Abstract
Objective
To investigate the synergic antibacterial activity of garlic and tazma honey against standard and clinical pathogenic bacteria.
Methods
Antimicrobial activity of tazma honey, garlic and mixture of them against pathogenic bacteria were determined. Chloramphenicol and water were used as positive and negative controls, respectively. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration of antimicrobial samples were determined using standard methods.
Results
Inhibition zone of mixture of garlic and tazma honey against all tested pathogens was significantly (P≤0.05) greater than garlic and tazma honey alone. The diameter zone of inhibition ranged from (18±1) to (35±1) mm for mixture of garlic and tazma honey, (12±1) to (20±1) mm for tazma honey and (14±1) to (22±1) mm for garlic as compared with (10±1) to (30±1) mm for chloramphenicol. The combination of garlic and tazma honey (30-35 mm) was more significantly (P≤0.05) effective against Salmonella (NCTC 8385), Staphylococcus aureus (ATCC 25923), Lyesria moncytogenes (ATCC 19116) and Streptococcus pneumonia (ATCC 63). Results also showed considerable antimicrobial activity of garlic and tazma honey. MIC of mixture of garlic and tazma honey at 6.25% against total test bacteria was 88.9%. MIC of mixture of garlic and tazma honey at 6.25% against Gram positive and negative were 100% and 83.33%, respectively. The bactericidal activities of garlic, tazma honey, and mixture of garlic and tazma honey against all pathogenic bacteria at 6.25% concentration were 66.6%, 55.6% and 55.6%, respectively.
Conclusions
This finding strongly supports the claim of the local community to use the combination of tazma honey and garlic for the treatment of different pathogenic bacterial infections. Therefore, garlic in combination with tazma honey can serve as an alternative natural antimicrobial drug for the treatment of pathogenic bacterial infections. Further in vivo study is recommended to come up with a comprehensive conclusion.
Keywords: Garlic, Honey, Antimicrobial activity, Bactericidal activity, Inhibition zone, Minimum inhibitory concentration, Minimum bactericidal concentration
1. Introduction
The use of traditional medicine to treat infection has been practiced since the origin of mankind and honey produced by honeybees (Apis mellifera) is one of the oldest traditional medicines considered as traditional remedy for microbial infections[1],[2]. Antimicrobial action of honey depends on the presence of hydrogen peroxide which is produced enzymatically in honey[3]. The glucose oxidase is secreted from the hypopharyngeal gland of the bee into the nectar to assist in the formation of honey from the nectar. The hydrogen peroxide and acidity produced by two reactions (glucose and oxygen chemically react to produce gluconic acid) has antimicrobial potential[3]. Another antimicrobial activity of honey also depends on osmolarity effect which inhibits bacteria. The high sugar concentration of honey ties-up water molecules, so that bacteria would have insufficient water to support their growth[4]. The antimicrobial activity of honey is also related to its geographical region and flower from which the final product is derived[5],[6]. As a result, most of antimicrobial studies against pathogenic bacteria were made on honey produced by the honey bee[7],[8]. However, the scientific work on the honey produced by Apis mellipodae (A. mellipodae, stingless bee) is limited[9],[10].
In Ethiopia, honey produced by A. mellipodae, is considered to be important in traditional treatment of respiratory ailments, surface infections, diarrhea and various other diseases in line with treatments using honey[4]. Ashenafi has evaluated the antibacterial effect of A. mellipodae honey on Salmonella typhimurium, Salmonella enteritidis, Escherichia coli (E. coli), Bacillus cereus and Staphylococcus aureus (S. aureus) at concentration of 10%, 15%, and 20% in brain heart infusion broth[4]. According to this study, retarded growth and inhibition were noted at concentration of 15%, and 20%. The A. mellipodae honey, according to Ahenafi, may be effective to treat food born infections at low concentration[4].
Garlic (Allium sativum) has a long history as a treatment for cold, cough and asthma, and is reported to strengthen the immune system[11]. Moreover, garlic is widely used to treat alzheimer's disease, cancer, cardiovascular disease including atherosclerosis, strokes, hypertension, thrombosis and hyperlipidemias, infections, enhance children's conditions and dermatologic applications, as well as reduce stress[12]–[14]. Generally, garlic has long been known to have antibacterial, antifungal, anticancer and antiviral properties[13]–[17]. The main antimicrobial constituent of garlic has been identified as the oxygenated sulfur compound, thio-2-propene-1-sulfinic acid S-allyl ester, which is referred to as allicin[18].
