Abstract
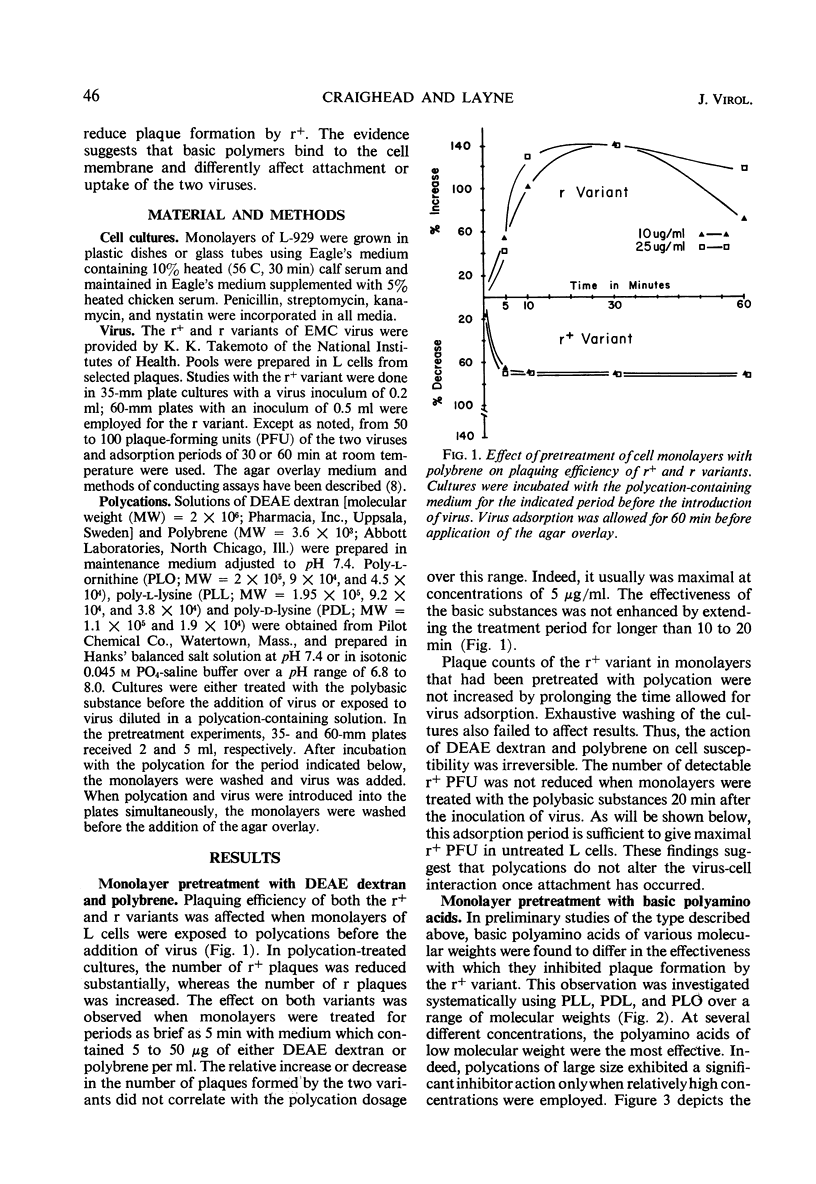
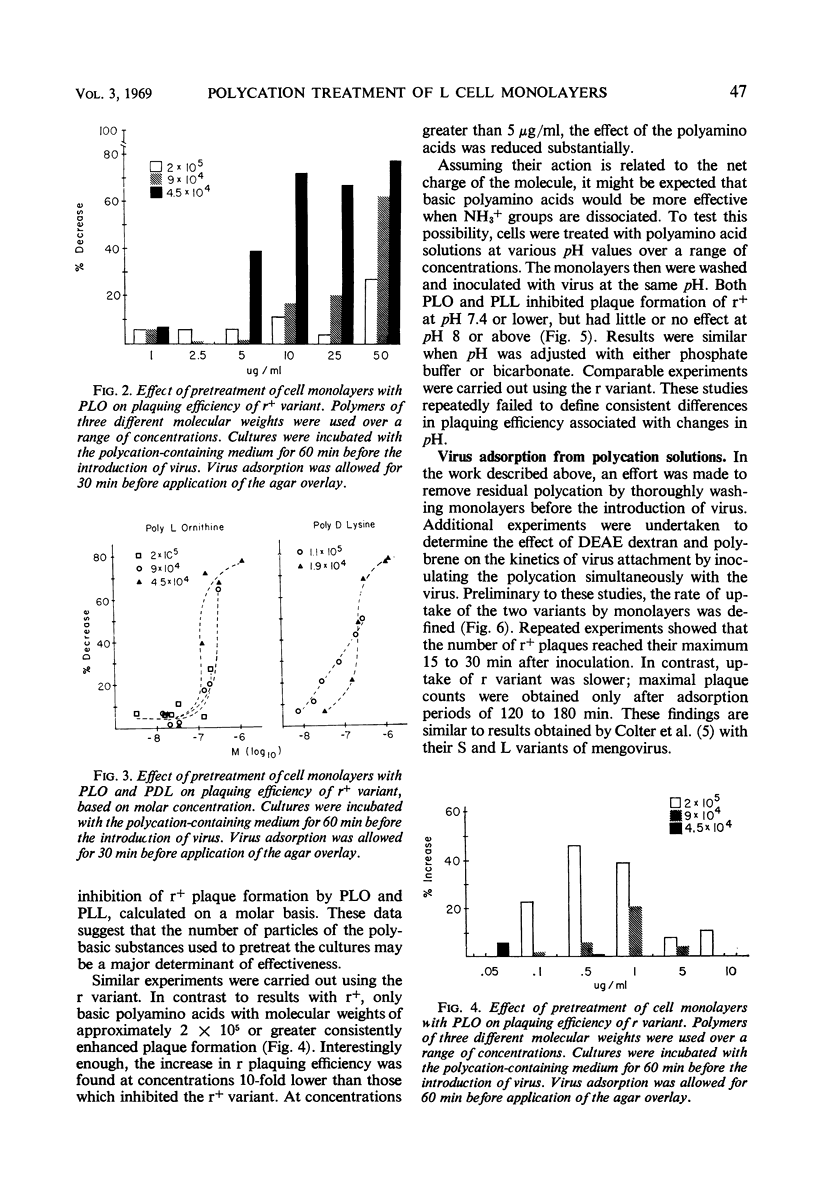
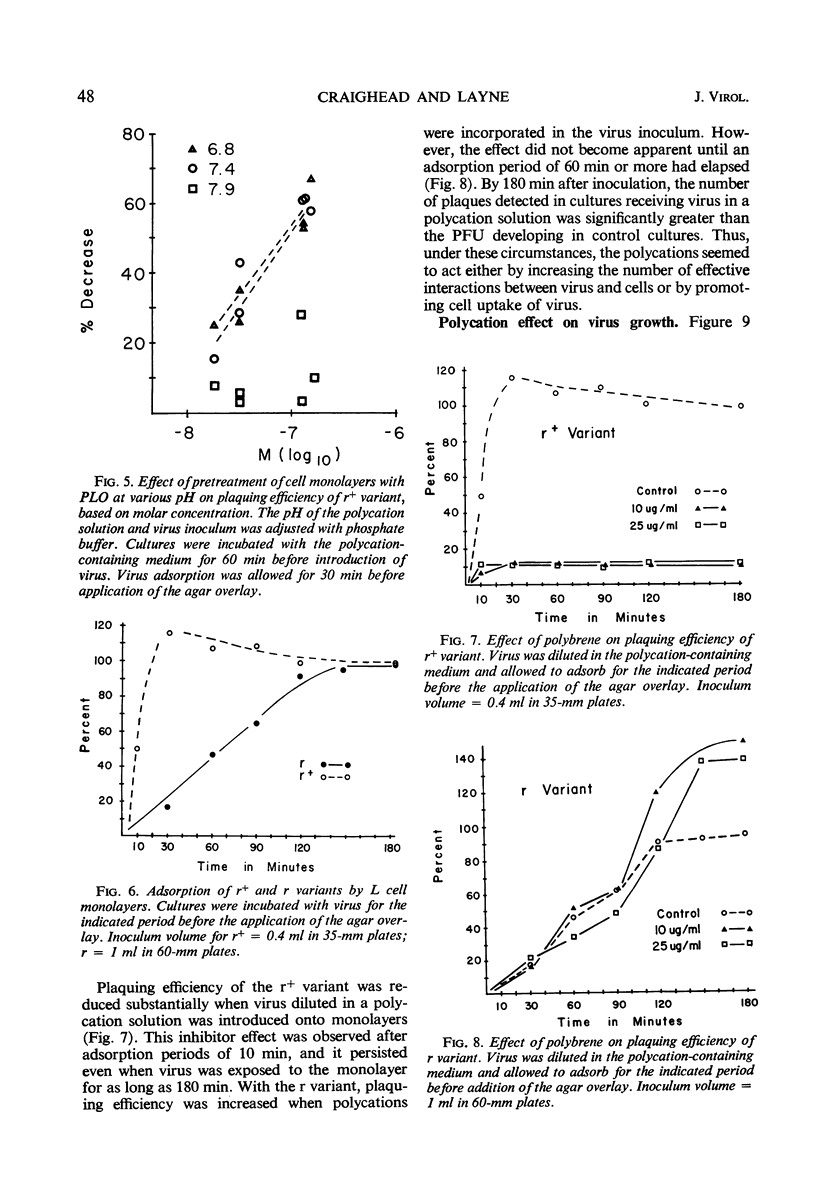
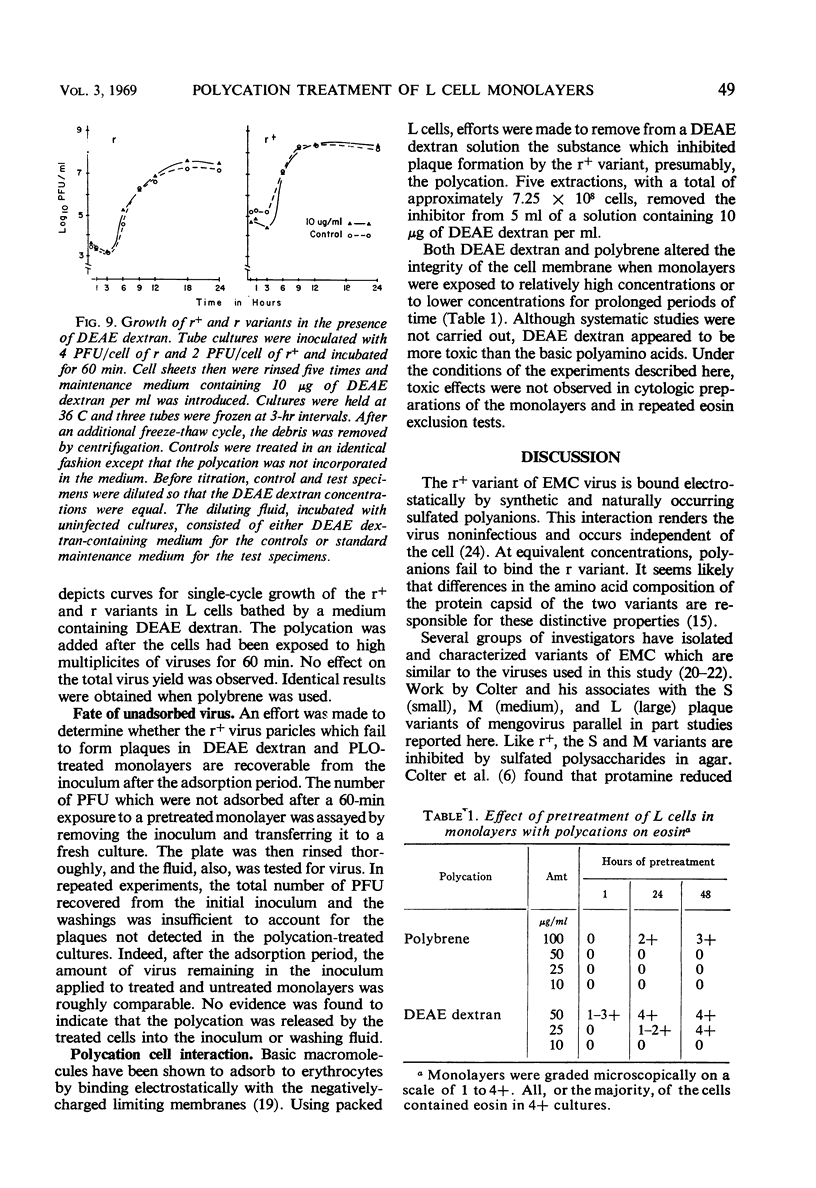
Polycation treatment of L cell monolayers affected plaquing efficiency of both the r+ and r variants of the encephalomyocarditis virus. Plaque formation by r+ variant was decreased markedly by three structurally different types of synthetic basic polymers, diethylaminoethyl dextran, hexadimethrene (polybrene), and basic polyamino acids. In contrast, these same substances increased substantially the number of plaques formed by the r variant. The effect on the two variants was observed when polycations were applied to the cells before or simultaneously with the introduction of virus. The molar concentration and size of the polymer proved important. Thus, basic polyamino acids of low molecular weight were significantly more inhibitory for the r+ variant than were those of high molecular weight. On the other hand, plaquing efficiency of the r variant was increased by relatively large polyamino acids, but not by polymers of small size. Basic polyamino acids inhibited r+ plaque formation to a greater degree at low than at high pH values. However, plaquing efficiency of the r variant in polycation-treated cultures was not affected by changes in pH. Basic polymers appear to bind to cell membranes and affect either attachment or uptake of the