Abstract
Purpose
Thirty percent to 90% of cancer survivors report impaired sleep quality post-treatment, which can be severe enough to increase morbidity and mortality. Lifestyle interventions, such as exercise, are recommended in conjunction with drugs and cognitive behavioral therapy for the treatment of impaired sleep. Preliminary evidence indicates that yoga—a mind-body practice and form of exercise—may improve sleep among cancer survivors. The primary aim of this randomized, controlled clinical trial was to determine the efficacy of a standardized yoga intervention compared with standard care for improving global sleep quality (primary outcome) among post-treatment cancer survivors.
Patients and Methods
In all, 410 survivors suffering from moderate or greater sleep disruption between 2 and 24 months after surgery, chemotherapy, and/or radiation therapy were randomly assigned to standard care or standard care plus the 4-week yoga intervention. The yoga intervention used the Yoga for Cancer Survivors (YOCAS) program consisting of pranayama (breathing exercises), 16 Gentle Hatha and Restorative yoga asanas (postures), and meditation. Participants attended two 75-minute sessions per week. Sleep quality was assessed by using the Pittsburgh Sleep Quality Index and actigraphy pre- and postintervention.
Results
In all, 410 survivors were accrued (96% female; mean age, 54 years; 75% had breast cancer). Yoga participants demonstrated greater improvements in global sleep quality and, secondarily, subjective sleep quality, daytime dysfunction, wake after sleep onset, sleep efficiency, and medication use at postintervention (all P ≤ .05) compared with standard care participants.
Conclusion
Yoga, specifically the YOCAS program, is a useful treatment for improving sleep quality and reducing sleep medication use among cancer survivors.
INTRODUCTION
In patients with cancer, sleep quality is impaired before treatment, worsens during treatment, and remains impaired after treatments are complete.1–6 Thirty percent to 90% of cancer survivors report some form of impaired sleep quality post-treatment,1–5,7 which can be severe enough to increase morbidity and mortality.1–5,7–18 Impaired sleep quality—excessive daytime napping, difficulty falling asleep, difficulty staying asleep, waking up too early—is among the most distressing adverse effects experienced by cancer survivors.1–4 Despite the ubiquity of impaired sleep and its negative consequences, sleep problems are both underdiagnosed and undertreated in post-treatment cancer survivors.4,8,9,19
Treatment options for impaired sleep include the use of sedatives and hypnotics, over-the-counter sleep aids, cognitive behavioral therapy (CBT), and lifestyle interventions.20 Unfortunately, sedatives and hypnotics lead to CNS toxicities, possible drug interactions with cancer therapeutics, dependency, rebound sleep impairment after discontinuation and, ultimately, do not cure sleep problems. CBT can be helpful but may not be appealing to everyone.1–4,8,9,21,22 Lifestyle interventions, such as exercise, provide an additional treatment option that some individuals may prefer, and current guidelines for the treatment of impaired sleep recommend using them in conjunction with drugs and CBT.1–4,7–9,19,23–29
Although research supports the use of exercise for improving sleep, data are limited among post-treatment cancer survivors, particularly with regard to yoga. Yoga is an increasingly popular mind-body practice also characterized as a mindfulness mode of exercise.30–33 Hatha yoga, the foundation of all yoga styles and the most popular form, includes both Gentle Hatha and Restorative yoga and is growing in acceptance for therapeutic use in traditional Western medicine.30–37 Gentle Hatha yoga focuses on physical aspects and is part of many styles of yoga, including Iyengar, Anusara, and others.30–34 Restorative yoga focuses on full relaxation and is part of the Iyengar style.38,39 The combination of Gentle Hatha and Restorative yoga may provide an effective approach for improving sleep, because it uses a holistic sequence of meditative, breathing, and physical alignment exercises requiring both the active and passive engagement of skeletal muscles.30,31,34,35,38,39 Despite yoga's popularity, only limited scientific evidence suggests that yoga may improve sleep among cancer survivors (studies include four community yoga program evaluations40–43 and seven phase I to II trials38,44–49). To the best of our knowledge, none of these studies specifically targeted sleep or enrolled participants with sleep impairments, and no large, multicenter phase III clinical trials have confirmed these findings.
The primary aim of this clinical trial was to determine the efficacy of a standardized yoga intervention for improving global sleep quality (primary outcome) compared with standard care for post-treatment cancer survivors experiencing sleep problems. It was hypothesized that cancer survivors in the yoga condition would have better global sleep quality than survivors in the standard care condition after completing 4 weeks of yoga. Adverse events, adherence, and enjoyment are also reported.
PATIENTS AND METHODS
Study Design
The University of Rochester Cancer Center (URCC) Community Clinical Oncology Program (CCOP) Research Base conducted a nationwide, multicenter, randomized controlled trial (RCT) examining the efficacy of yoga compared with standard care for improving global sleep quality in post-treatment cancer survivors. Survivors were recruited in cohorts (n = 20 to 30), stratified by sex and baseline sleep disturbance (two levels: ≤ 5 or > 5 on an 11-point symptom inventory scale anchored by 0 [no sleep disturbance] and 10 [worst possible sleep disturbance]), and randomly assigned to both groups at each CCOP. Group assignment was determined by a computer-generated random numbers table in blocks of two and an allocation ratio of 1:1. Allocation was concealed from coordinators until after they registered the participants by using a computerized Web site that generated an e-mail to the research base and CCOP site. The study primary investigator and biostatistician were blinded to allocation. Participants received their allocation assignment after completing baseline assessments.
Each institutional review board approved the study before participants were enrolled. Baseline measurements were completed during the week immediately before commencing the 4-week intervention, and final measurements were completed during the week immediately following completion of the intervention. As part of pre- and post-testing, participants completed all questionnaires at home and wore actigraphs on their wrists for seven consecutive days, 24 hours a day. Sleep medication use was not restricted in the study design but was monitored.
Study Participants
Cancer survivors were recruited by clinical research coordinators through the use of flyers in communities and direct contact during regularly scheduled clinic visits in 12 US cities by nine CCOPs from 2007 to 2010. Participants were enrolled 2 to 24 months postsurgery, postchemotherapy, and/or post-radiation therapy. To be eligible, survivors must (1) have a confirmed diagnosis of cancer; (2) have completed treatment for cancer; (3) have sleep disturbance (indicated by a response of ≥ 3 on a clinical symptom inventory by using an 11-point scale anchored by 0 [no sleep disturbance] and 10 [worst possible sleep disturbance]); (4) be able to read English; (5) be ≥ 21 years of age; (6) give written informed consent; (7) not have maintained a regular personal practice of yoga within the 3 months before enrolling onto the study or be planning to start yoga on their own during the next 4 weeks; (8) not have a confirmed diagnosis of sleep apnea; (9) not be receiving any form of treatment for cancer, with the exception of hormonal or monoclonal antibody therapy; and (10) not have metastatic cancer. Figure 1 shows the flow of cancer survivor recruitment, participation, and dropout. All participants provided informed consent before completing any study requirements.
