in their skin as they age, and does pigmentation confer protection against UV-induced mtDNA damage?
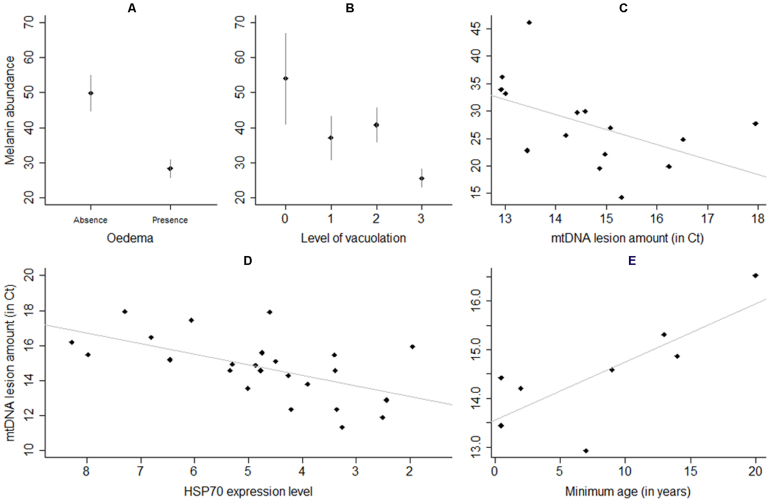
Using quantitative real time PCR methodology (qPCR), we screened for mtDNA damage from the skin of three whale species (fin, sperm, and blue whales; Fig. 1A) with different skin colour (Fig. 1A–C) and sea surface behaviour. The level of blue whale mtDNA damage was inversely predicted by melanin density of individual samples (t11 = −2.65, p = 0.02; see details in Table S1A; Fig. 2C), which suggests that greater pigmented individuals accumulate lower levels of damage. In addition, the abundance of melanin was also inversely related to the level of microscopic lesions (cytoplasmic vacuolation and intracellular oedema, methodology described previously8)) for whales across the three species studied (t95 = −2.11, p = 0.04 and t95 = −4.19, p<0.0001 respectively; see details in Table S2; Fig. 2A and B). Furthermore, as predicted under the assumption that mtDNA damage accumulates over time within the cell (aided by compromised mtDNA repair mechanisms10), the amount of whale skin mtDNA lesions increased with age for blue whales (t11 = 2.24, p = 0.05; see details in Table S1A; Fig. 2E).
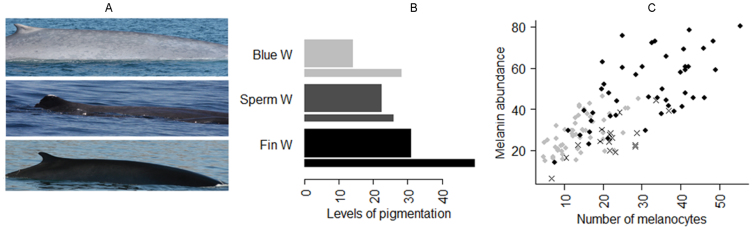
Figure 1. The contrasting skin colours of the three studied species.
(A) Photograph showing, from top to bottom, a blue whale (pale grey skin colour, the lightest species), a sperm whale (dark grey skin colour) and a fin whale (black skin colour, the darkest species). This photograph was taken by DG. (B) Differences in density of melanocytes (thick bars) and melanin (thin bars) amongst the three studied species (n = 53, n = 17, n = 45 for blue, sperm and fin whales respectively). (C) Association between melanin abundance and melanocyte counts in whales. Grey dots correspond to blue whales, black dots to fin whales and crosses to sperm whales. The counting area was determined as previously described (see8). Briefly, the number of melanocytes per 100 arbitrary units was determined in triplicate along the entire epidermal ridge. The number of epidermal ridges to count was established a priori based on a cumulative curve8.