viruses. The evidence suggests that the substances influence by different mechanisms the interaction of the r+ and r variants with cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- Amako K., Dales S. Cytopathology of Mengovirus infection. I. Relationship between cellular disintegration and virulence. Virology. 1967 Jun;32(2):184–200. doi: 10.1016/0042-6822(67)90269-3. [DOI] [PubMed] [Google Scholar]
- Breeze D. C. Comparative growth and selection of small plaque and large plaque encephalomyocarditis virus in the absence of inhibitors from agar. J Gen Virol. 1967 Jan;1(1):71–80. doi: 10.1099/0022-1317-1-1-71. [DOI] [PubMed] [Google Scholar]
- Bukrinskaia A. G., Gutel'man A. K., Burduchea O., Jên K. F. Deistvie gistonov na reproduktsiiu miksovirusov. Vopr Virusol. 1965 Nov-Dec;10(6):720–726. [PubMed] [Google Scholar]
- COLTER J. S., DAVIES M. A., CAMPBELL J. B. STUDIES OF THREE VARIANTS OF MENGO ENCEPHALOMYELITIS VIRUS. I. RATE OF ATTACHMENT TO L CELLS, AND EFFECT OF PH ON INFECTIVITY. Virology. 1964 Nov;24:474–480. doi: 10.1016/0042-6822(64)90187-4. [DOI] [PubMed] [Google Scholar]
- COLTER J. S., DAVIES M. A., CAMPBELL J. B. STUDIES OF THREE VARIANTS OF MENGO ENCEPHALOMYELITIS VIRUS. II. INHIBITION OF INTERACTION WITH L CELLS BY AN AGAR INHIBITOR AND BY PROTAMINE. Virology. 1964 Dec;24:578–585. doi: 10.1016/0042-6822(64)90210-7. [DOI] [PubMed] [Google Scholar]
