Abstract
Objective
To investigate the potential role of fresh Carica papaya (C. papaya) leaf extract on haematological and biochemical parameters and toxicological changes in a murine model.
Methods
In total 36 mice were used for the trial. Fresh C. papaya leaf extract [0.2 mL (2 g)/mouse] was given only to the test group (18 mice). General behavior, clinical signs and feeding patterns were recorded. Blood and tissue samples were collected at intervals. Haematological parameters including platelet, red blood cell (RBC), white blood cell (WBC), packed cell volume (PCV), serum biochemistry including serum creatinine, serum glutamic-oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT) were determined. Organs for possible histopathological changes were examined.
Results
Neither group exhibited alteration of behavior or reduction in food and water intake. Similarly, no significant changes in SGOT, SGPT and serum creatinine levels were detected in the test group. Histopathological organ changes were not observed in either group of mice except in three liver samples of the test group which had a mild focal necrosis. The platelet count (11.33±0.35)×105/µL (P=0.000 04) and the RBC count (7.97±0.61)×106/µL (P=0.000 03) were significantly increased in the test group compared to that of the controls. However, WBC count and PCV (%) values were not changed significantly in the test group. The platelet count in the test group started to increase significantly from Day 3 (3.4±0.18×105/µL), reaching almost a fourfold higher at Day 21 (11.3×105/µL), while it was 3.8×105/µL and 5.5×105/µL at Day 3 and Day 21 respectively in the control. Likewise, the RBC count in the test group increased from 6×106/µL to 9×106/ µL at Day 21 while it remained near constant in the control group (6×106/µL).
Conclusions
Fresh C. papaya leaf extract significantly increased the platelet and RBC counts in the test group as compared to controls. Therefore, it is very important to identify those chemicals of C. papaya leaves as it can be recommended to be used as a medication to boost thrombopoiesis and erythropoiesis in humans and in animals in which these cell lineages have been compromised.
Keywords: Carica papaya, Alternative medicine, Erythropoiesis, Thrombopoiesis, Toxicity
1. Introduction
Carica papaya (C. papaya, family Caricarceae, papaya) is one of the most popular and economically important plants in the world as its fruit is a common delicacy[1]. It is a soft wooded single-stemmed perennial tree, 2-10 m in height, with a crown of large palmate leaves emerging from the apex of the trunk. The soft, hollow, cylindrical trunk ranges from 30 cm in diameter at the base to about 5 cm in diameter at the crown[2]. Although native to Central America, it has been transported to many parts of the tropics.
The papaya plant is lactiferous as it contains specialized cells known as lactifers that occur in most tissues and secrete latex. Lactifiers secrete latex and are dispersed throughout most plant tissues. The papaya-latex is well known for being a rich source of the four cysteine endopeptidases namely papain, chymopapain, glycyl endopeptidase and caricain[3]. Leaves contain an alkaloid called carpaine and a glucoside named carposide[4].
Different parts of the papaya plants including fruit, dried fruit, leaves, dried leaves, stems, seeds and roots have long been used as ingredients in alternative medicine. For instance, the seeds are used for expelling worms and roots and seeds are used as an abortifacient agent. The leaves (especially fallen ones) are used variously for the treatment of fevers, pyrexia, diabetes, gonorrhoea, syphilis, inflammation and as a dressing for septic wounds[5].
Untested herbal medicines could be potentially injurious to human health. Many plants used in traditional and folk medicines are potentially toxic, mutagenic, and carcinogenic[6]–[11]. Toxicological studies of extracts from different parts of C. papaya plants such as seeds, fruit, roots and leaves have been carried out using several animal models. Acute and chronic toxicities of unripe fruit of the C. papaya have been documented[12].
Some of the traditional claims of efficacy that have been investigated scientifically using animal models and their efficacy have been proven[13],[14]. Recent studies showed that C. papaya leaf extract has potential anti-sickling (inhibition of sickle cell formation) properties[14]. Indran et al. have shown that there is a protective effect against gastric ulcers in rats[13]. Moreover, C. papaya flowers are known to have antibacterial activities[15]. The contraceptive efficacy of the seeds of C. papaya has been earlier demonstrated in rats, mice and rabbits[16]–[19]. Oral administration of C. papaya seed extract could induce reversible male infertility and could be used for pharmaceutical development of a male contraceptive.