According to Omoya and Akharaiyi[19],[20], the mixture of honey and ginger showed high antimicrobial activity against pathogenic Gram positive and negative bacteria. But still there is not any scientific report about the synergic effect of honey and garlic extract. In Ethiopia, people use mixture of A. mellipodae honey and garlic to treat different types of diseases such as cold, cough, asthma, diarrhoea and respiratory infections. People use A. mellipodae honey and garlic in various combinations, there is not any scientific report about the synergic effect of these substances. Therefore, it is necessary to investigate synergic antimicrobial effect of A. mellipodae honey and garlic mixture. The aim of the present investigation is to study the synergic antibacterial effect of A. mellipodae honey and garlic mixture against standard and clinical pathogenic bacteria. The outcome of this investigation may be used as baseline to formulate antimicrobial substance from the combination of honey and garlic extract.
2. Materials and methods
2.1. Preparation of garlic extract
Matured fresh garlic bulbs were purchased from Gondar town market. The cleaned bulbs of garlic were peeled, weighed (55 g) and homogenized aseptically using a sterile mortar and pestle. The homogenized bulbs were squeezed using sterile cheese cloth. Out of 100 g garlic bulb, 34.5 g juice which was considered as 100% in concentration was recovered[21]. From the garlic 50%, 25%, 12.5% and 6.25% (v/v) of extracts were prepared by diluting the concentrated juice with appropriate volumes of sterile distilled water for determination of antimicrobial activity, minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC). A. mellipodae honey was also diluted using the same procedure.
2.2. Source and dilution of honey
The A. mellipodae honey used in this study was obtained from South Gondar, Ethiopia. The A. mellipodae honey sample was first filtered with a sterile mesh to remove debris. Pure (100.0%) honey was referred to as ‘neat’. It was then diluted with sterile distilled water to different concentrations: 12.5%, 25.0% and 50.0% (v/v).
2.3. Test pathogenic bacteria
All standard bacterial cultures, namely, E. coli (ATCC 25922), Salmonella typhi (clinical isolate) (S. typhi), Listeria moncytogenes (ATCC 19116) (L. moncytogenes), S. aureus (ATCC 25923), Shigella flexneri (ATCC 12022) (S. flexneri), P. vulgaris (ATCC 881) (P. vulgaris), Salmonella NCTC 8385 and S. pneumonia (ATCC 63) were obtained from Ethiopia Health and Nutrition Research Institute, while Shigella dysenterae (S. dysenterae) (clinical isolate) was obtained from University of Gondar Medical and Health Sciences Hospital. Most of them are commonly involved in causing gastroenteritis, pneumonia, wound and urinary tract infections.
2.4. Determination of antibacterial activity of honey and garlic extracts
McFarland standard No. 0.5 was prepared according to Andrews[22]. Standard and clinical isolates of pathogenic bacteria were inoculated and spread over on Muller-Hinton (MH) agar and incubated for 24 h. 2-3 colonies were peaked up by wire loop aseptically into sterile saline solution and the turbidity was adjusted to the 0.5 McFarland's standard solution (a concentration of 1.5×108 CFU/mL)[22]. Each MH agar plate was uniformly seeded by means of sterile swab dipped into the suspension and streaked on the agar plate surface, and the plates was left on the bench for excess fluid being absorbed. Using a sterile cork borer (6 mm diameter, 4 mm deep and about 2 cm apart) wells were made in the agar medium. Using a micropipette 100 µL of sample with the concentration of 50% was added into the wells in the plate. The plates were incubated at 37 °C for 24 h. The mean diameters of inhibition zones were measured in mm and the results were recorded. The inhibition zones with diameter less than 12 mm were considered as having no antibacterial activity. A positive control well was equally filled with chloramphenicol (32 µg/mL) while sterile, distilled water served as negative control.
2.5. Antibiotic sensitivity testing
The test microorganisms were also tested for their antimicrobial activity against the commonly prescribed antibiotics using similar methods for the determination of antimicrobial activity of honey and garlic extracts.
2.6. Determination of MIC
The MIC of the extracts was determined according to methods described by Shahidi and Akinyemi et al[23],[24]. Extracts were diluted to concentrations ranging from 6.25% to 25% for garlic, honey and a mixture of garlic with honey (1:1, v/v). To each dilution of honey, garlic extract and a mixture of both, in nutrient broth tubes were seeded 100 µL of the standard and clinical bacterial inoculum. Negative control tubes with no bacterial inoculation were simultaneously maintained. Tubes were incubated aerobically at 37 °C for 24 h. The MIC was defined as the lowest concentration of garlic extract that completely inhibits the growth of the organism.
