Abstract
Purpose
To simplify the recommended staging evaluation by correlating tumor and clinical features with patterns of distant metastasis in newly diagnosed patients with embryonal rhabdomyosarcoma (ERMS) or alveolar rhabdomyosarcoma (ARMS).
Patients and Methods
Patient data from the Intergroup Rhabdomyosarcoma Study Group and the Children's Oncology Group over two periods were analyzed: 1991 to 1997 and 1999 to 2004. We used recursive partitioning analyses to identify factors (including histology, age, regional nodal and distant metastatic status, tumor size, local invasiveness, and primary site) that divided patients into subsets with the most different rates of metastatic disease.
Results
Of the 1,687 patients analyzed, 5.7% had lung metastases, 4.8% had bone involvement, and 6% had bone marrow (BM) involvement. Rhabdomyosarcoma (RMS) without local invasion (T1) had a low rate of metastasis for all distant sites, especially ERMS (0% bone, 0% BM). ARMS with local invasion (T2) had a higher rate of metastasis for all distant sites (13% lung, 18% bone, 23% BM). ERMS, T2 also had a higher rate of metastatic lung involvement (9%). The likelihood of bone or BM involvement increased in the presence of lung metastases (41% with, 6% without). Regional nodal metastases (N1) predicted a high rate of metastasis in all distant sites (14% lung, 14% bone, 18% BM). A staging algorithm was developed.
Conclusion
Staging studies in childhood RMS can be tailored to patients' presenting characteristics. Bone marrow aspirate and biopsy and bone scan are unnecessary in at least one third of patients with RMS.
INTRODUCTION
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children and adolescents and the fourth most common solid tumor in childhood.1 It is the only sarcoma for which behavior, treatment, and prognosis vary widely according to histology, primary site, and age.2–4 This has led to extensive evaluations and complex algorithms for treatment assignment.
In 1972, the Intergroup Rhabdomyosarcoma Study Group (IRSG) opened the first cooperative group study for children with RMS, IRS-I. Since IRS-II, all prospective RMS clinical trials for newly diagnosed patients in the United States have required staging evaluations including computed tomography (CT) of the chest, bone scan, and bone marrow (BM) aspirate and biopsy (usually bilateral). Similar study entry requirements exist within other large, international RMS consortia.5,6 The primary purpose of staging evaluations is to identify the minority (16%) of newly diagnosed patients with distant metastases from the majority with only locoregional disease. This distinction has important implications for treatment assignment and prognosis.
However, all staging procedures carry risk and expense. CT and bone scans expose patients to radiation and often require sedation in the young.7,8 BM aspiration and biopsy require sedation or anesthesia and can be complicated by pain, infection, or bleeding. The time to perform and obtain results from staging studies may delay the initiation of therapy. Moreover, false-positive tests lead to additional studies that impose further risk and expense. Selecting staging studies according to the risk of metastasis in a particular clinical situation could decrease the number of procedures and their associated sequelae while ensuring accurate staging.
We sought to learn whether we could predict the frequency of metastatic involvement in the lung, bone, and BM in newly diagnosed children and adolescents with RMS on the basis of specific initial tumor and clinical characteristics. We then used these results to develop an initial staging algorithm for this patient population.
PATIENTS AND METHODS
Patient Population
We analyzed data from the IRSG and the Children's Oncology Group from two periods: 1991 to 1997 (studies IRS-IV pilot, IRS-IV, IRS-V pilot, D9501, and D9602; n = 1,122) and 1999 to 2004 (studies D9602, D9802, and D9803; n = 797).9–16 These periods were chosen because clinical trials for all risk groups were open simultaneously, allowing ascertainment of all patients regardless of risk category. The treatment protocols were approved by the institutional review boards of participating centers, and informed consent for participation was obtained from the patients, parents, or legal guardians. Data were obtained from institutionally completed case report forms.