Today, many tropical and subtropical countries are engulfed by dengue infection which is caused by viruses belonging to the Flaviviridae family. There is no specific therapy for dengue even though the infection has a significant mortality. Sri Lanka is no exception where, dengue infection is a priority for the national health services for prevention and reducing mortality[20]. In desperation, many people have resorted to use papaya leaf extract covertly. This is applicable even to hospitalized dengue patients. However, recommending C. papaya leaf extract for dengue infection is unethical until it is proven by scientific research.
Therefore, in the present study, we have investigated the effects of oral intake of pure extract of C. papaya leaves on haematological/biochemical parameters and toxicological changes in the murine model.
2. Materials and methods
2.1. Experimental animals
Male white mice (average body weight 32-33 g) 6 weeks old, obtained from the Medical Research Institute, Sri Lanka were used as the model for haematological and toxicological investigation. Animals were kept in the animal house of the Faculty of Medicine, University of Peradeniya.
Three experimental trials were conducted during May 2011 to May 2012 using three sets of mice. Mice were divided into two groups, control and test, in all three trials. For the first and second trials, we used 48 mice (for each trial 12 mice/control and 12 mice/test) and for the third trial, 36 mice were used (18 control and 18 test). All mice were given a standard commercial diet with free access to water. All mice in both test and control groups were numbered by ear tattooing. The first trial was a pilot study to plan a proper study where the following variables were considered-dose of C. papaya leaf extract, timing of blood sampling and histopathological changes in liver. The second trial refined the methodology and the third provided the results presented here. Ethical clearance was obtained from the Ethical Review Committee of the Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, Sri Lanka.
2.2. Preparation of C. papaya leaf extract
Fresh, middle stage age, C. papaya leaves were picked daily for 7 d. Leaves were washed and the stems were removed before use. After weighing, leaves were blended without adding water or other liquids. Then the mixture was filtered to obtain a pure extract of C. papaya leaves. Finally, the volume of the extract was measured and the extracts were stored at 4 °C until use. Fresh extracts were prepared for each use.
2.3. Dosage of C. papaya leaf extract
In the first trial, we used 0.5 mL (5 g)/mouse/day and in second and third trial, we used 0.2 mL (2 g)/mouse/day. The first trial was conducted to determine a suitable daily dose per mouse. In the third trial, test group mice were fed with fresh C. papaya leaf extract for seven consecutive days (0.2 mL (2 g)/mouse/day), the first of these days being regarded as day one of the trial. Similarly, the control group was given water.
2.4. Experimental procedure
Body weights of all mice were recorded before feeding started and weighing was repeated every second day during the study period. Behavioral activities were recorded once a day.
Blood smears were prepared to evaluate the platelet and red blood cell (RBC) counts from both test and control groups on alternate days and on days of sacrifice (on Days 8, 14 and 21, an equal number of mice were sacrificed from each group for biochemical and histological examination). The numbers of platelets and RBCs were counted in 10 fields or more under oil immersion (×100). The procedure was repeated in thin or thick areas of the film if the distribution was uneven. Then, average numbers of platelets and RBCs were determined by dividing the total number by the number of fields viewed. Finally, the average number of platelets and RBCs was multiplied by the established field factor to determine the estimate count.
Six mice from each group (test and control) were sacrificed at Day 8, 14 and 21. Blood was collected. Packed cell volume (PCV) was measured. Serum was separated and stored at -80 °C until use. Serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase and creatinine levels were also measured. Furthermore, specimens (liver, lungs, kidney, heart, intestine and spleen) were collected for the histopathological and toxicological investigation.
2.5. Histopathological examination
Specimens were fixed in buffered formalin (10%). Paraffin tissue sections (thickness 2-7 µm) were prepared. Sections were de-paraffinized and re-hydrated. Samples were stained with hematoxylin and eosin (H&E) and observed under a light microscope.
2.6. Statistical analysis
A test for the normal distribution was performed for platelet and RBC counts in both control and test groups using the Shapiro-Wilk normality test. Wilcoxon signed rank test was applied for statistical comparison of the platelet counts. Welch two sample t-test was used for statistical comparison of RBC counts. All values were expressed as the mean±SEM). Differences were considered as significant at P<0.05.
3. Results
3.1. Body weight and behavioral changes
The average body weight of mice at the start for the third trial was (32.000±0.595) g in the test group and (32.70±0.70) g in the control group. However, it was observed that the average body weight was (36.20±1.63) g and (36.50±1.62) g in the test and control groups respectively by Day 21 after the start of treatment. Neither loss of body weight nor behavioral changes were observed in the test group.