Fig 1.
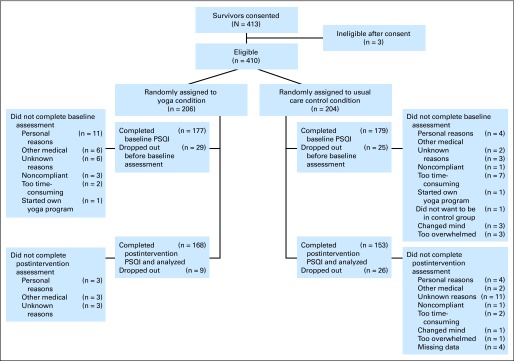
CONSORT diagram. PSQI, Pittsburgh Sleep Quality Index.
Yoga Intervention Experimental Condition
The yoga intervention used the standardized Yoga for Cancer Survivors (YOCAS) program, designed by researchers at the University of Rochester Medical Center. All sessions were taught in community-based sites (eg, yoga studios, community centers, community oncology practices) with an average group size of 12 (range, 10 to 15) in the late afternoon or evening after 4 pm (see Table 1 for a full description of the standardized YOCAS program).
Table 1.
Description of Standardized YOCAS Intervention Components
| Sequences | |
|---|---|
| Seated | Standing |
| Ynana mudra (mindfulness sitting meditation) | Adhomukha Svanasana (downward dog) |
| Parvatasana (seated mountain pose) | Uttanasana (standing forward extension) |
| Lateral extension with breath | Prasaritta Padotanasana (forward stretch extended legs) |
| Bharadvajasana (seated twist) | Balasana (lateral arm child pose) |
| Janu sirasana (head-to-knee pose) | Balasana (child pose with shoulder extension transition to supported backbend) |
| Modification: Adhomukha Paschimottanasana (supported forward bend from chair) | |
| Spinal waves | |
| Balasana (extended child pose) | |
| Transition | Restorative |
| Supine curl to floor | Supta Baddhakonasana (supported back bolster, belt, legs cobbler, blankets) |
| Savasana (lateral extension with open jaw) | Adhomukha Virasana (supported child pose with twist) |
| Jathara Parivartanasana (supine twist bilaterally) | Setubandha Sarvangasana (supported legs and back to shoulder blades, legs belted) |
| Suptapadangusthasana (supine leg stretch) | Viloma II (regulated exhalation) |
| Sethubandhasana (supine pelvic lift) | Viparita Karani (legs up wall, pelvis on bolster) |
| Savasana (corpse pose) | |
| Mudra | Pranayama |
| Ynana (Seal of Wisdom; link index finger and thumb together) | Equalize breath with pause postexhalation |
| Hmm breath | |
| Viloma II | |
| Mindfulness meditation | Visualization |
| Body scan and sensation | Mind turn inward to heart |
| Internal viewing | Dive beneath surface |
| Nostril breathing, gravity tailbone, tactile cues | Lying into back body |
| Affirmation: my senses turn inward and I relax into peace | |
NOTE. The Yoga for Cancer Survivors (YOCAS) intervention uses two forms of yoga: Gentle Hatha yoga and Restorative yoga. The YOCAS sessions are standardized, and each session includes physical alignment postures, breathing and mindfulness exercises. The intervention is delivered in an instructor-taught, group format, twice a week for 75 minutes each time over 4 weeks for a total of eight sessions of yoga.
The YOCAS intervention is delivered by Registered Yoga Alliance instructors. To ensure intervention standardization, quality, fidelity, and prevention of drift, each yoga instructor completes a standardized training session and is provided with a detailed YOCAS instructor manual and digital video disc (DVD). A coordinator at each Community Clinical Oncology Program site also completes the same standardized training session and performs a random independent observation of YOCAS sessions to ensure proper content is being taught. YOCAS sessions are conducted in community-based group settings, free-of-charge to participants.
Hatha and Restorative yoga include the three components of movement, breath, and awareness. The body component includes a movement component asana (postures). In the YOCAS program, asana includes seated, standing, transitional, and supine poses, with an emphasis on restorative poses using supports that were chosen on the basis of yoga theory postulating their positive influence on sleep. All asanas are given with modifications to address multi-levels of experience. The mindfulness component includes a breath component pranayama (breathing exercises) to regulate breathing and an awareness component that includes meditation instruction, visualization, and affirmation. Mindfulness is incorporated throughout the program as the practice of paying attention with nonjudgmental observation, to the present experience, for the purpose of attending to both external and internal impressions.
Standard Care Control Condition
The control condition used a standard care format. Cancer survivors assigned to this condition continued with the standard follow-up care provided by their treating oncologists as appropriate for their individual diagnoses. Participants in the control condition were offered the 4-week YOCAS program gratis after completing all study requirements.
Measures
Clinical and demographic information was collected by coordinators by using medical records and study-specific forms. Race and ethnicity data were collected for descriptive purposes by using the National Cancer Institute (NCI) Cancer Therapy Reporting Program criteria for clinical trials. Participants identified their race and ethnicity; categories were condensed to white, African American, and other for reporting purposes. Adherence and compliance were monitored through the use of daily patient diaries and attendance records kept by the yoga instructors. No make-up sessions were provided for missed classes. Intensity of the yoga was monitored by using the American College of Sports Medicine Rating of Perceived Exertion Scale.50 All participants were instructed to continue their routine daily activities during the 4-week intervention period but were asked not to start a new yoga or exercise regimen on their own during this 4-week period to avoid exercise contamination. A feedback form was used to assess enjoyment and helpfulness of the yoga intervention and whether participants would recommend it to others.
The primary measure of sleep quality was the Pittsburgh Sleep Quality Index (PSQI), a psychometrically validated, patient-reported, 19-item instrument.51 The global sleep quality score was the primary outcome and the subscale scores of global sleep quality characteristics were secondary end points (full details are presented in the Appendix, onlineonly).
Actigraphy was a secondary objective measure of global sleep quality characteristics including sleep onset latency, wake after sleep onset, and sleep efficiency (see Appendix for full details on this measure).
Adverse Events
Adverse events were monitored by the URCC Data Safety Monitoring Committee. All unexpected, serious, life-threatening, and fatal adverse events were reported.