Figure 2. Melanin abundance, HSP70 expression and age influence sensitivity to UV-induced damages in whales.
Relationship between whale melanin abundance and skin lesions: (A) intracellular oedema (n = 39 and n = 66 for absence and presence, respectively) (B) cytoplasmic vacuolation (n = 9, n = 23, n = 41, n = 35 for vacuolation levels of 0, 1, 2 and 3, respectively; see8), and (C) blue whale mitochondrial DNA lesions. (D) Inverse correlation between mitochondrial DNA (mtDNA) lesion density and HSP70 expression levels (ΔCt). (E) Direct correlation between mtDNA lesion density and individual minimum age of blue whales (calculated by taking into account the first year of observation reported for a particular individual in the Gulf of California; individuals with a minimum age of 1 were excluded; individuals that were observed in the Gulf of California on the year they were born, and as such their exact age was known, were included). Bars = ± 95% CI.
Melanin abundance was not only dependent on the quantity of melanocytes (F1,40 = 19.83, p<0.0001; Fig. 1C) but also on the transcriptional activity of TYR and P53 (F2,40 = 6.43, p = 0.004 and F1,40 = 4.06, p = 0.05, respectively), genes known to be involved in the process of melanogenesis23,24,25,26. The abundance of melanocytes8 and of melanin was highest for fin whales, the darkest of the species studied here (t95 = 3.99, p = 0.0001 and t95 = 3.42, p<0.001 when compared with sperm and blue whale, respectively; see details in Table S2; Fig. 1B and Fig. S1A). Intriguingly, both blue and sperm whales shared comparable levels of melanin (t95 = −0.93, p = 0.35; see details in Table S2), although melanocytes were more prevalent in sperm whales than in blue whales8, suggesting that melanin production capacity is restricted in sperm whales compared to blue whales.
The capacity of whales to respond to UV and the interspecies differences in the molecular mechanisms used in this process.
To accomplish this we tested three hypotheses. Firstly, we predicted that UV-induced damage would lead to changes in the expression of genes involved in genotoxic stress pathways in the studied whale species. Secondly, species with different skin colour and sea surface behaviour (time spent at the sea surface) might use different cellular mechanisms to protect themselves from UV exposure. Thirdly, whales would expectedly modulate UV-counteractive molecular mechanisms in response to the seasonal increase in UV levels.
As part of the process in addressing the first two hypotheses, the level of HSP70, an indicator of cellular stress15,16, was measured using qPCR. Transcription of HSP70 was significantly higher in sperm whales when compared with fin and blue whales (t30 = 4.57, p = 0.0001 and t30 = 3.75, p<0.001 respectively; see details in Table S3B; Fig. S1B). In addition, HSP70 expression and mtDNA lesions were found to be inversely related across species (t15 = −4.26, p<0.001, see details in Table S1B; Fig. 2D). To complete the process of addressing the two hypotheses, the expression of KIN, a cell cycle control protein up-regulated by UV was determined19,20. The results show that KIN expression was also higher in sperm whales (t33 = 3.12, p = 0.004 and t33 = 3.95, p<0.0005; see details in Table S3A; Fig. S1B).