To be included in the analysis, patients had to have embryonal RMS (ERMS) or alveolar RMS (ARMS) confirmed by central pathology review and data on the following variables: age (< 1, 1 to 9, 10+ years), primary site (favorable [orbit, nonparameningeal head and neck, genitourinary {GU} non–bladder/prostate {B/P}], unfavorable [extremity, GU B/P, parameningeal, retroperitoneal, other]), tumor size (≤ 5 cm, > 5 cm), local invasion (T1, primary tumor confined to the anatomic site of origin; T2, extension and/or fixation of the primary tumor to surrounding tissue), regional nodal status (N0, no regional nodal disease; N1, regional lymph nodes clinically involved), and metastatic status (metastases present or absent). Staging definitions were determined using the IRS TNM classification for pretreatment clinical assessment of disease, and IRS group was assigned at diagnosis according to the extent of residual tumor after initial surgery.17,18 All studies required CT or magnetic resonance imaging of the primary site, chest CT scan, bone scan, and BM aspirate and biopsy (usually bilateral) for staging evaluation. Sites of metastatic involvement could be determined based on the institution's assessment of imaging characteristics without histologic confirmation.
Statistical Methods
Descriptive statistics (counts and percentages) were performed for all eligible patients. We undertook a recursive partitioning analysis to identify subsets of patients with different rates of metastatic disease. Separate analyses were done for lung, bone, and BM metastases. Each analysis first identified the prognostic factor that divided patients into subsets with the most different rate of the metastatic disease being considered. Specifically, the prognostic factor was the variable that was most significantly associated with the given site of metastatic disease according to a χ2 test of independence in a contingency table. The process of using χ2 statistics to further partition the patients into risk categories continued recursively. The following risk factors were considered: age group, nodal status, tumor size, tumor invasiveness, histology, metastatic site, and primary site of disease. A P value less than .05 was used to determine statistical significance at each partitioning. The decision tree analysis was done using SAS, version 9.2 (SAS Institute, Cary, NC; Fig 1). Based on the recursive partitioning analysis, an initial staging algorithm was developed. A decision to omit a staging evaluation at a particular branch point was made if the likelihood of a positive test was less than 2%.
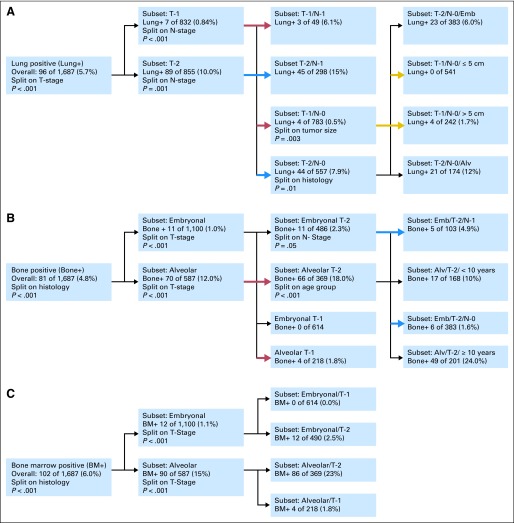
Fig 1.
Recursive partitioning analysis: (A) lung, (B) bone, and (C) bone marrow (BM) metastasis. Emb, embryonal; Alv, alveolar.
RESULTS
Patient and Tumor Characteristics
The initial characteristics of the 1,687 patients eligible for this analysis are listed in Table 1. Overall, 269 patients (16%) had metastases; 96 (5.7%) had lung metastases, 81 (4.8%) had bone involvement, and 102 (6%) had BM involvement.
Table 1.
Initial Presenting Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Total No. | 1,687 | |
| Study | ||
| IRS IV PILOT | 10 | 1 |
| IRS IV | 889 | 52 |
| IRS V PILOT | 37 | 2 |
| IRS D9501 | 19 | 1 |
| IRS D9602 | 249 | 15 |
| IRS D9802 | 99 | 6 |
| IRS D9803 | 384 | 23 |
| Sex | ||
| Male | 1,022 | 61 |
| Female | 665 | 39 |
| Age, years | ||
| < 1 | 60 | 4 |
| 1-9 | 1,086 | 64 |
| 10+ | 541 | 32 |
| Race | ||
| Nonwhite | 488 | 29 |
| White | 1,171 | 71 |
| Site | ||
| Extremity | 254 | 15 |
| GU B/P | 146 | 9 |
| GU non-B/P | 352 | 21 |
| Nonparameningeal head and neck | 122 | 7 |
| Orbit | 155 | 9 |
| Parameningeal | 386 | 23 |
| Retroperitoneal | 136 | 8 |
| Other | 136 | 8 |
| Histology | ||
| Embryonal/botryoid/spindle cell | 1,100 | 65 |
| Alveolar | 587 | 35 |
| Group | ||
| I | 305 | 18 |
| II | 250 | 15 |
| III | 863 | 51 |
| IV | 269 | 16 |
| Stage | ||
| I | 594 | 35 |
| II | 272 | 16 |
| III | 552 | 33 |
| IV | 269 | 16 |
| Tumor invasiveness | ||
| T1 | 832 | 49 |
| T2 | 855 | 51 |
| Size, cm | ||
| ≤ 5 | 820 | 49 |
| > 5 | 867 | 51 |
| Nodes | ||
| N0 | 1,340 | 79 |
| N1 | 347 | 21 |
| Lung metastases | ||
| No | 1,591 | 94 |
| Yes | 96 | 6 |
| Bone metastases | ||
| No | 1,606 | 95 |
| Yes | 81 | 5 |
| Bone marrow metastases | ||
| No | 1,585 | 94 |
| Yes | 102 | 6 |
Abbreviations: B/P, bladder/prostate; GU, genitourinary; IRS, International Rhabdomyosarcoma Study.