Statistical Analyses
On the basis of published data,51 and assuming a correlation coefficient of 0.50 between pre- and post-treatment observations and a standard deviation of 4.7, 160 patients per study arm provides 80% power to detect a difference between arms of 1.3 in the mean PSQI global sleep quality score at a significance level of 5% with a two-sided F test using analysis of covariance (ANCOVA). Analyses were performed with SAS Version 9.2 and R Version 2.13.1. Clinical and demographic variables were examined with two-tailed (α = .05) t tests for continuous variables and χ2 or Monte Carlo tests (if any expected cell counts were < 5)52 for categorical variables to test population differences between arms. ANCOVAs, with arm as the factor, baseline as the covariate, and arm by baseline interaction, were used to evaluate arm effects for the post-treatment PSQI global sleep quality score and actigraphy outcomes. Ordinal logistic regression (OLR; proportional odds), with arm as a factor, baseline as a covariate, and arm by baseline interaction terms, was used to evaluate arm effects for the post-treatment PSQI subscale scores. If the interaction was significant (P ≤ .10), it was retained in the model. Estimated within-group effects from the ANCOVAs and OLRs were expressed in terms of pre-post mean differences. Estimated between-group effects were expressed in terms of between-group mean differences or odds ratios for ANCOVAs and OLRs, respectively. All data were analyzed by using the intent-to-treat principle. Analyses were based on complete cases because analyses revealed results in which missing data were missing completely at random,53 and sensitivity analyses using multiple imputation revealed no significant differences when reporting the actual real data for complete cases versus estimated data with imputations (see Appendix and Appendix Table A1, online only, for a full description of intent-to-treat, missing completely at random, and sensitivity analyses).
RESULTS
Four hundred thirteen survivors were consented between 2007 and 2010. Three patients were deemed ineligible. The 410 eligible survivors were randomly assigned to yoga (n = 206) or standard care (n = 204), Figure 1 shows participant flow and loss to follow-up.
Baseline Characteristics of Participants
There were no significant differences between groups in baseline characteristics overall or by CCOP location, the probability of withdrawal or loss to follow-up by group assignment, or in completers versus noncompleters. Twenty-two percent of participants did not provide fully evaluable data; this is typical for clinical trials conducted in the NCI multisite CCOP network. Participants reported withdrawing largely for personal reasons, illness, and treatment-related issues, not because of dissatisfaction with the yoga intervention. Table 2 shows the baseline data for the 410 participants who were consented and eligible before random assignment, and the baseline data were separated into the two study arms. Both groups met the clinical cutoff criterion for impaired sleep quality at baseline with PSQI scores above 8.
Table 2.
Participant Demographics and Characteristics
| Characteristic | Total (N = 410) |
YOCAS (n = 206) |
Standard Care (n = 204) |
Test | Statistic | df | P | |||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |||||
| Female sex | 393 | 96 | 197 | 96 | 196 | 96 | χ2 | 0.00 | 1.0 | .983 |
| Age, years | t test | 0.37 | 400 | .709 | ||||||
| Mean | 54.1 | 54.3 | 54.0 | |||||||
| SE | 0.51 | 0.77 | 0.67 | |||||||
| Race/ethnicity | χ2 simulation | 3.31 | N/A | .185 | ||||||
| White | 383 | 93 | 197 | 96 | 186 | 91 | ||||
| African American | 24 | 6 | 8 | 4 | 16 | 8 | ||||
| Other | 3 | 1 | 1 | 1 | 2 | 1 | ||||
| Currently employed | 323 | 81 | 168 | 83 | 155 | 78 | χ2 | 1.24 | 1 | .266 |
| Marital status | χ2 simulation | 10.34 | N/A | .061 | ||||||
| Married or long-term committed relationship | 289 | 72 | 145 | 71 | 144 | 72 | ||||
| Divorced or separated | 60 | 15 | 28 | 14 | 32 | 15.8 | ||||
| Single | 35 | 9 | 18 | 8.9 | 17 | 9.5 | ||||
| Widowed | 18 | 4 | 6 | 6 | 12 | 6 | ||||
| Education | χ2 simulation | 0.92 | N/A | .930 | ||||||
| Completed 4 years of college or more | 189 | 47 | 96 | 47 | 93 | 47 | ||||
| Completed < 4 years of college | 141 | 35 | 72 | 36 | 69 | 35 | ||||
| High school graduate | 69 | 17 | 33 | 16 | 36 | 18 | ||||
| Less than a high school education | 3 | < 1 | 2 | < 1 | 1 | < 1 | ||||
| Cancer type | χ2 simulation | 13.42 | N/A | .168 | ||||||
| Breast | 309 | 75 | 152 | 74 | 157 | 77 | ||||
| Hematologic | 30 | 7 | 16 | 8 | 14 | 7 | ||||
| Gynecologic | 19 | 5 | 11 | 5 | 8 | 4 | ||||
| Alimentary | 24 | 6 | 7 | 3 | 17 | 8 | ||||
| Other | 28 | 7 | 20 | 10 | 8 | 4 | ||||
| Cancer stage | χ2 | 2.83 | 5 | .726 | ||||||
| 0 | 21 | 5 | 11 | 5 | 10 | 5 | ||||
| I | 145 | 36 | 66 | 33 | 79 | 39 | ||||
| II | 137 | 34 | 71 | 35 | 66 | 33 | ||||
| III | 64 | 16 | 32 | 16 | 32 | 16 | ||||
| IV | 11 | 3 | 7 | 4 | 4 | 2 | ||||
| Unknown | 26 | 6 | 15 | 7 | 11 | 5 | ||||
| Previous treatment | ||||||||||
| Surgery | 364 | 91 | 183 | 90 | 181 | 91 | χ2 | 0.01 | 1 | .916 |
| Chemotherapy | 284 | 71 | 145 | 72 | 139 | 70 | χ2 | 0.10 | 1 | .752 |
| Radiation therapy | 266 | 66 | 137 | 67 | 129 | 65 | χ2 | 0.21 | 1 | .646 |
| Hormone treatment | 28 | 7 | 13 | 6 | 15 | 8 | χ2 | 0.06 | 1 | .813 |
| Current hormone therapy | 206 | 51 | 99 | 49 | 107 | 54 | χ2 | 0.06 | 1 | .816 |
| Time since first treatment for cancer, months | t test | 1.67 | 235 | .097 | ||||||
| Mean | 16.3 | 14.9 | 17.7 | |||||||
| SE | 0.85 | 0.50 | 1.61 | |||||||
| Karnofsky performance status | t test | 0.28 | 318 | .776 | ||||||
| Mean | 87.4 | 86.9 | 87.8 | |||||||
| SE | 1.61 | 2.31 | 2.24 | |||||||
| Exercise stage of change | χ2 | 0.92 | 4 | .922 | ||||||
| Not exercising and do not intend to begin in next 6 months | 18 | 4 | 8 | 4 | 10 | 5 | ||||
| Not exercising but intend to begin in the next 6 months | 81 | 20 | 43 | 21 | 38 | 19 | ||||
| Not exercising but intend to begin in the next 30 days | 95 | 24 | 45 | 22 | 50 | 25 | ||||
| Exercising and have been for less than 6 months* | 87 | 22 | 44 | 22 | 43 | 22 | ||||
| Exercising and have been for more than 6 months* | 120 | 30 | 62 | 31 | 58 | 29 | ||||
Abbreviations: N/A, not applicable; YOCAS, Yoga for Cancer Survivors.