2.7. Determination of MBC
Dilutions showing no visible growth for the MIC was subcultured onto a fresh MH agar plate and incubated at 37 °C for 24 h. The lowest concentration of the extracts yielding no growth on the MH plate was recorded as MBC[20].
2.8. Data analysis
All data were analyzed using SPSS version 16.0. Means and standard devaviations of the triplicate analysis were calculated using analysis of variance (ANOVA) to determine the significant differences in the means, followed by Duncan's Multiple range test (P<0.05) when the F-test demonstrated significance. The statistically significant difference was defined as P<0.05.
3. Results
Inhibition zone of mixture of garlic extract and A. mellipodae honey, garlic extract and A. mellipodae honey alone against standard and clinical pathogenic bacteria is presented in Table 1. Inhibition zone of mixture of garlic extract and A. mellipodae honey against all tested pathogens was significantly (P≤0.05) greater than that of garlic extract and A. mellipodae honey alone. Inhibition zone of A. mellipodae honey against all pathogenic bacteria was statistically (P≤0.05) greater than inhibition zone of garlic extract but less than that of garlic extract and tenegn honey mixture. Inhibition zone of chloramphenicol against all tested pathogenic bacteria was greater than that of garlic extract and tenegn honey mixture.
Table 1. Comparison of inhibition zone among mixture of garlic extract and A. mellipodae honey, garlic extract and A. mellipodae honey (50%) against standard and clinical isolated pathogenic bacteria.
| Source of antimicrobial agents | Sum of squares | Df | Mean | F square | Sig. |
| Mixture of garlic extract and A. mellipodae honey (equal volume) (50%) | 696.000 | 8 | 87.000 | 87.000 | 0.00 |
| Garlic extract (50%) | 154.741 | 8 | 19.343 | 20.890 | 0.00 |
| Tazma honey (50) | 204.000 | 8 | 25.500 | 25.500 | 0.00 |
| Chloramphenicol (31 µg/mL) | 1082.741 | 8 | 135.343 | 146.170 | 0.00 |
Df: Degree of freedom; Sig.: Significance.
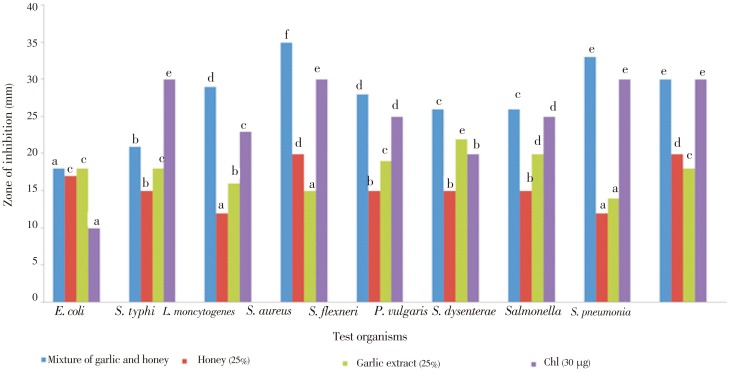
The inhibition zones of A. mellipodae honey and garlic extract mixture, garlic extract and A. mellipodae honey alone against standard and clinical pathogenic bacteria are presented in Figure 1. The inhibition zone of mixture of A. mellipodae and garlic extract (30-35 mm) against Salmonella NCTC 8385, S. aureus (ATCC 25923) and S. pneumonia (ATCC 63) were significantly (P≤0.05) greater than the rest test pathogenic bacteria. The diameter of inhibition zone ranged from (18±1) mm to (35±1) mm for mixture of garlic extract and A. mellipodae honey, (12 ±1) mm to (20±1) mm for A. mellipodae honey and (14±1) mm to 22±1) mm for garlic extract as compared with (10±1) mm to (30±1) mm for chloramphenicol at the various concentration used. The inhibition zone of mixture of tazema honey and garlic extract against E. coli (ATCC 25922) was statistically (P≤0.05) less than the other tested pathogens. The inhibition zone of mixture of tazema honey and garlic extract against S. flexneri (ATCC 12022), L. moncytogenes (ATCC 19116), S. pneumonia (ATCC 63) were significantly (P≤0.05) greater than S. dysenterae (clinical isolate), P. vulgaris (ATCC 881), E. coli (ATCC 25922), S. typhi (clinical isolate) and less than the rest pathogenic bacteria.
Figure 1. Antibacterial activity of different concentrations of garlic, hone and mixture of garlic and honey (v/v) by agar well diffusion method.