Overall Results of Recursive Partitioning Analyses
The most significant predictor of lung involvement resulting from the recursive partitioning analysis was tumor invasiveness (T1 v T2; P = .0001; Fig 1A; Table 2). In patients with T1 disease (n = 832), only 7 (0.8%) had lung metastases. A high rate of lung involvement (n = 89; 10%) was seen in patients with T2 disease (n = 855). As with bone and BM involvement, N1 status (n = 347) was strongly associated with lung involvement (n = 48; 14%).
Table 2.
Rate of Metastasis Based on Histology and T Stage
| Site and Characteristic | Rate of Involvement |
||
|---|---|---|---|
| No. | Total No. | % | |
| Lung | |||
| T1 | 7 | 832 | 0.8 |
| T2, N0, embryonal | 23 | 383 | 6 |
| T2, N0, alveolar | 21 | 174 | 12 |
| T2, N1 | 45 | 298 | 15 |
| Bone | |||
| Embryonal, T1 | 0 | 614 | 0 |
| Embryonal, T2 | 11 | 486 | 2 |
| Alveolar, T1 | 4 | 218 | 2 |
| Alveolar, T2 | 66 | 369 | 18 |
| Bone marrow | |||
| Embryonal, T1 | 0 | 614 | 0 |
| Embryonal, T2 | 12 | 486 | 2 |
| Alveolar, T1 | 4 | 218 | 2 |
| Alveolar, T2 | 86 | 369 | 23 |
The combination of regional lymph node involvement and tumor invasiveness further predicted differences in the incidence of lung metastases. Patients with T1, N0 disease (n = 783) had a low rate of lung involvement (n = 4; 0.5%). In comparison, those with T1, N1 disease (n = 49) had higher rates of lung metastasis (n = 3; 6.1%; P < .001). Similar findings were noted among patients with T2, N0 (n = 557) and T2, N1 (n = 298) disease (n = 44; 7.9% and n = 45; 15%, respectively). When the combination of tumor invasiveness, regional lymph node status, and histology variables was analyzed, invasiveness remained the most important variable. Lung metastasis rates were low for patients with T1, N0, ERMS (four of 595 patients; 0.7%) and ARMS (0 of 188 patients) and higher for patients with T2, N0, ERMS (23 of 383 patients; 6%) and ARMS (21 of 174 patients; 12%). No patients with T2, N0 tumors less than 5 cm had lung involvement (n = 541). When considering primary site, no orbital, T1 patients had lung involvement at presentation.
Regional lymph node involvement was also found to be an important variable in predicting bone and BM involvement (Table 3). Patients with T1, N0 (n = 783) disease had a low rate of bone (n = 3; 0.4%) and BM (n = 3; 0.4%) involvement. Among N1 patients (n = 347), 158 (46%) had evidence of distant metastasis: 49 (14%) had bone involvement and 61 (18%) had BM involvement. Forty-four of the N1 patients with bone disease (90%) and 56 with BM disease (92%) had alveolar histology.
Table 3.