Participants were excluded if they were practicing yoga within the 3 months prior to enrolling onto the study or planning to start yoga on their own during the time they were enrolled. Current participation in other types of exercise was permitted.
Attendance and Adherence
Attendance records showed that survivors assigned to the yoga arm attended an average of 6.5 of the 8 prescribed sessions (Fig 2). The average total dose of yoga for the entire 4-week intervention period was 480 minutes of the prescribed 600 minutes. Although not required, participants in the yoga condition were told that they could practice the yoga they learned in class on their own outside of class. On the basis of daily diaries, survivors in the yoga arm reported a total of three sessions combining class-based and home-based yoga each week for an average of 182 minutes with a perceived exertion rating of 3.4 (moderate). Exercise contamination in the control condition was minimal; seven participants reported an average of 20 minutes of yoga one time each week with a perceived exertion rating of 1.0 (very weak) during the intervention period.
Fig 2.
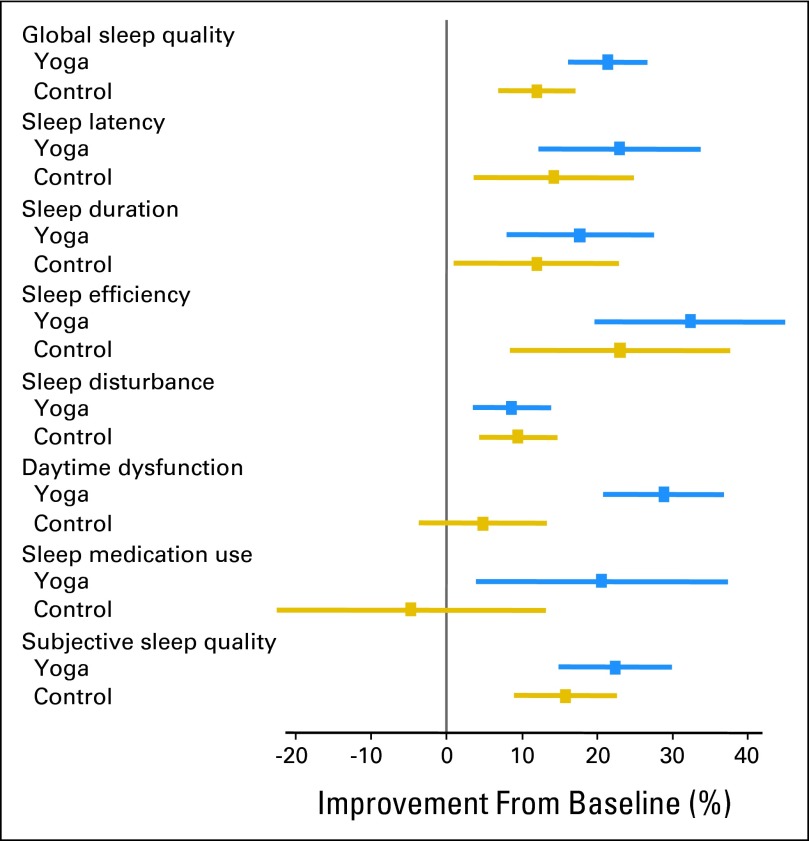
Percent improvement in global sleep quality and subscales from baseline to postintervention on the Pittsburgh Sleep Quality Index.
Patient-Reported Sleep Quality Variables From PSQI
Participants in the yoga condition demonstrated significantly greater improvements in the primary outcome of global sleep quality (P < .01) at postintervention compared with control participants. In addition, the yoga participants demonstrated significantly greater improvements in the characteristics that define global sleep quality (secondary outcomes) including daytime dysfunction (P < .01), subjective sleep quality (P < .05), and sleep medication use (P < .05) at postintervention compared with participants in the control condition (Table 3 and Fig 2).
Table 3.
Means, ORs, and SEs for PSQI and Actigraphy Data
| Outcome | YOCAS Group |
Control Group |
YOCAS-Control |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Preintervention |
Postintervention |
Within-Group Difference |
P | No. | Preintervention |
Postintervention |
Within-Group Difference |
P | Baseline Reference |
Between-Group Difference |
P‡ | |||||||||
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Minutes | % | Mean* or OR† | SE | ||||||
| PSQI | |||||||||||||||||||||
| Global sleep quality | 168 | 9.20 | 0.25 | 7.23 | 0.26 | −1.96 | 0.25 | < .001 | 153 | 8.96 | 0.28 | 7.89 | 0.26 | −1.07 | 0.23 | .005 | −0.79* | 0.30 | .009 | ||
| Sleep latency | 168 | 1.48 | 0.08 | 1.14 | 0.08 | −0.34 | 0.08 | .004 | 158 | 1.47 | 0.09 | 1.26 | 0.09 | −0.21 | 0.08 | .089 | 0.84† | 0.18 | .416 | ||
| Sleep duration | 168 | 1.05 | 0.06 | 0.86 | 0.06 | −0.19 | 0.05 | .020 | 158 | 1.01 | 0.06 | 0.89 | 0.06 | −0.12 | 0.06 | .182 | 0.93† | 0.21 | .737 | ||
| Sleep efficiency | 168 | 1.13 | 0.08 | 0.76 | 0.07 | −0.36 | 0.07 | .001 | 158 | 1.10 | 0.09 | 0.85 | 0.08 | −0.25 | 0.08 | .035 | 0.82† | 0.18 | .363 | ||
| Sleep disturbance | 168 | 1.66 | 0.05 | 1.51 | 0.05 | −0.14 | 0.04 | .031 | 157 | 1.75 | 0.05 | 1.59 | 0.05 | −0.17 | 0.05 | .012 | 0.84† | 0.21 | .484 | ||
| Daytime dysfunction | 168 | 1.30 | 0.05 | 0.93 | 0.05 | −0.38 | 0.05 | < .001 | 160 | 1.18 | 0.05 | 1.13 | 0.05 | −0.06 | 0.05 | .460 | 0.38† | 0.09 | < .001 | ||
| Sleep medication use | 168 | 1.01 | 0.10 | 0.80 | 0.10 | −0.21 | 0.09 | .137 | 160 | 0.81 | 0.10 | 0.84 | 0.10 | 0.04 | 0.07 | .788 | 0.56† | 0.16 | .046 | ||
| Subjective sleep quality | 168 | 1.57 | 0.05 | 1.22 | 0.05 | −0.35 | 0.06 | < .001 | 157 | 1.62 | 0.05 | 1.37 | 0.05 | −0.26 | 0.06 | .001 | 0.63† | 0.15 | .047 | ||
| Actigraphy | |||||||||||||||||||||
| Sleep onset latency | 157 | 30.49 | 2.72 | 28.56 | 2.18 | −1.94 | 2.17 | .579 | 147 | 34.5 | 2.94 | 31.59 | 3.12 | −2.90 | 2.64 | .499 | −7.05* | 2.98 | .813 | ||
| Wake after sleep onset§ | 157 | 63.05 | 2.02 | 61.17 | 1.75 | −1.88 | 1.46 | .482 | 147 | 66.16 | 2.17 | 66.45 | 2.42 | 0.29 | 1.43 | .929 | 66 | −3.33* | 2.10 | .078 | |
| 80 | −7.39* | 1.28 | < .001 | ||||||||||||||||||
| 100 | −13.18* | 1.52 | < .001 | ||||||||||||||||||
| 150 | −27.68* | 3.01 | < .001 | ||||||||||||||||||
| 200 | −42.17* | 4.72 | < .001 | ||||||||||||||||||
| Overall | < .001 | ||||||||||||||||||||
| Sleep efficiency§ | 157 | 76.65 | 0.86 | 77.01 | 0.66 | 0.36 | 0.74 | .736 | 147 | 76.00 | 0.71 | 76.26 | 0.74 | 0.26 | 0.61 | .804 | 20 | 14.80* | 2.99 | .002 | |
| 40 | 9.69* | 1.97 | .002 | ||||||||||||||||||
| 60 | 4.57* | 1.05 | .004 | ||||||||||||||||||
| 75 | 0.74* | 1.68 | .354 | ||||||||||||||||||
| Overall | .008 | ||||||||||||||||||||
NOTE. Mean differences are reported at different baseline values when a significant arm-baseline interaction is present (ie, actigraphy end points). Pittsburgh Sleep Quality Index (PSQI) values represent actual scores on the measure, and actigraphy data are presented in minutes for sleep onset latency and wake after sleep onset, and as a percent for sleep efficiency. Within-group differences are expressed as mean difference for PSQI and actigraphy data. Between-group differences are expressed as mean differences for the global sleep quality PSQI score and actigraphy and as odds ratios (ORs) for the PSQI subscales with controls as the reference.