- CRAIGHEAD J. E. SOME PROPERTIES OF THE ENCEPHALOMYOCARDITIS, COLUMBIA SK AND MENGO VIRUSES. Proc Soc Exp Biol Med. 1965 Jun;119:408–412. doi: 10.3181/00379727-119-30196. [DOI] [PubMed] [Google Scholar]
- Connolly J. H. Effect of histones and protamine on the infectivity of Semliki Forest virus and its ribonucleic acid. Nature. 1966 Nov 19;212(5064):858–858. doi: 10.1038/212858a0. [DOI] [PubMed] [Google Scholar]
- ELLEM K. A., COLTER J. S. The isolation of three variants of Mengo virus differing in plaque morphology and hemagglutinating characteristics. Virology. 1961 Nov;15:340–347. doi: 10.1016/0042-6822(61)90365-8. [DOI] [PubMed] [Google Scholar]
- FENWICK M. L., COOPER P. D. Early interactions between poliovirus and ERK cells: some observations on the nature and significance of the rejected particles. Virology. 1962 Oct;18:212–223. doi: 10.1016/0042-6822(62)90007-7. [DOI] [PubMed] [Google Scholar]
- Gasic G. J., Berwick L., Sorrentino M. Positive and negative colloidal iron as cell surface electron stains. Lab Invest. 1968 Jan;18(1):63–71. [PubMed] [Google Scholar]
- KODZA H., JUNGEBLUT C. W. Effect of receptor-destroying enzyme on the growth of EMC virus in tissue culture. J Immunol. 1958 Jul;81(1):76–81. [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Bishop J. M. The effect of polycations on the interaction of viral RNA with mammalian cells: studies on the infectivity of single- and double-stranded poliovirus RNA. Virology. 