Rate of Metastasis Based on Nodes, Histology, and T Stage
| Characteristic | Site and Rate of Involvement |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung |
Bone |
Bone Marrow |
|||||||
| No. | Total No. | % | No. | Total No. | % | No. | Total No. | % | |
| N0, T1, n = 783 | 4 | 783 | 0.5 | 3 | 783 | 0.4 | 3 | 783 | 0.4 |
| N0, T2, n = 557 | |||||||||
| Embryonal | 23 | 383 | 6 | 6 | 383 | 1.6 | 7 | 383 | 1.8 |
| Alveolar | 21 | 174 | 12 | 23 | 174 | 13 | 31 | 174 | 18 |
| N1, n = 347 | 48 | 347 | 14 | 49 | 347 | 14 | 61 | 347 | 18 |
The recursive partitioning analyses identified histology (ERMS v ARMS) as the most important variable for bone and BM involvement (Figs 1B and 1C). In patients with ERMS (n = 1,100), 11 (1%) had bone involvement and 12 (1.1%) had BM involvement. Among those with ARMS (n = 587), in contrast, 70 (12%) had bone involvement (P = .0001) and 90 (15%) had BM involvement (P = .0001). After histology, the next most significant variable with risk of initial bone and BM involvement was tumor invasiveness. Differences in incidence at each metastatic site were noted when histology and tumor invasiveness were analyzed together (Table 2). ERMS, T1 disease (n = 614) was associated with no bone or BM involvement, whereas ERMS, T2 disease (n = 486) was associated with a 2% risk (n = 11) of bone involvement (P = .0002) and a 2% risk (n = 12) of BM involvement (P = .0001). Patients with ARMS, T1 disease (n = 218), had a 2% risk of both bone and bone marrow involvement (four patients each), whereas ARMS, T2 patients (n = 369) had a much higher incidence of bone and BM involvement (n = 66 [18%] and n = 86 [23%], respectively; P = .0001). When incorporating nodal status, only 1.6% of ARMS, T1, N0 patients had bone (n = 3) or BM (n = 3) involvement.
Lung Metastases and Bone or BM Involvement
In patients who presented with lung involvement (n = 96), 39 (41%) were found to have either bone or BM involvement. In patients without lung involvement (n = 1,591), 96 (6%) had bone or BM involvement. Patients with T2, N0 ERMS without lung involvement (n = 360) had low rates of bone or BM involvement (n = 2, 0.6% and n = 3, 0.8%, respectively) compared with patients with T2, N0 ERMS with lung involvement (n = 23; n = 4; 17.4% and n = 4; 17.4%, respectively). The one patient with T1 ARMS with lung involvement and 3% (six of 217) of the patients with T1 ARMS without lung involvement had bone or BM involvement. Bone or BM involvement was frequent in patients with T2 ARMS (60% with lung involvement, 26% without).
Association of Bone and BM Involvement
In patients with bone involvement (n = 81), 48 (59%) had concomitant BM involvement, whereas 33 (41%) had bone disease only. In patients with BM involvement (n = 102), 48 (47%) had concomitant bone involvement, whereas 54 (53%) had BM disease only. For this reason, bone and BM involvement were analyzed separately.
Staging Algorithm
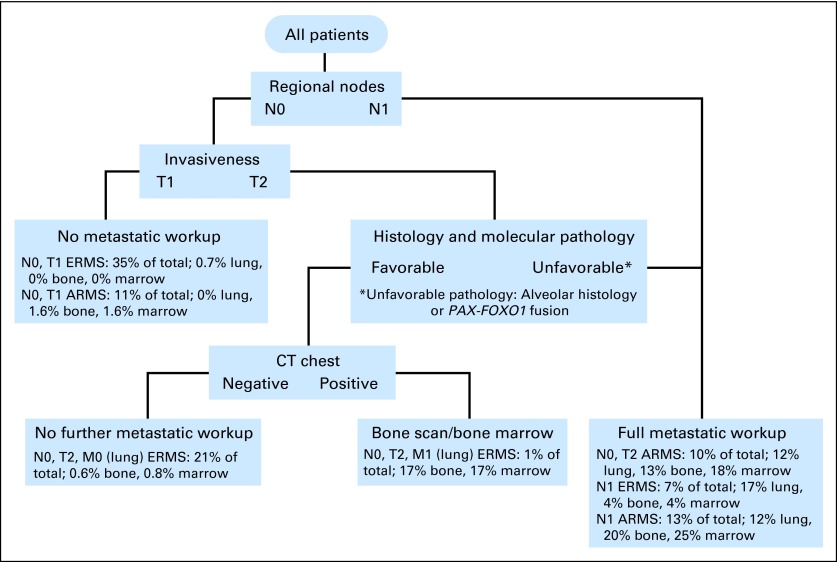
Given these results (summarized in Table 4), an initial staging proposal was devised. This algorithm assumes that upfront clinical and histologic characteristics are known (Fig 2) and that tests for which positivity rates are less than 2% in a patient subpopulation warrant omission.