Chl: Chloramphenicol. Values are means of triplicate determinations. Values of the same colour bars followed by different letters are significantly different (P<0.05).
The inhibition zone of A. mellipodae honey (17-20 mm) against S. aureus (ATCC 25923), S. pneumonia (ATCC 63) and E. coli (ATCC 25922) were significantly (P≤0.05) greater than the rest pathogenic bacteria. The inhibition zone of tazema honey to S. typhi (clinical isolate), S. flexneri (ATCC 12022) and P. vulgaris (ATCC 881) were greater (P≤0.05) than L. moncytogenes (ATCC 19116) and Salmonella NCTC 8385.
The inhibition zone of garlic extract (20-22 mm) against P. vulgaris (ATCC 881) and S. dysenterae (clinical isolate) were statistically (P≤0.05) greater than the rest tested pathogenic bacteria, while the inhibition zone of garlic extract against Salmonella NCTC 8385 and S. aureus (ATCC 25923) were significantly (P≤0.05) less than the rest pathogenic bacteria. The inhibition zone of chloramphenicol to S. flexneri (ATCC 12022), S. dysenterae (clinical isolate), S. typhi (clinical isolate), Salmonella NCTC 8385, S. pneumonia (ATCC 63) and S. aureus (ATCC 25923) were statistically greater than the rest pathogenic bacteria. Results of this study also showed a considerable antimicrobial activity of garlic and A. mellipodae honey.
The diameter of the inhibition zones against commonly prescribed antibiotics are shown in Table 2. The mean inhibition zone of mixture of garlic extract and A. mellipodae honey (27.33 mm) was greater than the mean inhibition zone of commercially used antibiotics (24.03 mm) against tested pathogens. Those commercial antibiotics are widely used to treat the infections caused by both gram negative and gram positive bacteria. The mixture of garlic extract and A. mellipodae honey may serve as a new choice of antibacterial agents against commonly encountered pathogens.
Table 2. Antibiogram of commonly used commercial antibiotics against the test bacteria.
| Test bacteria | Antibiotics (µg) | Maximum zone of inhibition (mm) |
| E. coli (ATCC 25922) | C30 | 10 |
| SXT | 16 | |
| Na | 22 | |
| S. pneumonia (ATCC 63) | C30 | 30 |
| SXT | 30 | |
| Na | 24 | |
| L. moncytogenes (ATCC 19116) | C30 | 23 |
| SXT | 24 | |
| Na | 28 | |
| S. flexneri (ATCC 12022) | C30 | 25 |
| SXT | 27 | |
| Na | 28 | |
| Salmonella NCTC 8385 | C30 | 30 |
| CN10 | 30 | |
| E15 | 10 | |
| SXT | 18 | |
| Na | 26 | |
| S. aureus (ATCC 25923) | C30 | 32 |
| CN10 | 30 | |
| E15 | 30 | |
| SXT | 20 | |
| Na | 22 | |
| S. dysenterae (clinical isolate) | C30 | 25 |
| CN10 | 30 | |
| SXT | 10 | |
| Na | 20 | |
| E15 | 15 | |
| P. vulgaris (ATCC 881) | C30 | 20 |
| CN10 | 28 | |
| E15 | 30 | |
| S. dysenterae (clinical isolate) | C30 | 24 |
| CN10 | 24 | |
| E15 | 32 | |
| SXT | 25 | |
| Na | 23 | |
| The mean inhibition zone | Total antibiotics | 24.03 |
C: Chloramphenicol, CN: Cephalexin, E: Erythromycin, SXT: Trimethoprim-sulfamethoxaxole, Na: Nalidixic acid.
MIC of different concentration of garlic, A. mellipodae and mixture of garlic and A. mellipodae honey (v/v) are shown in Table 3. MIC of mixture of garlic extract and A. mellipodae honey at 6.25% against total test bacteria was 88.9%. MIC of mixture of garlic extract and A. mellipodae honey at 6.25% against Gram positive and negative were 100% and 83.33%, respectively. MIC of mixture of garlic extract and A. mellipodae honey against L. moncytogenes (ATCC 19116) was 12.50%, but in the rest pathogenic bacteria, the MIC was reduced by half (6.25%). On the other hand, MIC of A. mellipodae honey against E. coli (ATCC 25922), Salmonella NCTC 8385, L. moncytogenes (ATCC 19116) and S. dysenterae (clinical isolate) were 12.50%, but in the rest tested pathogenic bacteria it was 6.25%. MIC of garlic extract against all pathogenic bacteria was 6.25%.