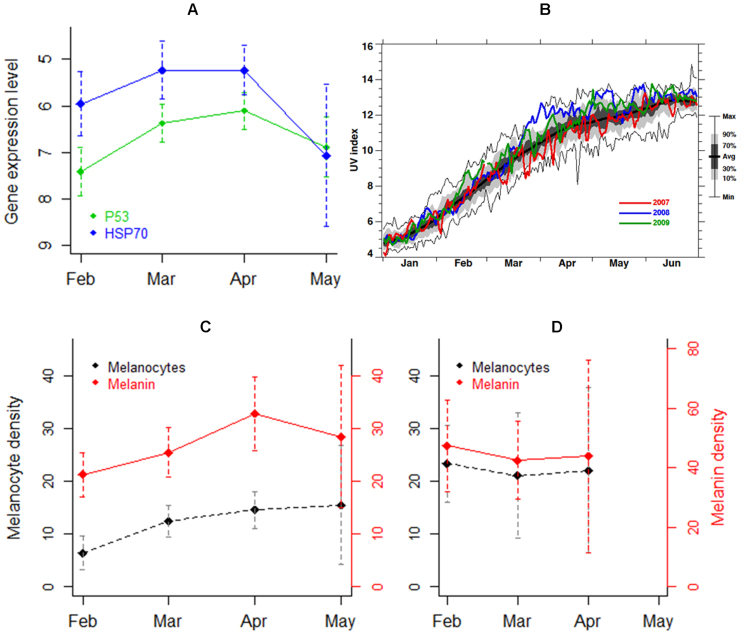
To address the third hypothesis of seasonal changes in stress response, we found that when studying temporal changes in gene expression levels, the transcription levels of P53 and HSP70 formed an ascending curve between February and May, peaking in March/April (LR = 11.93, p<0.01 and LR = 8.96, p = 0.03, respectively; see details in Table S3; Fig. 3A). Following the seasonal increase in UV radiation that reached the Gulf of California (Fig. 3B), blue whales increased melanocyte abundance (F1,47 = 3.07, p = 0.04; see details in Table S4; Fig. 3C), suggesting that individuals of this species are capable of modulating their degree of pigmentation. Although statistically non-significant, melanin density appeared to follow the same increasing trend over months suggesting tanning ability (see details in Table S4; Fig. 3C). Intriguingly, we did not observe analogous changes in the pigmentation of the (comparatively darker) fin whale (Fig. 3D).
Figure 3. Monthly fluctuations in gene expression and pigmentation levels follow seasonal variation in UV.
(A) Monthly differences in mean expression levels of P53 and HSP70 genes in blue and fin whales (data pooled; expressed as ΔCt, y axis inverted; n = 11, 13, 12 and 6 for February, March, April and May, respectively). Sperm whales were not included as they were sampled exclusively in May. (B) UV index recorded between January and June over the Gulf of California, Mexico (data average records for 26°–28°N and 109°–112°W) for the years 2007 (red), 2008 (blue) and 2009 (green). Calculation (a simply function of total column ozone and the solar zenith angle) was conducted under local noon and clear sky conditions and does not consider cloud or aerosol effects. Observation years extend from 1979–2010 (32-year running average shown by a thick black line). The lower and upper thin black lines show the minimum and maximum value observed, respectively. The grey shading shows the probability distribution function (i.e., 80% of the observations are within the light grey shading, while 40% are within the dark shading). Plot obtained using total ozone observations from the Total Ozone Mapping Spectrometer (TOMS) and Ozone Monitoring Instrument (OMI). This figure was constructed by Eric Nash and Paul Newman from NASA Goddard Space Flight Center. (C) Monthly variation of blue whale melanocyte and melanin abundance during 2007 (n = 3, 13, 7 and 3 for February, March, April and May, respectively). (D) Monthly variation of fin whale melanocyte and melanin abundance during 2008 (n = 6, 3 and 2 for February, March and April, respectively). Bars ± 95% CI.