Abbreviation: YOCAS, Yoga for Cancer Survivors.
Mean values were obtained from analysis of covariance models.
ORs were obtained from ordinal logistic regression models.
Indicates a simultaneous test of the arm main effect and the arm-baseline interaction (ie, overall test of arm).
Indicates a significant arm-baseline interaction.
Participants in the yoga condition demonstrated significant improvements in sleep quality, including global sleep quality (P < .01), sleep latency (P < .01), sleep duration (P < .05), sleep efficiency (P < .01), sleep disturbances (P < .05), subjective sleep quality (P < .01), and daytime dysfunction (P < .01), but not sleep medication use over the 4-week intervention period. Participants in the standard care condition also demonstrated significant improvements in global sleep quality (P < .01), sleep efficiency (P < .05), sleep disturbance (P ≤ .01), and subjective sleep quality (P < .01), but not in sleep latency, sleep duration, daytime dysfunction, or sleep medication use.
Clinical Significance and Medication Use
Participants in both groups demonstrated average baseline global sleep quality scores of 9.0 (above the accepted clinical criterion of ≥ 8.0) on the PSQI, indicating clinically impaired sleep quality. Yoga participants exhibited large improvements in sleep quality (d = 0.62) from pre- to postintervention, suggesting a clinically meaningful improvement,54–57 although the control group did not (d = 0.37). In addition, participants in the yoga group reduced their sleep medication use by 21% per week, but participants in the control condition increased their sleep medication use by 5% per week resulting in a significant difference in medication use between groups at postintervention. Ninety percent of cancer survivors found yoga useful for improving their sleep quality, and 100% would recommend yoga to other cancer survivors experiencing sleep problems with 63% highly recommending it, further supporting clinically meaningful improvements.
Objective Sleep Quality Variables From Actigraphy
Yoga participants showed significantly greater improvements in wake after sleep onset (P < .01) and sleep efficiency (P < .01) at postintervention compared with control participants. Interactions showed that participants in the yoga group who demonstrated 60 minutes or more of wakefulness after sleep onset or a sleep efficiency of ≤ 60% at baseline derived the greatest reductions in wake after sleep onset and the greatest improvements in sleep efficiency.
Adverse Events
One patient had a serious adverse event—supraventricular tachycardia, considered grade 2 and unrelated to the study intervention—during the study period. No other serious adverse events were reported.
DISCUSSION
This RCT demonstrates that the YOCAS program is a useful therapy for post-treatment cancer survivors with impaired sleep. Yoga participants demonstrated significantly greater improvements in global sleep quality and, secondarily, subjective sleep quality, daytime dysfunction, wake after sleep onset, sleep efficiency, and medication use at postintervention compared with standard care participants. Although both groups showed significant improvements in global sleep quality, sleep efficiency, sleep disturbances, and subjective sleep quality, improvements were greater in the yoga participants, and only the yoga participants also showed significant improvements in sleep latency, sleep duration, and daytime dysfunction. Furthermore, participants in the yoga group decreased sleep medication use by 21%, while the standard care group increased sleep medication use by 5% resulting in significant differences between the groups at postintervention. These results suggest that the improvements in global sleep quality experienced by yoga participants may be related to reductions in daytime dysfunction characterized by less daytime napping and lower fatigue ultimately resulting in better sleep continuity, while the improvements in global sleep quality in the control group may be due, at least in part, to continued use of sleep medication. Importantly, our objective actigraphy results (although changes were small because of high variability) corroborate the PSQI patient-reported outcomes, which demonstrate improvements in sleep quality stemming from yoga participation. These results are generalizable to post-treatment cancer survivors receiving follow-up care in US CCOPs.
Results from this first nationwide, multicenter, phase III RCT comparing yoga to standard care for improving sleep quality confirm previous findings38,40–49 that suggest yoga is efficacious for improving sleep quality among cancer survivors. Results extend previous findings by integrating Gentle Hatha and Restorative yoga postures in a standardized yoga program and demonstrating the ability to effectively disseminate and administer a standardized yoga intervention in a variety of community settings across the United States to successfully treat impaired sleep quality among cancer survivors.