Table 3. MIC determination of different concentrations of garlic, hone and mixture of garlic and honey.
| Test organisms | MIC (% of dilution) |
||
| Mixture of honey and garlic | Honey | Garlic extract | |
| E. coli (ATCC 25922) | 6.25 | 12.50 | 6.25 |
| Salmonella NCTC 8385 | 6.25 | 12.50 | 6.25 |
| L. moncytogenes (ATCC 19116) | 12.50 | 12.50 | 12.50 |
| S. aureus (ATCC 25923) | 6.25 | 6.25 | 6.25 |
| S. flexneri (ATCC 12022) | 6.25 | 6.25 | 6.25 |
| P. vulgaris (ATCC 881) | 6.25 | 6.25 | 6.25 |
| S. dysenterae (clinical isolate) | 6.25 | 12.50 | 6.25 |
| S. typhi (clinical isolate) | 6.25 | 6.25 | 6.25 |
| S. pneumonia (ATCC 63) | 6.25 | 6.25 | 6.25 |
| Total test bacteria at 6.25% concentration | 88.90 | 55.60 | 88.90 |
| Total test bacteria at 12.5% concentration | 11.10 | 44.40 | 11.10 |
| Total Gram positive bacteria at 6.25% concentration | 100.00 | 100.00 | 100.00 |
| Total Gram negative bacteria at 6.25% concentration | 83.33 | 50.00 | 83.33 |
MBC of different concentrations of garlic, A. mellipodae and mixture of garlic and A. mellipodae honey (v/v) was presented in Table 4. The bactericidal activity of garlic extract, A. mellipodae honey, and mixture of garlic extract and A. mellipodae honey against all tested pathogenic bacteria at 6.25% concentration were 66.6%, 55.6% and 55.6%, respectively. MBC of mixture of garlic extract and A. mellipodae honey against E. coli (ATCC 25922), Salmonella NCTC 8385, L. moncytogenes (ATCC 19116) and S. aureus (ATCC 25923) was 12.50%, while against the rest pathogenic bacteria it was 6.25%. On the other hand, MBC of A. mellipodae honey against E. coli (ATCC 25922), L. moncytogenes (ATCC 19116), P. vulgaris (ATCC 881) and S. dysenterae (clinical isolate) was 12.50%, while against the rest pathogenic tested bacteria, it was 6.25%. MBC of garlic extract against Salmonella NCTC 8385, L. moncytogenes (ATCC 19116) and S. aureus (ATCC 25923) was 12.5% but against the rest pathogenic bacteria was 6.25%. MBC of garlic extract, A. mellipodae honey and combination of garlic extract and A. mellipodae honey against L. moncytogenes (ATCC 19116) was 12.50%. Therefore, all those antimicrobial agents under this investigation were relatively not efficient to kill such tested pathogenic bacteria in low concentration.
Table 4. MBC determination of different concentrations of garlic, honey and mixture of garlic and honey.
| Test organisms | MBC (% of dilution) |
||
| Mixture of honey and garlic | Honey | Garlic extract | |
| E. coli (ATCC 25922) | 12.50 | 12.50 | 6.25 |
| Salmonella NCTC 8385 | 12.50 | 6.25 | 12.50 |
| L. moncytogenes (ATCC 19116) | 12.50 | 12.50 | 12.50 |
| S. aureus (ATCC 25923) | 12.50 | 6.25 | 12.50 |
| S. flexneri (ATCC 12022) | 6.25 | 6.25 | 6.25 |
| P. vulgaris (ATCC 881) | 6.25 | 12.50 | 6.25 |
| S. dysenterae (clinical isolate) | 6.25 | 12.50 | 6.25 |
| S. typhi (clinical isolate) | 6.25 | 6.25 | 6.25 |
| S. pneumonia (ATCC 63) | 6.25 | 6.25 | 6.25 |
| Total test bacteria at 6.25% concentration | 55.60 | 55.60 | 66.60 |
| Total test bacteria at 12.5% concentration | 44.40 | 44.40 | 33.33 |
4. Discussion
The synergic antimicrobial effect of garlic extract and A. mellipodae honey mixture against all pathogenic bacteria was found to be greater (P≤0.05) than other antimicrobial agents (garlic extract and A. mellipodae honey alone). That may be the reason why the local society or community widely use the mixture of garlic and A. mellipodae honey to treat different pathogenic bacterial infections. The traditional knowledge about the treatment of infectious disease using natural products or substances should be investigated using scientific methods. The mean inhibition zone of mixture of garlic extract and A. mellipodae honey (27.33 mm) was greater than the mean inhibition zone (24.03 mm) of commercially used antibiotics against tested pathogens. It may be the basic reason why the local society or community widely preferred to use the mixture of garlic and A. mellipodae honey to treat different pathogenic bacterial infections. Therefore, mixture of garlic extract and A. mellipodae honey may be effective to treat both gram positive and negative bacterial infections at low concentrations. According to this study, mixture of garlic extract and A. mellipodae honey has larger inhibition zone against tested pathogenic bacteria than garlic extract and A. mellipodae honey. Although mixture of garlic extract and A. mellipodae honey has high antimicrobial activity, garlic extract and A. mellipodae honey alone also have good potential of a broad spectrum of activity against both Gram-positive and Gram-negative bacteria.