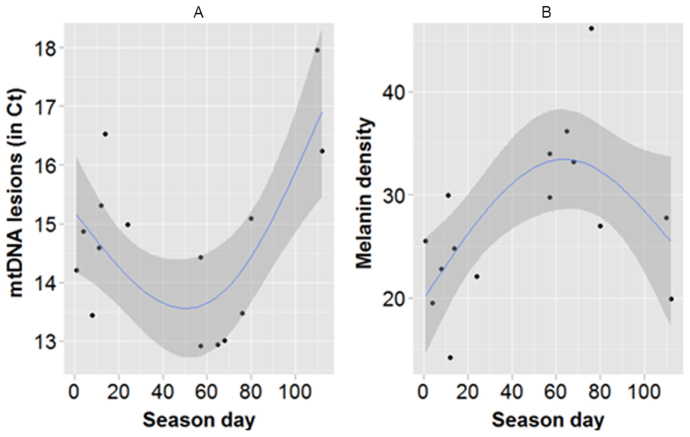
Another, non-exclusive, hypothesis for the observed differences in pigmentation plasticity amongst species might entail their distinct migratory behaviour. While fin whales are year-long residents of the Gulf of California27, blue whales migrate annually from higher to lower latitudes28, where levels and intensity of UV are greater29. Consequently, when blue whales arrive at the Gulf of California they are exposed suddenly to higher levels of UV. It is possible that the higher prevalence of microscopic lesions14 and mtDNA lesions that occur at the beginning of the season (Fig. 4A, Table S1A) reflects the time needed for UV acclimatization to occur30, especially as blue whale pigmentation seems to then increase gradually (Fig. 4B).
Figure 4. Seasonal variation of levels of mitochondrial DNA (mtDNA) lesions (A) and melanin density (B) of blue whales.
Season day indicates the moment the biopsy was taken (day 0 through 20 = February, 21 through 50 = March, 51 through 80 = April and more than 81 = May and June). Only individuals biopsied for the first time in that season were included. Each dot represents a different individual. Lines and shaded areas represent the gam regressions and their corresponding 95% CI, respectively.
Discussion
Those blue whales with higher levels of melanin showed lower levels of mtDNA damage; analogous correlations have been recorded recently in humans, strongly suggesting that melanin confers protection against mtDNA UV-induced damage31. Those whales with higher melanin also showed a reduced level of microscopic lesions. Our results thus offer further evidence that melanisation protects animals such as whales against UV exposure, at both the molecular and cellular levels8. The level of mtDNA damage also increased with age in those whales for which information was available (the blue whales), which is also observed in humans and other species10, and could result in decreased bioenergetic capacity with age.
The transcription of HSP70 was significantly higher in sperm whales compared to blue and fin whales, which could suggest a potential inhibitory effect of HSP70 on melanin production, as has been observed in mice that over-express HSP7032. Paradoxically, HSP70 appears to have a geno-protective effect for cells exposed to UV. Evidence for this assumption is supported by the observation that those whales with higher levels of HSP70 showed decreased mtDNA lesions across species. Expression of KIN was also higher in the sperm whales. Taken together, transcription patterns of HSP70 and KIN might reflect the markedly dissimilar sea-surface behaviour of sperm whale compared to the other two species. For instance, foraging dive intervals at the surface are approximately five times longer for sperm whales than for blue and fin whales33,34, and during socialization, sperm whales can remain at the surface for up to six hours at a time34, thus increasing exposure to damaging UV. In humans and laboratory animals, levels of expression of repair genes such as HSP70 and KIN increase in a time-dependent manner following UV irradiation15,20, and up-regulation of HSP70 and KIN can be observed between 6 h and 8 h post UV irradiation20. In this sense, the recorded expression levels suggest that sperm whales activate genotoxic stress pathways that involve over-expression of HSP70 and KIN in response to long and persistent exposure to UV.
The gene expression level of HSP70 and P53 showed an ascending increase from February to May, with a peak in March/April. These trends mimic the temporal variation in UV recorded between February and April for the Gulf of California suggesting that overexpression of repair genes is dose-dependent in whales as is known to occur in humans and laboratory animals15,20. It is reasonable to hypothesize that the comparatively lower levels of expression recorded in May are due to acclimatization to UV exposure, as UV levels plateaued in April. This phenomenon has been described in humans, whose sensitivity to sunburn decreases with increasing frequency and duration of solar exposure30.