Table 4.
Estimates of the Rates of Lung, Bone, and Bone Marrow Metastases for Various Patient Subsets
| Patient Subset | No. of Patients | % With Lung Metastases | % With Bone Metastases | % With Bone Marrow Metastases |
|---|---|---|---|---|
| All patients | 1,687 | 5.7 | 4.8 | 6.0 |
| N0, T1, embryonal | 595 | 0.7 | 0 | 0 |
| N0, T1, alveolar | 188 | 0 | 1.6 | 1.6 |
| N0, T2, embryonal, no lung disease | 360 | 0 | 0.6 | 0.8 |
| N0, T2, embryonal, lung disease | 23 | 100 | 17.4 | 17.4 |
| N0, T2, alveolar | 174 | 12 | 13 | 18 |
| N1, embryonal, no lung disease | 101 | 0 | 3.0 | 2.0 |
| N1, embryonal, lung disease | 21 | 100 | 9.5 | 14.3 |
| N1, alveolar | 225 | 12 | 19.6 | 24.9 |
Fig 2.
Rhabdomyosarcoma initial staging algorithm using clinical and histologic characteristics. CT, computed tomography; ARMS, alveolar rhabdomyosarcoma; ERMS, embryonal rhabdomyosarcoma.
DISCUSSION
In childhood RMS, staging evaluations include standard diagnostic tests that historically have been disease-specific rather than risk-specific. So, a substantial number of patients with low-stage disease are subjected to potentially avoidable testing. Using a recursive partitioning statistical method, we have demonstrated that presenting clinical and tumor features in children and adolescents with RMS can predict rates and sites of distant metastatic involvement, allowing for a risk-based approach to initial disease evaluation.
Our analysis demonstrates that patients with T1 tumors have a low rate of metastasis at all sites. More specifically, those with ERMS, T1 disease are at particularly low risk of metastatic BM and bone disease (none of 614 patients). This subgroup, which constitutes 36% of all patients within our entire cohort, does not require bone scan or BM evaluation.
Conversely, those with ARMS or T2 disease have an increased likelihood of presenting with bone and BM involvement. Patients with T2 ARMS had a particularly high rate of distant metastasis for all disease sites, suggesting that bone scan and BM evaluations should still be required in the initial staging evaluation. Patients with node-negative T1 ARMS and T2 ERMS had less than a 2% rate of bone or BM involvement. Although higher compared with the very low-risk ERMS, T1 subgroup, the overall rates are low. We examined the association among all three targeted metastatic sites to further determine the significance of this level of risk involvement and found that the likelihood of bone and BM involvement increased considerably in the presence of lung metastases. Thus the decision to perform a bone scan or BM in these subsets of patients could reasonably be based on lung imaging results.
Patients with regional lymph node involvement had a high rate of metastases in all distant sites. This was most evident in patients with N1 ARMS, who should undergo complete initial staging evaluations with CT chest, bone scan, and BM. Although comparatively lower, the metastatic rates in patients with N1 ERMS are sufficiently high to warrant a full staging evaluation.
Lung involvement was common in all patients with T2 disease, including ERMS histology, indicating that chest CT should be performed in these patients as part of the initial staging evaluation. In contrast, those patients with node-negative, ERMS or ARMS T1 disease are at very low risk of metastatic lung involvement (only four of 783 patients). Because the incidence of lung involvement in patients with N0, T1 disease is so low, substituting a chest radiograph for a staging chest CT in this more favorable risk subgroup would provide a substantial reduction in diagnostic radiation exposure and a much lower rate of false-positive results.
An argument could be made that even though only 0.5% of T1 patients (four of 783) had lung involvement, the positive CT scans led to intensification of their therapy, which improved their outcome. This assumes, however, that all four patients had true-positive chest CT scans. The reported frequency of false-positive initial chest CT findings in children with sarcoma has ranged from 43% to 58%.19–21 Thus the likelihood of a false-positive chest CT is much greater than the likelihood of a true positive. The presence of combined additional tumor variables such as size less than 5 cm and highly favorable sites (ie, orbit) may further minimize the risk of incorrect staging. With this in mind, we propose that N0, T1 patients have upfront chest radiographs, for the purposes of later comparison, and that chest CT scans be omitted. Whether chest radiographs could ultimately replace chest CT scans for all patients would need to be studied prospectively.