In this study, aqueous extract of garlic inhibited the growth of enteric pathogens such as E. coli, S. typhi, S. flexineri and other pathogens at low concentration which is in line with the report of Shobana et al. and Sadeghian and Ghazvini[25]–[27]. All tested pathogens were also sensitive to A. mellipodae honey. However, the mixture of garlic extract and A. mellipodae honey was more effective against test pathogens than garlic extract and A. mellipodae honey separately. Garlic in combination with A. mellipodae honey can serve as alternative natural antimicrobial drug for the treatment of pathogenic bacterial infections.
As it is clearly presented and shown in the data for determination of MIC and MBC, synergic antimicrobial action of garlic and A. mellipodae honey mixture may attribute to allicin in garlic, and osmolarity, acidity, hydrogen peroxide generation and phytochemical components considered in honey, as reported by Molan[28],[29]. Allicin is identified as the oxygenated sulfur compound, thio-2-propene-1-sulfinic acid S-allyl ester, which is generally referred to as allicin[18]. The main antimicrobial effect of allicin is due to its chemical reaction with thiol groups of various enzymes, e.g. alcohol dehydrogenase, thioredoxin reductase, and RNA polymerase, which can affect essential metabolism of cysteine proteinase activity involved in the virulence of E. histolytica[30]. In brief, allicin interferes with RNA production and lipid synthesis. If RNA cannot be produced, or produced in less amount then protein synthesis will be severely affected. In short, no more protein synthesis takes place without the action of mRNA, rRNA and tRNA. Allicin also affects lipid synthesis and as a result phospholipid biolayer of the cell wall cannot be formed correctly in both gram positive and gram negative bacteria. All these contribute a lot to impact on the growth of bacteria with the presence of allicin or AGE[21].
The antimicrobial role of honey is attributed to its high osmolarity, acidity (low pH) and content of hydrogen peroxide (H2O2) and non-peroxide components, i.e., the presence of phytochemical components like methylglyoxal[31]–[33]. The antimicrobial agents in honey are predominantly hydrogen peroxide, of which the concentration is determined by relative levels of glucose oxidase, synthesized by the bee and catalase originating from flower pollen[32]. The hydrogen peroxide and acidity produced by the reaction of glucose and oxygen (glucose + O2→gluconic acid + H2O2) in honey may contribute a lot to inhibitory and bactericidal activity against pathogenic bacteria.
The cumulative effect of allicin in garlic and high osmolarity, acidity (low pH), content of hydrogen peroxide (H2O2) as well as the presence of phytochemical components like methylglyoxa in honey may play a great role in the inhibitory and bactericidal activity against pathogenic bacteria. This finding strongly supports the claim of the local community to use the combination of A. mellipodae honey and garlic for the treatment of different pathogenic bacterial infections. This paves a way to consider and acknowledge the traditional knowledge for the treatment of infectious diseases using natural resources like honey and garlic.
Acknowledgments
I am thankful to University of Gondar for financial assistant (UOG/Budget/no.6215) for the successful completion of research work. I also would like to express my appreciation to the Department of Biotechnology for the facilities provided during this study. I would like to thank Muluken Dejen for her contribution to purchase A. mellipodae honey from South Gondar.
Comments
Background
In Ethiopia, people use A. mellipodae honey and garlic mixture to treat different types of diseases such as cold, cough, asthma, diarrhoea and respiratory infections. But still there is no any scientific report about the synergic effect of any type of honey and garlic extract. People use A. mellipodae honey and garlic in various combinations, there is no any scientific report about the synergic effect of these substances. Therefore, there is a need to investigate synergic antimicrobial effect of A. mellipodae honey and garlic mixture.