Blue whales appeared able to increase melanocyte abundance in response to the increase in UV from February to May, suggesting that individuals of this species are capable of modulating their degree of pigmentation. Although statistically non-significant, melanin density appeared to follow the same increasing trend over months suggesting tanning ability (Table S4; Fig. 3C). Tanning has already been described for some wild species. For instance, hammerhead sharks gradually increase integument melanin in response to an increase in direct UV exposure35. Other fish species36 as well as freshwater zooplankton37, have also demonstrated sun-tanning capability. Interestingly in this concept, we did not observe analogous changes in the pigmentation of the comparatively darker fin whale. It is possible that fin whale constitutive skin pigmentation is sufficient to counteract the harmful effects of UV. Indeed, fin whales have recorded the lowest prevalence of sunburn lesions compared to blue and sperm whales8.
The observed differences in pigmentation plasticity amongst species might entail their distinct migratory behaviour. The higher prevalence of microscopic lesions14 and mtDNA lesions at the beginning of the season might reflect the time needed for UV acclimatization30, especially as blue whale pigmentation seems to then increase gradually. Unexpectedly, towards the end of the season, blue whale melanin density appeared to diminish while mtDNA lesions peaked once more. Although statistically non-significant, it is tempting to speculate that the trend might reflect skin turnover, or cell survival, linked to a focal overload of genetic and cellular skin damage within an overall field effect of UV-exposure38,39. On the whole, the observed trends suggest that blue whales are able to modulate pigmentation in response to the fluctuating levels of UV through the season, suggesting marked phenotypic plasticity.
Despite the opportunity to study these long-lived oceanic predators over decades, our findings demonstrate how poorly we understand the impact of UV exposure and the basic processes that are engaged by this environmental insult. The discovery of an apparent plastic pigmentation response as well as the use of distinct strategies to counteract harmful exposure to UV amongst whale species raise questions about the selective pressure that sun exposure has exerted on these marine mammals.
Methods
Ethics statement
Sampling was approved by the Ethics Committee Regulations of the Zoological Society of London and complied with national and international regulations on animal welfare.
Whale sample collection
Cetacean surveys and sample collection (106, 55 and 23 skin biopsies of blue, fin and sperm whales respectively) were conducted in the Gulf of California, Mexico, between January and June 2007–2009 as was described in detail previously8. Resampled individuals were excluded from the analyses, the first sample obtained per individual being the one included. Minimum age was estimated by the age at the first sighting of a particular individual using the CICIMAR sighting history of photo-identified blue whale's data set.
Skin pigmentation indices using histology analysis
Melanocyte density, used as a surrogate measure of constitutive pigmentation8,13, and melanin abundance were calculated for each individual within a standardized area using digital photographs of H&E stained skin sections. Histological and melanocyte density quantification methods were performed as described previously8. Melanin abundance was measured using the image processing program Image J (for details see Fig. S2 and Fig. S3). Tissue analyses were conducted on all samples that were unaffected by technical issues such as tissue fixation or section size. Details on sample numbers are shown in each figure legend or table caption.
Gene expression assays
Gene expression assays were described in detail previously14. Briefly, total RNA was extracted using the RNeasy® Mini Kit (Qiagen, UK). Complementary DNA (cDNA) was obtained by reverse transcription using the QuantiTect® Reverse Transcription Kit (Qiagen, UK). All qPCRs were performed in a 7300 Real-Time PCR System (Applied Biosystems, UK) using Power SYBR Green PCR Master Mix (Applied Biosystems, UK). As internal control genes we used the genes coding for the ribosomal proteins S18 (RSP18) and ribosomal proteins L4 (RPL4), which were selected as best intra- and interspecies control genes among four genes tested using the packages BestKeeper, geNorm and NormFinder14. Target gene (HSP70, P53, KIN and TYR) primers were designed using sequences obtained in the NCBI GenBank database (for sequence details of the primers and corresponding targeted gene sections see Table S5). In total, we obtained high quality gene expression data for 60 samples including 22 blue whales, 22 fin whales and 16 sperm whales.