3.2. Histopathology
There were no gross pathological changes observed on organs during the post mortem examination of mice from either group. Examination of histological sections of the liver, lung, kidney, spleen, heart and the intestine found no remarkable changes other than mild focal lytic cell necrosis in three liver sections obtained from the test group (3 of 18 samples). However, in the first trial, when high dose of C. papaya leaf extract was used, liver histology showed focal lytic necrosis of all test groups indicating that there can be dose dependent toxicity.
3.3. Serology
Serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase and creatinine levels were not altered from their normal range in both groups (Table 1).
Table 1. Haematological and biochemical parameters in the groups after giving C. papaya leaves extract (mean±SD).
| Parameters |
Day 1 |
Day 7 |
Day 14 |
Day 21 |
||||
| Control group | Test group | Control group | Test group | Control group | Test group | Control group | Test group | |
| Platelet count (×105 /µL) | 3.67±0.16 | 3.36±0.16 | 4.52±0.15 | 9.00±0.35 | 5.21±0.13 | 10.86±0.38 | 5.53±0.12 | 11.33±0.35 |
| RBC (×106 /µL) | 6.23±0.17 | 5.87±0.19 | 5.95±0.18 | 6.63±0.32 | 6.61±0.28 | 7.95±0.59 | 6.00±0.31 | 7.97±0.61 |
| WBC (×103 /µL) | 7.45±0.23 | 7.61±0.13 | 7.16±0.21 | 7.62±0.32 | 7.34±0.15 | 7.71±0.61 | 7.52±0.11 | 8.01±0.42 |
| SGOT (U/L) | 88.67±7.60 | 118.67±25.91 | 96.17±40.00 | 110.17±23.00 | 90.00±16.47 | 90.00±13.40 | ||
| SGPT (U/L) | 28.50±2.70 | 24.17±3.70 | 17.83±4.90 | 27.67±9.97 | 47.50±7.40 | 42.83±3.32 | ||
| Serum creatinine (mg/dL) | 0.12±0.12 | 0.12±0.02 | 0.03±0.04 | 0.1±0.62E-18 | 0.1±6.2E-18 | 0.10±0.12 | ||
| PCV (%) | 41.8±0.48 | 40.83±1.85 | 43.7±2.72 | 40.83±1.19 | 41.00±4.32 | 44.83±1.79 | ||
3.4. Hematological investigation
No significant changes were observed in PCV in the test group compared to that of the control group. Values always remained within the normal range (39%-49%).
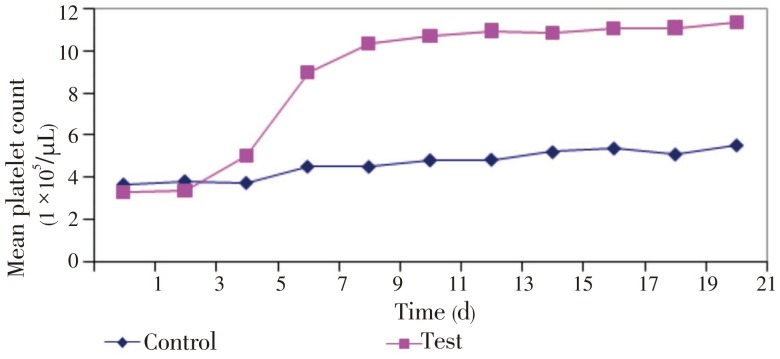
Average platelet counts of the test and control groups were (3.36±0.16)×105/µL and (3.67±0.16)×105/µL respectively before the experiment. There was no significant difference in the platelet counts during the first 3 days in either group. However, platelet counts within the test group started to rise steadily after Day 3 and reached a peak level at Day 13 (10.94×105/µL). Subsequently, the platelet count remained relatively constant at a range of 10×105 to 11×105/µL. The platelet count of the test group was (11.33±0.35)×105/µL at the end of the experiment compared to that of the control group (5.53±0.12)×105/µL (Figure 1).
Figure 1. Trends of platelet count rise in test and control groups.
Control and test groups had nearly similar RBC counts [6.23±0.17)×106/µL and (5.87±0.19)×106/µL in control and test mice respectively] on Day 1 (just before feeding started). The average RBC counts in the control group remained at a constant level of (6.00±0.31)×106/µL with minor fluctuations. In contrast, the average RBC count in the test group increased steadily during the first two weeks with (6.63±0.32)×106/µL at Day 8 and (7.95±0.59)×106/µL at Day 14. Thereafter, it remained at constant level around 7.97×106/µL (Table 1).