A proposal to eliminate certain initial staging studies could raise concern about a subset of high-risk patients who would be deemed as having nonmetastatic disease when metastases were actually present, which could ultimately compromise outcome. However, our proposal to eliminate upfront bone scan and BM would affect only a select group of patients with very low-risk characteristics. A similar very low-risk population of children with RMS was identified in a study by Dantonello et al.6 Primary tumor-, treatment- and patient-related factors were evaluated to determine their ability to predict pattern and risk of relapse in localized RMS on consecutive trials of the Cooperative Weichteilsarkom Studiengruppe. None of the 440 patients with ERMS tumors less than 5 cm in size and just six of the total 803 patients with ERMS tumors (0.7%) experienced a bone or BM relapse.
Using the risks established by our recursive partitioning analyses model, we have simplified the recommended staging evaluation by correlating tumor and clinical features with patterns of distant metastasis in newly diagnosed patients with RMS. This has facilitated the development of an initial staging algorithm. Because nodal status, tumor invasiveness, and histologic subtype are the most powerful predictors of metastatic disease, these variables represent the initial bifurcations in the pathway. Subsequent divergences along the algorithm reflect the associations among various risk factors when the results of particular evaluations are known. The algorithm is designed conservatively, so that any characteristics associated with an increased risk of metastases lead to closer investigation. We decided a metastatic site positivity rate of less than 2% was sufficiently low to justify omitting specific staging evaluations while fully acknowledging that others might see the value in testing for rates between 0.6 and 1.6%. By providing all the data (summarized in Table 4), one could choose to further reduce the likelihood of missing a metastatic site at the expense of adding more evaluations that carry their own risks.
Although it has been well established that ARMS has a worse outcome than ERMS,11,22–24 the diagnosis of ARMS can be challenging. On IRS-IV, there was only 70% concordance between institutional and review pathologists.11 For our purposes, it is important to identify tumors with pathologic features that are associated with aggressive behavior because those cases merit a more extensive initial work-up. Because the majority of that work-up will be done before the central review pathology is completed and acknowledging inter-institutional pathology interpretation, we have chosen to label the histology branch point of our proposed staging algorithm as “unfavorable histology and molecular pathology.” This would include alveolar histology or PAX-FOXO1 fusion (t(1;13), t(2;13)).22,25,26 Additional refinement to these criteria may be necessary as we further define pathologically high-risk and standard-risk ARMS in the future as more definitive diagnostic criteria for ARMS are developed.
Monetary and nonmonetary costs are reasons to avoid unnecessary tests. Chest CT, bone scan, and BM aspiration and biopsy are expensive and uncomfortable and carry risks including radiation exposure as well as complications of sedation or anesthesia in the subset of children unable to complete the tests while fully awake. False-positive results lead to more investigations, such as additional imaging and/or biopsies with their own costs, discomforts, and risks. Using the proposed algorithm, approximately two thirds of new patients with RMS would be spared bone scans and BM evaluations, and half would be spared chest CT. This would lead to a substantial reduction in radiation exposure for the population as a whole, as well as reduced sedation- and procedure-related complication risks. Additional indirect benefits would include shorter time to study enrollment and initiation of therapy and lower diagnosis-associated costs.
The use of newer, noninvasive modalities such as fluorodeoxyglucose positron emission tomography (PET) scans have an ability to detect lymph node, bone, and BM involvement in patients with metastatic RMS, often with higher sensitivity and specificity compared with conventional modalities.27–32 The results of further studies to determine whether PET/CT could replace bone scans and BM biopsies in high-risk patients might change the recommended staging evaluation. However, our data indicate that there is no reason to perform PET/CT scans in (for example) patients with embryonal histology orbital tumors. In general, limiting the use of duplicative staging modalities will be an important issue of future study but should be evaluated prospectively.
In conclusion, staging BM aspirate and biopsy and bone scan is unnecessary in at least the one third of patients with noninvasive ERMS. Omission of chest CT should also be considered in those with node-negative, noninvasive RMS. Restricting the extent of the metastatic work-up on the basis of predictors of metastatic spread may decrease costs, complications, and false-positive findings.
Footnotes
Supported by cooperative group Grants No. U10 CA98543-08, U10 CA98413-08, CA-42326, and CA-54498 and by the Daniel P. Sullivan Fund (R.B.W.).