These results also provide important information regarding potentially meaningful clinical thresholds for screening patients and making treatment recommendations. Specifically, we found that even patients who self-report mild to moderate global sleep quality impairment report sleep benefits when completing the YOCAS program. In addition, patients who objectively demonstrate more than 1 hour of wakefulness in the middle of the night, very poor sleep efficiency (60% or lower), or some combination of these characteristics derive the greatest benefits from participation in the YOCAS program, specifically, improved sleep with reduced use of medication.
Despite its positive results, this study has limitations. The study did not control for specific components such as time or attention because this would have required the use of a placebo yoga intervention for the YOCAS program; however, no validated approach for placebo-controlled yoga interventions exists. The results are not generalizable to all types of yoga. Of the participants who expressed interest and enrolled onto the study, the majority were female, white, married, well-educated breast cancer survivors, limiting generalizability. Although this is typical of studies conducted through the NCI CCOP Network, it suggests that focused attention is needed to find ways to increase under-represented cancer survivors' interest in yoga to improve their sleep quality. The lack of a triple-blind study design was dealt with, in part, by including objective actigraphy assessments and blinding the study principal investigator and biostatistician throughout the primary analyses. There were no long-term follow-ups to assess sustainability of benefits. Lastly, 22% of enrolled participants were lost to follow-up and/or did not provide fully evaluable data. Although there were no significant differences between completers and noncompleters on demographic characteristics, future trials need to look for ways to improve retention.
In conclusion, our findings indicate that yoga, specifically the Gentle Hatha and Restorative yoga components in the YOCAS program, improves sleep quality among post-treatment cancer survivors. Further phase III studies are needed that replicate these findings, increase the length and intensity of yoga to increase the magnitude of sleep benefits, conduct long-term follow-up assessments to determine the sustainability of sleep benefits, compare yoga to established effective treatments for sleep (eg, cognitive behavioral therapy and pharmaceuticals), compare yoga with appropriate placebos to understand the contributions of the individual mind-body components, and investigate the biopsychosocial mechanisms through which yoga improves sleep quality. This information will help identify the optimal dose of yoga for sleep problems. Additional research also should examine the impact of yoga on cancer recurrence and survival rates.
Supplementary Material
Acknowledgment
Presented as featured podium presentations at the 46th Annual Meeting of the American Society of Clinical Oncology (ASCO; Best of ASCO), Chicago, IL, June 4-8, 2010; 32nd Annual Meeting of the Society of Behavioral Medicine (Complimentary and Integrative Medicine Research Award), Washington, DC, April 27-30, 2011; Multinational Association of Supportive Care in Cancer International Symposium, Athens, Greece, June 23-25, 2011; and 58th Annual Meeting of the American College of Sports Medicine, Denver, CO, May 31-June 4, 2011.
We thank Susan Rosenthal, MD, for her support on this project. We extend special thanks to the cancer survivors, yoga instructors, and research staff in the University of Rochester Community Clinical Oncology Program (CCOP) Research Base and at each of the National Cancer Institute (NCI) CCOP affiliates who recruited and followed participants in this study. We thank the NCI and the Office of Cancer Complementary and Alternative Medicine for their funding support of this project.
Appendix
Study Measures
Pittsburgh Sleep Quality Index.
The primary measure of sleep quality was the Pittsburgh Sleep Quality Index (PSQI), a psychometrically validated, patient-reported, 19-item instrument.51 The PSQI instrument provides a global sleep quality score with additional subscale scores for specific characteristics of global sleep quality, including sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, daytime dysfunction, sleep medication use, and subjective sleep quality. The global sleep quality score was the primary outcome and the subscale scores were secondary end points. Global sleep quality scores ≥ 5 among healthy adults and among eight or more patients with cancer are reliable clinical cutoffs indicating poor sleep quality (Buysse DJ, et al: Psychiatry Res 28:193-213, 1989; Carpenter JS and Andrykowski MA: J Psychosom Res 45:5-13, 1998)51 Regression analyses indicate that our screening criterion of > 3 corresponds to a PSQI global sleep quality score of 6, and actual baseline PSQI scores averaged 9.0 to 9.2, exceeding the validated cutoff criterion for clinically impaired sleep among cancer survivors.
Actigraphy.
Actigraphy was a secondary objective measure of characteristics of global sleep quality, including sleep onset latency, wake after sleep onset, and sleep efficiency. Actigraphy is a validated, cost-effective method for assessing sleep and its components; it is accurate to within ± 10% of polysomnography, the gold standard (Ancoli-Israel S, et al: Sleep 26:342-392, 2003; Berger AM, et al: J Pain Symptom Manage 36:191-199, 2008; de Souza L, et al: Sleep 26:81-85, 2003).5 Actigraphy collects physical activity data in all three planes of motion and aggregates these data into single activity counts which are summed over specific time intervals; these data are then used to estimate components of sleep. The Actiwatch 64 (Mini Mitter: A Respironics Company, Bend, OR) was used to assess sleep efficiency, sleep latency, and wake after sleep onset.
Statistical Procedures
Intent-to-treat and missing data analyses.
According to Piantadosi's Clinical Trial Text (Piantadosi S: Clinical Trials: A Methodologic Perspective (ed 2). Hoboken, NJ, Wiley, 2005) and Fisher et al (Fisher LD, et al: Intention to treat in clinical trials, in Peace KE (ed): Statistical Issues in Drug Research and Development. New York, NY, Marcel Dekker, 1990, pp 331-350) the concept of intent-to-treat (ITT) includes three related but distinct criteria—inclusion of all randomly assigned patients in the groups to which they were randomly assigned regardless of their adherence with the entry criteria, regardless of the treatment they actually received, and regardless of subsequent withdrawal from treatment or deviation from the protocol. As stated in the article, we used ITT analyses and included all study participants in the arms to which they were randomly assigned, regardless of adherence to entry criteria, the treatment they actually received, and whether they withdrew from the study. For example, we did not drop or (re)move participants in the control arm who actually started a yoga program on their own or in the yoga arm who did not participate in the yoga intervention at all. It is often thought that in order to properly comply with all of these ITT principles, any missing data must always be imputed and reported. However, it is appropriate to impute missing data and report it only when that missing data induces bias into the results through its missingness. Thus, because we had participants who did not provide all of the requested data (some who withdrew and some who did not withdraw but refused to answer specific questions), we have to consider our missing data and make statistically appropriate decisions about whether or not to use the actual real data or to do imputations and use the estimated data for patients with missing data in this study. To do this, we first examined our missing data for all outcomes to determine whether it was missing completely at random (MCAR). Using Little's MCAR test (Little RJ: J Am Stat Assoc 83:1198-1202, 1988), there is no evidence that the data are not MCAR (P = .93). Therefore, there is no expected bias from these missing data when reporting a complete case analysis. Despite this, we also did multiple imputations and conducted sensitivity analyses. We used multiple imputation (SAS PROC MI: MCMC, Multiple Cains, EM Posterior Mode, Jeffrey's Prior, 100 imputations) to generate 100 complete data sets, analyzed each data set, and combined the results (PROC MIANALYZE). The reported differences between the sensitivity results of the imputation versus the complete case analyses were minor. For example, the results in Table A1 were obtained for the PSQI Global Sleep Quality Score (the primary outcome).