Research frontiers
This finding strongly supports the claim of the local community to use the combination of A. mellipodae honey and garlic for the treatment of different pathogenic bacterial infections. So, garlic in combination with A. mellipodae honey can serve as alternative natural antimicrobial drug for the treatment of pathogenic bacterial infections. Further in vivo study is recommended to come up with a comprehensive conclusion.
Related reports
There are different reports on the separate issues of antimicrobial effects on honey and garlic. However, a report on the synergistic effect of honey and garlic is scarce. This finding fills this research gap and may help base information for further in vivo research.
Innovations and breakthroughs
The finding of the study paves a way to consider and acknowledge the traditional knowledge for the treatment of infectious diseases using natural resources like honey and garlic.
Applications
Garlic in combination with A. mellipodae honey can be used as antimicrobial agent to different pathogenic bacteria. As recommended by the author it needs further validation and then it would be important for the community as it is routinely used as food.
Peer review
This is a very good finding in which the author investigated the synergistic antimicrobial activity of mixture of garlic extract and A. mellipodae honey against pathogenic bacteria. The results are interesting that garlic in combination with A. mellipodae honey can serve as alternative natural antimicrobial drug for the treatment of pathogenic bacterial infections.
Footnotes
Foundation Project: Supported by University of Gondar (UOG/Budget/no.6215), Gondar, Ethiopia.
Conflict of interest statement: I declare that I have no conflict of interest.
References
- 1.Mandal MD, Mandal S. Honey: its medicinal property and antibacterial activity. Asian Pac J Trop Biomed. 2011;1(2):154–160. doi: 10.1016/S2221-1691(11)60016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Namita P, Mukesh R. Medicinal plants used as antimicrobial agents: A review. Int Res J Pharm. 2012;3(1):31–40. [Google Scholar]
- 3.Patel RV, Thaker VT, Patel VK, Shukla P, Bhatnagar P, Patel A. In vitro study of changing antibiotic sensitivity and resistance by honey on gingival inflammation during orthodontic treatment-a preliminary report. Orthodontic Cyber J. 2010 [Online] Available from: http://orthocj.com/2010/06/in-vitro-study-antibiotic-sensitivity-resistance-honey/. [Assessed on 2 April, 2013]. [Google Scholar]
- 4.Efem SEE, Udoh KT, Iwara CI. The antimicrobial spectrum of honey and its clinical significance. Infect. 1992;20(4):227–229. doi: 10.1007/BF02033065. [DOI] [PubMed] [Google Scholar]
- 5.Taghizadeh M, Saffari M, Pourbabaei M, Mahboubi M. Antimicrobial activity of different honey samples against Pseudomonas aeruginosa in vitro. Biharean Biol. 2011;5(2):113–115. [Google Scholar]
- 6.Omoya FO, Akharaiyi FC. A pasture honey trial for antibacterial potency on some selected pathogenic bacteria. J Nat Prod. 2010;3:5–11. [Google Scholar]
- 7.Sherlock O, Dolan A, Athman R, Power A, Gethin G, Cowman S, et al. et al. Comparison of the antimicrobial activity of Ulmo honey from Chile and Manuka honey against methicillin-resistant Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. BMC Complement Altern Med. 2010;10:47. doi: 10.1186/1472-6882-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chute RK, Deogade NG, Kawale M. Antimicrobial activity of Indian honey against clinical isolates. Asiatic J Biotech Res. 2010;1:35–38. [Google Scholar]
- 9.Ashenafi M. The in vitro antibacterial activity of “Tazma Mare” honey produced by stingless bee (Apis mellipodae) Ethiop J Health Dev. 1994;8(2):109–117. [Google Scholar]
- 10.Temaru E, Shimura S, Amano K, Karasama T. Antimicrobial activity of honey from stingless honeybees (Hymenopetra; Apidae; Meliponinae) Pol J Microbol. 2007;56(4):281–285. [PubMed] [Google Scholar]
- 11.Borek C. Antioxidant health effects of aged garlic extract. J Nutr. 2001;131(3):1010–1015. doi: 10.1093/jn/131.3.1010S. [DOI] [PubMed] [Google Scholar]
- 12.Bongiorno PB, Fratellone PM, LoGiudice P. Potential health benefits of garlic (Allium Sativum): A narrative review. J Complem Integr Med. 2008;5(1):1–24. [Google Scholar]