1968 May;35(1):9–17. doi: 10.1016/0042-6822(68)90300-0. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. THE BASIS FOR THE SIZE DIFFERENCES IN PLAQUES PRODUCED BY VARIANTS OF ENCEPHALOMYOCARDITIS (EMC) VIRUS. Virology. 1963 Aug;20:559–566. doi: 10.1016/0042-6822(63)90280-0. [DOI] [PubMed] [Google Scholar]
- Moehring J. M., Moehring T. J. The response of cultured mammalian cells to diphtheria toxin. II. The resistant cell: enhancement of toxin action by poly-L-ornithine. J Exp Med. 1968 Mar 1;127(3):541–554. doi: 10.1084/jem.127.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S. Interaction of respiratory syncytial virus with polyions: enhancement of infectivity with diethylaminoethyl dextran. Proc Soc Exp Biol Med. 1968 May;128(1):163–166. doi: 10.3181/00379727-128-32969. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- RUBINI J. R., STAHMANN M. A., RASMUSSEN A. F., Jr Agglutination of red cells by synthetic lysine polypeptides. Proc Soc Exp Biol Med. 1951 Apr;76(4):659–662. doi: 10.3181/00379727-76-18587. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. A membrane effect of basic polymers dependent on molecular size. Nature. 1967 Aug 26;215(5104):934–936. doi: 10.1038/215934a0. [DOI] [PubMed] [Google Scholar]
- Ryser H. J., Hancock R. Histones and basic polyamino acids stimulate the uptake of albumin by tumor cells in culture. Science. 1965 Oct 22;150(3695):501–503. doi: 10.1126/science.150.3695.501. [DOI] [PubMed] [Google Scholar]