Mitochondrial DNA lesions
To isolate DNA for assessment of mtDNA damage, we used phenol chloroform extraction followed by ethanol precipitation. UV-induced mitochondrial DNA (mtDNA) lesions were detected and quantified using quantitative real-time PCR (qPCR) as described previously12. The principle of the assay resides, under quantitative conditions, in the fact that damaged mtDNA amplify at a lower efficiency rate than undamaged mtDNA and thus the threshold crossing point (Ct) value obtained is directly proportional to the level of damage12.
Statistical analysis
Gene expression levels were analysed using the relative quantification method (level of expression of the target gene relative to internal control genes) that is based on the ΔCt method (Cttarget gene - geometric mean Ctcontrol genes)14. As lower ΔCt values represent higher levels of expression, it was easier for the interpretation of the results to negatively transform these values. Linear models were constructed to investigate interspecific, intraspecific and temporal variation in levels of pigmentation and mtDNA lesion abundance. For gene expression analyses, linear mixed effect models were constructed14,21. Models were built in R and we used a top-down strategy to determine which variables explained a significant fraction of the data (see supplementary information for more details)21. Any violation of normality or homoscedasticity assumption was corrected by logarithmic transformation of the response variable.
Author Contributions
K.A.-W. and M.B.-M. are co-senior and corresponding authors of the study with the laboratory research conducted in their laboratories. The project was designed and developed by K.A.-W., D.G. and L.M.-L. and M.B.-M., D.G. & L.M.-L. conducted field work and collected samples. L.M.-L. performed the experiments and analysed the data with the help of K.A.-W., D.G., A.B., M.B.-M., E.W. and R.K. Mitochondrial DNA damage assays were conceived and conducted by A.B. and M.B.-M. M.B.-M. provided reagents, gave technical support and conceptual advice. L.M.-L., K.A.-W., M.B.-M. and A.B. co-wrote the manuscript. All authors discussed the results and implications and commented on the manuscript at all stages.
Supplementary Material
Supplementary Info 1
Acknowledgments
Members of the Cetacean Ecology Laboratory (CICIMAR) greatly assisted us during fieldwork. We thank Azuzena Ugalde de la Cruz for cross-referencing whale identity with the CICIMAR blue whale catalogue; Jon Spencer-Todd and Kyunglee Lee for helping with melanocyte counts and DNA extractions; Kate Ciborowski and Solenn Patalano for offering valuable advice on technical aspects of qPCR; Eric Nash and Paul Newman from NASA Goddard Space Flight Center, who kindly provided UV index data to construct figure 3B; and Edel O'Toole and Paul Jepson, who provided valuable comments that greatly improved our manuscript.
Footnotes
L.M.M.L was funded by a NERC PhD Studentship (NE/F00818X/1) awarded at Queen Mary University of London. Fieldwork was partially funded by the IPN (Instituto Politécnico National de Mexico to which CICIMAR is affiliated) and CONACYT (CB-2006-61982). MtDNA work was conducted in and financed by the Institute of Cellular Medicine of Newcastle University, and the UK NIHR Biomedical Research Centre in Ageing and Age-related Disease to the Newcastle upon Tyne Hospitals NHS Foundation Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This study is registered as project WLE/0474 at the Institute of Zoology. Samples were collected under permits SGPA/DGVS/00506/08, SGPA/DGVS/09760/08 and SGPA/DGVS/08021/06 issued by SEMARNAT.