- 13.Ekweney UN, Elegalan NN. Antibacterial activity of ginger and garlic extracts on Escherichia coli and Salmonella typhi. Int J Mol Med Adv Sci. 2005;1(4):411–416. [Google Scholar]
- 14.Cellini LE, Campli Di, Masulli M, Di Bartolomeo S, Allocati N. Inhibition of Helicobacter pyroli by garlic extract (Allium sativum) FEMS Immunol Med Microbiol. 1996;13(4):273–277. doi: 10.1111/j.1574-695X.1996.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida S, Kasuga S, Hayashi N, Ushiroguchi T, Matsuura H, Nakagawa S. Antifungal activity of ajoene derived from garlic. Appl Environ Microbiol. 1987;53(3):615–617. doi: 10.1128/aem.53.3.615-617.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan XY, Lif Q, Yu RJ, Wang H. [Comparison of the cytotoxic effects of fresh garlic diallyltrisulphide, 5-lurouracil, mitomycin and cis-DDP on two lines of gastric cancer cells] Zhonghua Zhong Liu Za Zhi. 1985;7(2):103–105. Chinese. [PubMed] [Google Scholar]
- 17.Block E. The chemistry of garlic and onions. Sci Am. 1985;252(3):114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 18.Cavallito CJ, Bailey JH. Allicin, the antibacterial principle of Allium sativum. isolation, physical properties and antibacterial acrion. J Am Chem Soc. 1944;66(11):1950–1951. [Google Scholar]
- 19.Omoya FO, Akharaiyi FC. Mixture of honey and ginger extract for antibacterial assessment on some clinical isolates. Int Res J Pharm. 2012;2(5):127–132. [Google Scholar]
- 20.Patel RV, Thaker VT, Patel VK. Antimicrobial activity of ginger and honey on isolates of extracted carious teeth during orthodontic treatment. Asian Pac J Trop Biomed. 2011;1:S58–S61. [Google Scholar]
- 21.Durairaj S, Srinivasan S, Lakshmanaperumalsamy P. In vitro antibacterial activity and stability of garlic extract at different pH and temperature. Electr J Biol. 2009;5(1):5–10. [Google Scholar]
- 22.Andrews JM. BSAC standard disc susceptibility testing method (version 5) J Antimicrob Chemother. 2006;58(3):511–529. doi: 10.1093/jac/dkl277. [DOI] [PubMed] [Google Scholar]
- 23.Shahidi GA. Evaluation of antimicrobial properties of Iranian medicinal plants against Micrococcus luteus, Serratia marceceans, Klebsiella pneumonia and Bordetella branchoseptica. Asian J Plant Sci. 2004;3(1):82–86. [Google Scholar]
- 24.Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA. Screening of crude extracts of six medicinal plants used in Southwest Nigerian orthodox medicine for anti-methicillin resistant Staphylococcus aureus activity. BMC Complement Altern Med. 2005;5:6. doi: 10.1186/1472-6882-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shobana S, Vidhya VG, Ramya M. Antibacterial activity of garlic varieties (ophioscordon and sativum) on enteric pathogens. Curr Res J Biol Sci. 2009;1(3):123–126. [Google Scholar]
- 26.Sadeghian A, Ghazvini K. Antimicrobial activity of garlic extract against Shigella. Iran J Med Sci. 2002;27(3):142–144. [Google Scholar]
- 27.Kadir Batcioglu K, Kargin FO, Satilmis B, Gul M, UyumLu AB, Buyuktuncel E, et al. Comparison of in vivo chemoprotective and in vitro antimicrobial activity of different garlic (Allium sativum) preparations. J Med Plants Res. 2012;6(14):2885–2894. [Google Scholar]
- 28.Molan PC. The antibacterial activity of honey: 1. The nature of the antibacterial activity. Bee World. 1992;73:5–28. [Google Scholar]
- 29.Goncagul G, Ayaz E. Antimicrobial effect of garlic (Allium sativum) and traditional medicine. J Anim Vet Adv. 2010;9(1):1–4. doi: 10.2174/157489110790112536. [DOI] [PubMed] [Google Scholar]
- 30.Ankri S. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;2:125–129. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 31.Weston RJ. The contribution of catalase and other natural products to the antibacterial activity of honey: a review. Food Chem. 2000;71:235–239. [Google Scholar]
- 32.Mavric E, Wittmann S, Barth G, Henle T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of manuka (Leptospermum scoparium) honeys from New Zealand. Mol Nutr Food Res. 2008;52(4):483–489. doi: 10.1002/mnfr.200700282. [DOI] [PubMed] [Google Scholar]
- 33.Mukherjee S, Chaki S, Das S, Sen S, Dutta SK, Dastida SG. Distinct synergistic action of piperacillin and methylyoxal against Pseudomonas aeruginosa. Indian J Exp Biol. 2011;49(7):547–551. [PubMed] [Google Scholar]