Our results clearly indicated that there was a significant increase (P<0.05) in average platelet counts in the test group, as against a slight increase in the control group (Table 1). RBC count was also significantly (P<0.05) increased in the test group compared to controls. There were no morphological changes observed in blood cells of mice in test group.
4. Discussion
This study clearly showed increasing platelet and RBC in healthy mice after feeding with a short course of papaya leaf extract. These animals remained healthy with normal weight gain during the experiment. No adverse effects were observed in the test group as evidenced by normal biochemical tests and normal histological examination of vital organs. However, at higher doses potential effects on the liver cannot be excluded. In Sri Lanka, various extracts from different parts of C. papaya have long been used in alternative medicine. Currently, C. papaya leaf juice is used to treat dengue patients and also for wound healing purposes. However, C. papaya leaf juice confers benefits in these diseases has not been tested scientifically in Sri Lanka. Therefore, studies to verify beneficial and/or harmful effects of C. papaya leaf extract in animal models will be the platform for future clinical research. As this study was done in healthy animals, we have no idea of platelet change in the thrombocytopenic state such as that induced by dengue fever.
In the present study, we used fresh C. papaya leaves to obtain pure extracts without adding any solvent or chemical. However, in previous animal studies[21],[22], C. papaya leaf extracts were prepared using dried leaves processed using various methods. Phytochemical analyses have indicated that C. papaya leaf extract contains chemical compounds such as alkaloids, tannins, antraquinone, cardenolides, steroids, saponins, phenolics, reducing sugars, flavonoids and cardiac glycosides[23]–[25]. These compounds can affect various biological processes in the body in ways that might have harmful or beneficial effects.
Bodyweight of both groups was increased by 4 g during the trial. Behavioral changes were not observed and animals moved freely within cages during the study. All mice (including both control and test groups) survived until the scarification. Therefore, it is suggested that oral feeding of C. papaya leaf extract (2 g/mouse/day for 7 d) may not cause adverse effects on animal behavior, appetite and bodyweight.
Similarly, feeding of C. papaya leaf extract didn't cause gross or histopathological changes in the test group, although mild focal necrosis was detected in a few liver sections (3 mice of 18) in this group. This might be due to the leaf extract fed to the mice, or some other unknown reason. Furthermore, a recent study proved that feeding of an aqueous extract of freeze dried C. papaya leaves did not cause acute toxicity in rats[22]. However, it is important to carry out further studies, feeding different doses for a long period of time to determine the chronic toxicity of C. papaya leaf extract. Liver enzyme profiles and creatinine levels did not significantly differ between test and control groups. Our results suggest that oral administration of C. papaya leaf extract may not cause sub acute and/or acute toxicity in the mouse model.
Interestingly, there was a significant rise in some haematalogical parameters, such as platelet and RBC counts, only in the test group. Other parameters, such as white blood cell count and PCV, did not show significant increase in either group.
Initially, platelet counts in the test and control groups were (3.36±0.16)×105/µL and (3.67±0.16)×105/µL respectively and (11.33±0.35)×105/µL and (5.53±0.12)×105/µL respectively at the end of the experiment. The effect on platelet counts by oral administration of C. papaya leaf extract is statistically significant (P<0.001). In addition, the RBC count in the test group increased significantly in comparison with the control (P<0.001).
The findings of the present study strongly suggest that there could be some active compounds in C. papaya leaves that can enhance haemopoiesis and thrombopoiesis in animals. Recently in Pakistan, a dengue patient was treated with aqueous extract of C. papaya leaves (25 mL, twice daily for 5-consecutive day) and exhibited a rise in platelet count[25]. Chemical analysis of C. papaya leaves showed the presence of considerable amounts of carpaine, malic acid, quinic acid, manghaslin and clitorin, minor quantities of various malic acid derivatives, nicotiflorin, rutin and unidentified constituents[26]. Therefore, we consider that it is very important to carry out further investigations to identify the active compounds in C. papaya leaf extracts which are responsible for the activation of haemopoiesis and thrombopoiesis.