References
- McKenzie R. L., Aucamp P. J., Bais A. F., Bjorn L. O. & Ilyas M. Ozone depletion and climate change: impacts on UV radiation. Photoch. Photobio. Sci. 10, 182–198 (2011). [DOI] [PubMed] [Google Scholar]
- Manney G. L. et al. Unprecedented Arctic ozone loss in 2011. Nature. 478, 469–475 (2011). [DOI] [PubMed] [Google Scholar]
- Norval M. et al. The human health effects of ozone depletion and interactions with climate change. Photoch. Photobio. Sci. 10, 199–225 (2011). [DOI] [PubMed] [Google Scholar]
- Sweet M. et al. Evidence of Melanoma in Wild Marine Fish Populations. PloS One. 7(8)</emph>, e41989 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein A. R., Romansic J. M., Kiesecker J. M. & Hatch A. C. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers distrib. 9, 123–140 (2003). [Google Scholar]
- Dahms H. U. & Lee J. S. UV radiation in marine ectotherms: molecular effects and responses. Aquat. Toxicol. 97, 3–14 (2010). [DOI] [PubMed] [Google Scholar]
- Llabrés M. et al. Impact of elevated UVB radiation on marine biota: a meta-analysis. Global Ecol. Biogeogr. 22(1)</emph>, 131–144 (2013). [Google Scholar]
- Martinez-Levasseur L. M. et al. Acute sun damage and photoprotective responses in whales. Proc. R. Soc. B 278, 1581–1586 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucab J. E., Phillips D. H. & Arlt V. M. Linking environmental carcinogen exposure to TP53 mutations in human tumours using the human TP53 knock-in (Hupki) mouse model. FEBS J. 277, 2567–2583 (2010). [DOI] [PubMed] [Google Scholar]
- Birch-Machin M. A. Mitochondria and skin disease. Clin. Exp. Dermatol. 25, 141–146 (2000). [DOI] [PubMed] [Google Scholar]
- Birch-Machin M. A. & Swalwell H. How mitochondria record the effects of UV exposure and oxidative stress using human skin as a model tissue. Mutagenesis. 25, 101–107 (2010). [DOI] [PubMed] [Google Scholar]
- Bowman A., Martinez-Levasseur L. M., Acevedo-Whitehouse K., Gendron D. & Birch-Machin M. A. The simultaneous detection of mitochondrial DNA damage from sun-exposed skin of three whale species and its association with UV-induced microscopic lesions and apoptosis. Mitochondrion. 13(4)</emph>, 342–349 (2013). [DOI] [PubMed] [Google Scholar]
- Costin G. E. & Hearing V. J. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 21, 976–994 (2007). [DOI] [PubMed] [Google Scholar]
- Martinez-Levasseur L. M., Gendron D., Knell R. J. & Acevedo-Whitehouse K. Control and target gene selection for studies on UV-induced genotoxicity in whales. BMC Res. Notes. In Press (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Coba F. et al. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J. Dermatol. Sci. 55, 161–169 (2009). [DOI] [PubMed] [Google Scholar]
- Matsuda M. et al. Prevention of UVB Radiation-induced Epidermal Damage by Expression of Heat Shock Protein 70. J. Biol. Chem. 285, 5848–5858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latonen L. & Laiho M. Cellular UV damage responses--functions of tumor suppressor p53. Biochim. Biophys. Acta. 1755, 71–89 (2005). [DOI] [PubMed] [Google Scholar]
- Ikehata H. Influences of p53 deficiency on the apoptotic response, DNA damage removal and mutagenesis in UVB-exposed mouse skin. Mutagenesis. 25, 397–405 (2010). [DOI] [PubMed] [Google Scholar]
- Biard D. S. F. et al. Enhanced expression of the Kin17 protein immediately after low doses of ionizing radiation. Rad. Res. 147, 442–450 (1997). [PubMed] [Google Scholar]
- Masson C. et al. Global genome repair is required to activate KIN17, a UVC-responsive gene involved in DNA replication. Pro. Natl. Acad. Sci. U.S.A. 100, 616–621 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur A., Ieno E., Walker N., Saveliev A. & Smith G. Mixed Effects Models and Extensions in Ecology with R. Springer, New York (2009). [Google Scholar]