Because the data were determined to be MCAR and the multiple imputation sensitivity analyses do not indicate a bias in reporting complete data or change the nature of the reported results, we report the actual real data for complete cases instead of estimated data using multiple imputations because this is the most appropriate and accurate method of representing the real data from this clinical trial.
Table A1.
Comparison of Complete Cases Versus Imputed Cases
| Parameter | Complete Case Analysis |
Multiple Imputation |
||||||
|---|---|---|---|---|---|---|---|---|
| Estimate | SE | T | Pr > T | Estimate | SE | T | Pr > T | |
| Intercept | 1.98 | 0.46 | 4.30 | < 0.001 | 2.73 | 0.45 | 6.13 | < 0.001 |
| PSQI baseline | 0.57 | 0.04 | 12.82 | < 0.001 | 0.58 | 0.04 | 13.12 | < 0.001 |
| Yoga-control | −0.79 | 0.30 | 2.65 | 0.0085 | −0.83 | 0.29 | −2.81 | 0.0053 |
Abbreviations: Pr > T, alpha score; PSQI, Pittsburgh Sleep Quality Index; T, t test score.
Footnotes
Supported by Grant No. U10CA037420 from the National Cancer Institute (NCI) with supplemental funding from the Office of Cancer Complementary and Alternative Medicine, and Grants No. K07CA120025, R25CA102618, and 5K07CA132916 from NCI.
The Yoga for Cancer Survivors (YOCAS) program cannot be used without permission.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00397930.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Karen M. Mustian, Gary R. Morrow
Financial support: Karen M. Mustian
Provision of study materials or patients: Karen M. Mustian, Pavan S. Reddy, Marianne K. Melnik
Collection and assembly of data: Karen M. Mustian, Pavan S. Reddy, Marianne K. Melnik
Data analysis and interpretation: Karen M. Mustian, Lisa K. Sprod, Oxana G. Palesh, Michelle Janelsins, Luke J. Peppone, Kavita Chandwani, Charles Heckler, Gary R. Morrow
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Berger AM, Farr LA, Kuhn BR, et al. Values of sleep/wake, activity/rest, circadian rhythms, and fatigue prior to adjuvant breast cancer chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savard J, Simard S, Blanchet J, et al. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 4.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger AM, Grem JL, Visovsky C, et al. Fatigue and other variables during adjuvant chemotherapy for colon and rectal cancer. Oncol Nurs Forum. 2010;37:E359–E369. doi: 10.1188/10.ONF.E359-E369. [DOI] [PubMed] [Google Scholar]
- 6.Palesh OG, Mustian KM, Peppone LJ, et al. Impact of paroxetine on sleep problems in 426 cancer patients receiving chemotherapy: A trial from the University of Rochester Cancer Center Community Clinical Oncology Program. Sleep Med. 2012;13:1184–1190. doi: 10.1016/j.sleep.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schultz PN, Klein MJ, Beck ML, et al. Breast cancer: Relationship between menopausal symptoms, physiologic health effects of cancer treatment and physical constraints on quality of life in long-term survivors. J Clin Nurs. 2005;14:204–211. doi: 10.1111/j.1365-2702.2004.01030.x. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864–5866. doi: 10.1200/JCO.2009.24.5993. [DOI] [PubMed] [Google Scholar]
- 9.Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J Clin Oncol. 2011;29:3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 10.Lehrer S, Green S, Ramanathan L, et al. Obesity and deranged sleep are independently associated with increased cancer mortality in 50 US states and the District of Columbia. Sleep Breath. doi: 10.1007/s11325-013-0811-x. [epub ahead of print on February 7, 2013] [DOI] [PubMed] [Google Scholar]
- 11.Miller YE, Karoor V, Dempsey EC, et al. Sleep-disordered breathing, hypoxemia, and cancer mortality. Am J Respir Crit Care Med. 2013;187:330–331. doi: 10.1164/ajrccm.187.3.330. [DOI] [PubMed] [Google Scholar]
- 12.Østhus AA, Aarstad AK, Olofsson J, et al. Prediction of survival by pretreatment health-related quality-of-life scores in a prospective cohort of patients with head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. 2013;139:14–20. doi: 10.1001/jamaoto.2013.1056. [DOI] [PubMed] [Google Scholar]
- 13.Omachi TA, Blanc PD, Claman DM, et al. Disturbed sleep among COPD patients is longitudinally associated with mortality and adverse COPD outcomes. Sleep Med. 2012;13:476–483. doi: 10.1016/j.sleep.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levine M. ACP Journal Club: Hypnotic drugs were associated with increased risk for mortality. Ann Intern Med. 2012;156:JC6–JC13. doi: 10.7326/0003-4819-156-12-201206190-02013. [DOI] [PubMed] [Google Scholar]
- 15.Vgontzas AN, Fernandez-Mendoza J, Liao D, et al. Insomnia with objective short sleep duration: The most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10(suppl 1):S7–S11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Ancoli-Israel S, Kripke DF. Prevalent sleep problems in the aged. Biofeedback Self Regul. 1991;16:349–359. doi: 10.1007/BF00999989. [DOI] [PubMed] [Google Scholar]
- 18.Ancoli-Israel S, Martin JL. Insomnia and daytime napping in older adults. J Clin Sleep Med. 2006;2:333–342. [PubMed] [Google Scholar]
- 19.Savard J, Morin CM. Insomnia in the context of cancer: A review of a neglected problem. J Clin Oncol. 2001;19:895–908. doi: 10.1200/JCO.2001.19.3.895. [DOI] [PubMed] [Google Scholar]
- 20.Schutte-Rodin S, Broch L, Buysse D, et al. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: A systematic review and meta-analysis. Psychother Psychosom. 2012;81:206–216. doi: 10.1159/000335379. [DOI] [PubMed] [Google Scholar]
- 22.Belleville G, Cousineau H, Levrier K, et al. Meta-analytic review of the impact of cognitive-behavior therapy for insomnia on concomitant anxiety. Clin Psychol Rev. 2011;31:638–652. doi: 10.1016/j.cpr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Thomasouli MA, Brady EM, Davies MJ, et al. The impact of diet and lifestyle management strategies for obstructive sleep apnoea in adults: A systematic review and meta-analysis of randomised controlled trials. Sleep Breath. doi: 10.1007/s11325-013-0806-7. [epub ahead of print on January 30, 2013] [DOI] [PubMed] [Google Scholar]