Presented in part at the Annual Meeting of the Connective Tissue Oncology Society, November 10-13, 2010, Paris, France.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Aaron R. Weiss, James R. Anderson, Douglas S. Hawkins, Suzanne L. Wolden, Richard B. Womer
Provision of study materials or patients: Suzanne L. Wolden
Collection and assembly of data: Aaron R. Weiss, James R. Anderson, David M. Parham, Richard B. Womer
Data analysis and interpretation: Aaron R. Weiss, Elizabeth R. Lyden, James R. Anderson, Douglas S. Hawkins, Sheri L. Spunt, David O. Walterhouse, David M. Parham, David A. Rodeberg, Simon C. Kao, Richard B. Womer
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Gurney JG, Young JL, Roffers SD, et al. Soft tissue sarcomas. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute, SEER Program; 1999. pp. 111–124. [Google Scholar]
- 2.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13:610–630. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 3.Joshi D, Anderson JR, Paidas C, et al. Age is an independent prognostic factor in rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. Pediatr Blood Cancer. 2004;42:64–73. doi: 10.1002/pbc.10441. [DOI] [PubMed] [Google Scholar]
- 4.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Carli M, Colombatti R, Oberlin O, et al. European intergroup studies (MMT4-89 and MMT4-91) on childhood metastatic rhabdomyosarcoma: Final results and analysis of prognostic factors. J Clin Oncol. 2004;22:4787–4794. doi: 10.1200/JCO.2004.04.083. [DOI] [PubMed] [Google Scholar]
- 6.Dantonello TM, Int-Veen C, Winkler P, et al. Initial patient characteristics can predict pattern and risk of relapse in localized rhabdomyosarcoma. J Clin Oncol. 2008;26:406–413. doi: 10.1200/JCO.2007.12.2382. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DJ, Hall EJ. Computed tomography: An increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 8.Robbins E. Radiation risks from imaging studies in children with cancer. Pediatr Blood Cancer. 2008;51:453–457. doi: 10.1002/pbc.21599. [DOI] [PubMed] [Google Scholar]
- 9.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group Study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitfeld PP, Lyden E, Raney RB, et al. Ifosfamide and etoposide are superior to vincristine and melphalan for pediatric metastatic rhabdomyosarcoma when administered with irradiation and combination chemotherapy: A report from the Intergroup Rhabdomyosarcoma Study Group. J Pediatr Hematol Oncol. 2001;23:225–233. doi: 10.1097/00043426-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Crist WM, Anderson JR, Meza JL, et al. Intergroup Rhabdomyosarcoma Study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 12.Pappo AS, Lyden E, Breitfeld P, et al. Two consecutive phase II window trials of irinotecan alone or in combination with vincristine for the treatment of metastatic rhabdomyosarcoma: The Children's Oncology Group. J Clin Oncol. 2007;25:362–369. doi: 10.1200/JCO.2006.07.1720. [DOI] [PubMed] [Google Scholar]
- 13.Pappo AS, Lyden E, Breneman J, et al. Up-front window trial of topotecan in previously untreated children and adolescents with metastatic rhabdomyosarcoma: An intergroup rhabdomyosarcoma study. J Clin Oncol. 2001;19:213–219. doi: 10.1200/JCO.2001.19.1.213. [DOI] [PubMed] [Google Scholar]
- 14.Raney RB, Walterhouse DO, Meza JL, et al. Results of the Intergroup Rhabdomyosarcoma Study Group D9602 protocol, using vincristine and dactinomycin with or without cyclophosphamide and radiation therapy, for newly diagnosed patients with low-risk embryonal rhabdomyosarcoma: A report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2011;29:1312–1318. doi: 10.1200/JCO.2010.30.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler E, Lyden E, Ruymann F, et al. Efficacy of ifosfamide and doxorubicin given as a phase II “window” in children with newly diagnosed metastatic rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. Med Pediatr Oncol. 2001;37:442–448. doi: 10.1002/mpo.1227. [DOI] [PubMed] [Google Scholar]