- Sturm R. A., Teasdale R. D. & Box N. F. Human pigmentation genes: identification, structure and consequences of polymorphic variation. Gene. 277, 49–62 (2001). [DOI] [PubMed] [Google Scholar]
- Polanowski A. M., Robinson-Laverick S. M., Paton D. & Jarman S. M. Variation in the Tyrosinase Gene Associated with a White Humpback Whale (Megaptera novaeangliae). J. Hered. 103(1)</emph>, 130–133 (2012). [DOI] [PubMed] [Google Scholar]
- Lin J. Y. & Fisher D. E. Melanocyte biology and skin pigmentation. Nature. 445, 843–850 (2007). [DOI] [PubMed] [Google Scholar]
- Oren M. & Bartek J. The sunny side of p53. Cell. 128, 826–828 (2007). [DOI] [PubMed] [Google Scholar]
- Murase D. et al. The essential role of p53 in hyperpigmentation of the skin via regulation of paracrine melanogenic cytokine receptor signaling. J. Biol. Chem. 284, 4343–4353 (2009). [DOI] [PubMed] [Google Scholar]
- Bérubé M., Urbán-Ramírez J., Dizon A. E., Brownell R. L. & Palsbøll P. J. Genetic identification of a small and highly isolated population of fin whales (Balaenoptera physalus) in the Sea of Cortez, Mexico. Conserv. Genet. 3, 183–190 (2002). [Google Scholar]
- Calambokidis J., Barlow J., Ford J. K. B., Chandler T. E. & Douglas A. B. Insights into the population structure of blue whales in the Eastern North Pacific from recent sightings and photographic identification. Mar. Mammal Sci. 25, 816–832 (2009). [Google Scholar]
- Ilyas M. Climate augmentation of erythemal UV-B radiation dose damage in the tropics and global change. Curr. Sci. 93, 1604–1608 (2007). [Google Scholar]
- Sayre R. M., Desrochers D. L., Wilson C. J. & Marlowe E. Skin type, minimal erythema dose (MED), and sunlight acclimatization. J. Am. Acad. Dermatol. 5, 439–443 (1981). [DOI] [PubMed] [Google Scholar]
- Swalwell H., Latimer J., Haywood R. M. & Birch-Machin M. A. Investigating the role of melanin in UVA/UVB- and hydrogen peroxide-induced cellular and mitochondrial ROS production and mitochondrial DNA damage in human melanoma cells. Free Radical Bio. Med. 52, 626–634 (2012). [DOI] [PubMed] [Google Scholar]
- Hoshino T. et al. Suppression of Melanin Production by Expression of HSP70. J. Biol. Chem. 285, 13254–13263 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll D. A., Acevedo-Gutiérrez A., Tershy B. R. & Urbán-Ramírez J. The diving behavior of blue and fin whales: is dive duration shorter than expected based on oxygen stores? Comp. Biochem. Physiol. 129, 797–809 (2001). [DOI] [PubMed] [Google Scholar]
- Whitehead H. Sperm Whales: Social Evolution in the Ocean. Chicago: The University of Chicago Press. 456p (2003). [Google Scholar]
- Lowe C. & Goodman-Lowe G. Suntanning in hammerhead sharks. Nature. 383, 677 (1996). [DOI] [PubMed] [Google Scholar]
- Adachi K. et al. The histological analysis, colorimetric evaluation, and chemical quantification of melanin content in ‘suntanned' fish. Pigm. Cell Res. 18, 465–468 (2005). [DOI] [PubMed] [Google Scholar]
- Hansson L. A. Induced pigmentation in zooplankton: a trade-off between threats from predation and ultraviolet radiation. Proc. R. Soc. B 267, 2327–2331 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatve M., Ortonne J. P., Birch-Machin M. A. & Gupta G. Management of field change in actinic keratosis. Brit. J. Dermatol. 157 (suppl.2), 21–24 (2007). [DOI] [PubMed] [Google Scholar]
- Dakubo G., Jakupciak J., Birch-Machin M. A. & Parr R. Clinical implications and utility of field cancerization. Cancer Cell Int. 7, 2 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Info 1