In conclusion, it is clear that an oral feeding of pure extract of C. papaya leaves causes considerable increases in platelet and RBC counts in the murine model without causing any acute/subacute toxicity. Therefore, we suggest that C. papaya leaf extract may be used as a medicine to boost haemopoiesis and thrombopoiesis when these have been suppressed by disease. However, this is a preliminary study and more work is needed to isolate and to identify the biologically active ingredients of C. papaya leaves that are responsible for these effects.
Acknowledgments
This research was financially supported by the National Research Council of Sri Lanka (Research Grant No. 09-05).
Comments
Background
This study is to evaluate the effect of hematology, biochemical and toxicology changes in mice after administration of C. papaya leaf extract. C. papaya is one of the medicinal plants have been used worldwide as a remedy, food, cosmetic and widely cultivated around the world. People in rural area have used papaya leaf as alternative to treat dengue and dengue hemorrhagic fever. They believe this plant's leaves can increase the platelet level of dengue patient shortly after receiving juice, boiled or raw of the leaves.
Research frontiers
Studies are being performed to evaluate the hematology, biochemical and toxicity of fresh C. papaya leaf extract in mice.
Related reports
In these studies acute, subacute and subchronic toxicity studies showed that the C. papaya leaf juice given to the Sprague Dawley rats didn't showed any toxicity effect (Halim et al., 2011; Afzan et al., 2012). The clinical trial using juice and methanol extract of C. papaya showed the extract elevated the platelet level and maintained the hematocrit stability in dengue patient during treatment (Yunita et al., 2012; Subenthiran et al., 2013). Other studies reported that the extract of C. papaya leaves administered to thrombocytopenic rat model at doses of 400 mg/kg and 800 mg/kg for 15 d showed significant alteration in platelet count, which was considered effective in the treatment of dengue (Patil et al., 2013).
Innovations and breakthroughs
This study has shown that mice received C. papaya leaf extract showed significant increase in RBC and platelet at a dose of 2 g/kg.
Applications
It will be significant to know, what kind of compound have made the elevation of platelet and RBC level in treated mice, since anti-platelet antibodies generate after dengue virus infection cause destruction of platelets.
Peer review
This is a interesting study of the efferct of C. papaya leaves extract on the haematological and biochemical parameters in a murine model. The findings are impressive and useful for continuous study related to C. papaya. C. papaya leaf could be an alternative to treat dengue and malaria fever instead of prescription drugs.
Footnotes
Foundation Project: Supported by the National Research Council of Sri Lanka (Research Grant No. 09-05).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ong H, Chua S, Milow P. Ethno-medicinal plants used by the Temuan villagers in Kampung Jeram Kedah, Negeri Sembilan, Malaysia. Ethno Med. 2011;5:95–100. [Google Scholar]
- 2.Jiao Z, Deng J, Li G, Zhang Z, Cai Z. Study on the compositional differences between transgenic and non-transgenic papaya (Carica papaya L.) J Food Comp Anal. 2010;23:640–647. [Google Scholar]
- 3.Thomás GE, Rodolfo HG, Juan MD, Georgina SF, Luis CG, Ingrid RB, et al. et al. Proteolytic activity in enzymatic extracts from Carica papaya L. cv. Maradol harvest by-products. Process Biochem. 2009;44:77–82. [Google Scholar]
- 4.Rivera-Pastrana DM, Yahia EM, González-Aquilar GA. Phenolic and carotenoid profiles of papaya fruit (Carica papaya L.) and their contents under low temperature storage. J Sci Food Agric. 2010;90:2358–2365. doi: 10.1002/jsfa.4092. [DOI] [PubMed] [Google Scholar]
- 5.Gill LS. Ethnomedical uses of plants in Nigeria. Benin, Nigeria: Uniben Press; 1992. [Google Scholar]
- 6.Valerio LG, Jr, Gonzales GF. Toxicological aspects of the South American herbs cat's claw (Uncaria tomentosa) and maca (Lepidium meyenii): a critical synopsis. Toxicol Rev. 2005;24:11–35. doi: 10.2165/00139709-200524010-00002. [DOI] [PubMed] [Google Scholar]