- 16.Walterhouse DO, Lyden ER, Breitfeld PP, et al. Efficacy of topotecan and cyclophosphamide given in a phase II window trial in children with newly diagnosed metastatic rhabdomyosarcoma: A Children's Oncology Group study. J Clin Oncol. 2004;22:1398–1403. doi: 10.1200/JCO.2004.05.184. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence W, Jr, Anderson JR, Gehan EA, et al. Pretreatment TNM staging of childhood rhabdomyosarcoma: A report of the Intergroup Rhabdomyosarcoma Study Group—Children's Cancer Study Group. Pediatric Oncology Group. Cancer. 1997;80:1165–1170. [PubMed] [Google Scholar]
- 18.Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I: A final report. Cancer. 1988;61:209–220. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 19.Absalon MJ, McCarville MB, Liu T, et al. Pulmonary nodules discovered during the initial evaluation of pediatric patients with bone and soft-tissue sarcoma. Pediatr Blood Cancer. 2008;50:1147–1153. doi: 10.1002/pbc.21454. [DOI] [PubMed] [Google Scholar]
- 20.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239:514–520. doi: 10.1148/radiol.2392050631. [DOI] [PubMed] [Google Scholar]
- 21.Picci P, Vanel D, Briccoli A, et al. Computed tomography of pulmonary metastases from osteosarcoma: The less poor technique—A study of 51 patients with histological correlation. Ann Oncol. 2001;12:1601–1604. doi: 10.1023/a:1013103511633. [DOI] [PubMed] [Google Scholar]
- 22.Crist WM, Garnsey L, Beltangady MS, et al. Prognosis in children with rhabdomyosarcoma: A report of the intergroup rhabdomyosarcoma studies I and II—Intergroup Rhabdomyosarcoma Committee. J Clin Oncol. 1990;8:443–452. doi: 10.1200/JCO.1990.8.3.443. [DOI] [PubMed] [Google Scholar]
- 23.Mazzoleni S, Bisogno G, Garaventa A, et al. Outcomes and prognostic factors after recurrence in children and adolescents with nonmetastatic rhabdomyosarcoma. Cancer. 2005;104:183–190. doi: 10.1002/cncr.21138. [DOI] [PubMed] [Google Scholar]
- 24.Meza JL, Anderson J, Pappo AS, et al. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: The Children's Oncology Group. J Clin Oncol. 2006;24:3844–3851. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen PH, Lynch JC, Qualman SJ, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: A report from the Children's Oncology Group. J Clin Oncol. 2002;20:2672–2679. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- 26.Breneman JC, Lyden E, Pappo AS, et al. Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol. 2003;21:78–84. doi: 10.1200/JCO.2003.06.129. [DOI] [PubMed] [Google Scholar]
- 27.Iagaru A, Goris ML. Rhabdomyosarcoma diffusely metastatic to the bone marrow: Suspicious findings on 99mTc-MDP bone scintigraphy confirmed by (18)F-18 FDG PET/CT and bone marrow biopsy. Eur J Nucl Med Mol Imaging. 2008;35:1746. doi: 10.1007/s00259-008-0864-4. [DOI] [PubMed] [Google Scholar]
- 28.Klem ML, Grewal RK, Wexler LH, et al. PET for staging in rhabdomyosarcoma: An evaluation of PET as an adjunct to current staging tools. J Pediatr Hematol Oncol. 2007;29:9–14. doi: 10.1097/MPH.0b013e3180307693. [DOI] [PubMed] [Google Scholar]
- 29.Ricard F, Cimarelli S, Deshayes E, et al. Additional benefit of F-18 FDG PET/CT in the staging and follow-up of pediatric rhabdomyosarcoma. Clin Nucl Med. 2011;36:672–677. doi: 10.1097/RLU.0b013e318217ae2e. [DOI] [PubMed] [Google Scholar]
- 30.Seshadri N, Wright P, Balan KK. Rhabdomyosarcoma with widespread bone marrow infiltration: Beneficial management role of F-18 FDG PET. Clin Nucl Med. 2007;32:787–789. doi: 10.1097/RLU.0b013e318148b434. [DOI] [PubMed] [Google Scholar]
- 31.Tateishi U, Hosono A, Makimoto A, et al. Comparative study of FDG PET/CT and conventional imaging in the staging of rhabdomyosarcoma. Ann Nucl Med. 2009;23:155–161. doi: 10.1007/s12149-008-0219-z. [DOI] [PubMed] [Google Scholar]
- 32.Völker T, Denecke T, Steffen I, et al. Positron emission tomography for staging of pediatric sarcoma patients: Results of a prospective multicenter trial. J Clin Oncol. 2007;25:5435–5441. doi: 10.1200/JCO.2007.12.2473. [DOI] [PubMed] [Google Scholar]