- Ryser H. J. Studies on protein uptake by isolated tumor cells. 3. Apparent stimulations due to pH, hypertonicity, polycations, or dehydration and their relation to the enhanced penetration of infectious nucleic acids. J Cell Biol. 1967 Mar;32(3):737–750. doi: 10.1083/jcb.32.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMULL C. E., LUDWIG E. H. Enhancement of the plaque-forming capacity of poliovirus ribonucleic acid with basic proteins. J Bacteriol. 1962 Nov;84:1035–1040. doi: 10.1128/jb.84.5.1035-1040.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO K. K., LIEBHABER H. Virus-polysaccharide interactions. I. An agar polysaccharide determining plaque morphology of EMC virus. Virology. 1961 Aug;14:456–462. doi: 10.1016/0042-6822(61)90338-5. [DOI] [PubMed] [Google Scholar]
- Tilles J. G. Enhancement of interferon titers by poly-L-ornithine. Proc Soc Exp Biol Med. 1967 Jul;125(3):996–999. doi: 10.3181/00379727-125-32259. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. DEAE-dextran: enhancement of cellular transformation induced by avian sarcoma viruses. Virology. 1967 Sep;33(1):175–177. doi: 10.1016/0042-6822(67)90109-2. [DOI] [PubMed] [Google Scholar]
- Wallis C., Melnick J. L. Mechanism of enhancement of virus plaques by cationic polymers. J Virol. 1968 Apr;2(4):267–274. doi: 10.1128/jvi.2.4.267-274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