- 7.Oduola T, Adeniyi FA, Ogunyemi EO, Bello IS, Idowu TO, Subair HG. Toxicity studies on an unripe Carica papaya aqueous extract: biochemical and haematological effects in Wistar albino rats. J Med Plants Res. 2007;1:1–4. [Google Scholar]
- 8.Lohiya NK, Manivannan B, Garg S. Toxicological investigations on the methanol sub-fractionof the seeds of Carica papaya as a male contraceptive in albino rats. Reprod Toxicol. 2006;22:461–468. doi: 10.1016/j.reprotox.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Oduola T, Bello I, Idowu T, Avwioro G, Adeosun G, Olatubosun LH. Histopathological changes in Wistar albino rats exposed to aqueous extract of unripe Carica papaya. N Am J Med Sci. 2010;2:234–237. doi: 10.4297/najms.2010.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol. 2010;127:760–767. doi: 10.1016/j.jep.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Teschke T, Genthner A, Wolff A. Kava hepatotoxicity: comparison of aqueous, ethanolic, acetonic kava extracts and kava-herbs mixtures. J Ethnopharmacol. 2009;123:378–384. doi: 10.1016/j.jep.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 12.Oduola T, Adeniyi FA, Ogunyemi EO, Bello IS, Idowu TO. Antisickling agent in an extract of unripe pawpaw (Carica papaya): is it real? Afr J Biotechnol. 2006;5:1947–1949. [Google Scholar]
- 13.Indran M, Mahmood AA, Kuppusamy UR. Protective effect of Carica papaya L leaf extract against alcohol induced acute gastric damage and blood oxidative stress in rats. West Indian Med J. 2008;57:323. [PubMed] [Google Scholar]
- 14.Imaga NO, Gbenle GO, Okochi VI, Akanbi SO, Edeoghon SO, Oigbochie V, et al. et al. Antisickling property of Carica papaya leaf extract. Afr J Biochem Res. 2009;3:102–106. [Google Scholar]
- 15.Zakaria ZA, Jais AM, Sulaiman MR, Mohamed Isa SS, Riffin S. The in vitro antibacterial of methanol and ethanol extracts of Carica papaya flowers and Mangifera indica leaves. Pharmacol Toxicol. 2006;3:278–283. [Google Scholar]
- 16.Das RP. Effect of papaya seed on the genital organ and fertility of male rats. Indian J Exp Biol. 1980;18:408–409. [PubMed] [Google Scholar]
- 17.Udoh P, Essien I, Udoh F. Effects of Carica papaya (paw paw) seeds extract on the morphology of pituitary-gonadal axis of male Wistar rats. Phytother Res. 2005;19:1065–1068. doi: 10.1002/ptr.1388. [DOI] [PubMed] [Google Scholar]
- 18.Vyas DK, Jacob D. Effect of papaya (C. papaya) seeds on the reproductive structures and fertility of the male rabbit. Indian Zool. 1984;8:105–108. [Google Scholar]
- 19.Udoh FV, Udoh PB, Umoh EE. Activity of alkaloid extract of Carica papaya seeds on reproductive functions in male Wistar rats. Pharm Biol. 2005;43:563–567. doi: 10.1080/13880200590951810. [DOI] [PubMed] [Google Scholar]
- 20.Epidemiology Unit. Epidemiological Bulletin. Sri Lanka: Ministry of Health; 2011. [Google Scholar]
- 21.Owoyele BV, Adebukola OM, Funmilayo AA, Soladoye AO. Anti-inflammatory activities of ethanolic extract of Carica papaya leaves. Inflammopharmacology. 2008;16:168–173. doi: 10.1007/s10787-008-7008-0. [DOI] [PubMed] [Google Scholar]
- 22.Harlim SZ, Abdullah NR. Study of acute toxicity of Carica papaya leaf extract in Sprague Dawley rats. Med Plants Res. 2011;5:1867–1872. [Google Scholar]
- 23.Canini A, Alesiani D, D'Arcangelo G, Tagliatesta P. Gas chromatography-mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J Food Comp Anal. 2007;20:584–590. [Google Scholar]
- 24.Awe IS, Sodipo OA. Purification of saponins of root of Bhlighia sapida Koenig-Holl. Nig J Biochem Mol Biol. 2001;16:201–204. [Google Scholar]
- 25.Ahmad N, Fazal H, Ayaz M, Abbasi BH, Mohamma I, Fazal L. Dengue fever treatment with Carica papaya leaves extracts. Asian Pac J Trop Biomed. 2011;1:330–333. doi: 10.1016/S2221-1691(11)60055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adlin A, Noor RA, Siti ZH. Repeated dose 28-days oral toxicity study of Carica papaya. leaf extract in Sprague Dawley rats. Molecules. 2012;17:4326–4342. doi: 10.3390/molecules17044326. [DOI] [PMC free article] [PubMed] [Google Scholar]