- 24.Yang PY, Ho KH, Chen HC, et al. Exercise training improves sleep quality in middle-aged and older adults with sleep problems: A systematic review. J Physiother. 2012;58:157–163. doi: 10.1016/S1836-9553(12)70106-6. [DOI] [PubMed] [Google Scholar]
- 25.Youngstedt SD, O'Connor PJ, Dishman RK. The effects of acute exercise on sleep: A quantitative synthesis. Sleep. 1997;20:203–214. doi: 10.1093/sleep/20.3.203. [DOI] [PubMed] [Google Scholar]
- 26.Kubitz KA, Landers DM, Petruzzello SJ, et al. The effects of acute and chronic exercise on sleep: A meta-analytic review. Sports Med. 1996;21:277–291. doi: 10.2165/00007256-199621040-00004. [DOI] [PubMed] [Google Scholar]
- 27.Mustian KM, Katula JA, Gill DL. Exercise: Complementary therapy for breast cancer rehabilitation. Women & Therapy. 2002;25:105–118. [Google Scholar]
- 28.Mustian KM, Griggs JJ, Morrow GR, et al. Exercise and side effects among 749 patients during and after treatment for cancer: A University of Rochester Cancer Center Community Clinical Oncology Program Study. Support Care Cancer. 2006;14:732–741. doi: 10.1007/s00520-005-0912-6. [DOI] [PubMed] [Google Scholar]
- 29.Mustian KM, Sprod LK, Janelsins M, et al. Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: A review. Oncol Hematol Rev. 2012;8:81–88. doi: 10.17925/ohr.2012.08.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bower JE, Woolery A, Sternlieb B, et al. Yoga for cancer patients and survivors. Cancer Control. 2005;12:165–171. doi: 10.1177/107327480501200304. [DOI] [PubMed] [Google Scholar]
- 31.Elkins G, Fisher W, Johnson A. Mind-body therapies in integrative oncology. Curr Treat Options Oncol. 2010;11:128–140. doi: 10.1007/s11864-010-0129-x. [DOI] [PubMed] [Google Scholar]
- 32.Mustian KM, Morrow GR, Carroll JK, et al. Integrative nonpharmacologic behavioral interventions for the management of cancer-related fatigue. Oncologist. 2007;12:52–67. doi: 10.1634/theoncologist.12-S1-52. [DOI] [PubMed] [Google Scholar]
- 33.Mustian KM, Sprod LK, Palesh OG, et al. Exercise for the management of side effects and quality of life among cancer survivors. Curr Sports Med Rep. 2009;8:325–330. doi: 10.1249/JSR.0b013e3181c22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: Results of a national survey. Altern Ther Health Med. 2004;10:44–49. [PubMed] [Google Scholar]
- 35.Smith KB, Pukall CF. An evidence-based review of yoga as a complementary intervention for patients with cancer. Psychooncology. 2009;18:465–475. doi: 10.1002/pon.1411. [DOI] [PubMed] [Google Scholar]
- 36.Muktibodhananda S. Hatha Yoga Pradipika. Poughkeepsie, NY: Nesma Books India; 2000. [Google Scholar]
- 37.Lasater J. Relax and Renew Restful Yoga for Stressful Times. Berkeley, CA: Publishers Group West; 1995. [Google Scholar]
- 38.Danhauer SC, Mihalko SL, Russell GB, et al. Restorative yoga for women with breast cancer: Findings from a randomized pilot study. Psychooncology. 2009;18:360–368. doi: 10.1002/pon.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danhauer SC, Tooze JA, Farmer DF, et al. Restorative yoga for women with ovarian or breast cancer: Findings from a pilot study. J Soc Integr Oncol. 2008;6:47–58. [PubMed] [Google Scholar]
- 40.Duncan MD, Leis A, Taylor-Brown JW. Impact and outcomes of an Iyengar yoga program in a cancer centre. Curr Oncol. 2008;15(suppl 2):s109. doi: 10.3747/co.v15i0.284. es72-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joseph CD. Psychological supportive therapy for cancer patients. Indian J Cancer. 1983;20:268–270. [PubMed] [Google Scholar]
- 42.Rosenbaum E, Gautier H, Fobair P, et al. Cancer supportive care, improving the quality of life for cancer patients. A program evaluation report. Support Care Cancer. 2004;12:293–301. doi: 10.1007/s00520-004-0599-0. [DOI] [PubMed] [Google Scholar]
- 43.Speed-Andrews AE, Stevinson C, Belanger LJ, et al. Pilot evaluation of Iyengar yoga program for breast cancer survivors. Cancer Nurs. 2010;33:369–381. doi: 10.1097/NCC.0b013e3181cfb55a. [DOI] [PubMed] [Google Scholar]
- 44.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer. 2012;118:3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carson JW, Carson KM, Porter LS, et al. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: Results from a randomized trial. Support Care Cancer. 2009;17:1301–1309. doi: 10.1007/s00520-009-0587-5. [DOI] [PubMed] [Google Scholar]
- 46.Chandwani KD, Thornton B, Perkins GH, et al. Yoga improves quality of life and benefit finding in women undergoing radiotherapy for breast cancer. J Soc Integr Oncol. 2010;8:43–55. [PubMed] [Google Scholar]
- 47.Cohen L, Warneke C, Fouladi RT, et al. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100:2253–2260. doi: 10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 48.Ulger O, Yağli NV. Effects of yoga on the quality of life in cancer patients. Complement Ther Clin Pract. 2010;16:60–63. doi: 10.1016/j.ctcp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Vadiraja SH, Rao MR, Nagendra RH, et al. Effects of yoga on symptom management in breast cancer patients: A randomized controlled trial. Int J Yoga. 2009;2:73–79. doi: 10.4103/0973-6131.60048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. Baltimore, MD: Lippincott, Williams, & Wilkins; 2010. [Google Scholar]
- 51.Beck SL, Schwartz AL, Towsley G, et al. Psychometric evaluation of the Pittsburgh Sleep Quality Index in cancer patients. J Pain Symptom Manage. 2004;27:140–148. doi: 10.1016/j.jpainsymman.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Hope AC. A simplified Monte Carlo significance test procedure. J R Stat Soc. 1968;B:582–598. [Google Scholar]
- 53.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- 54.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 55.Sloan JA. Assessing the minimally clinically significant difference: Scientific considerations, challenges and solutions. COPD. 2005;2:57–62. doi: 10.1081/copd-200053374. [DOI] [PubMed] [Google Scholar]
- 56.Jacobson NS, Truax P. Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 57.Cella D, Hahn EA, Dineen K. Meaningful change in cancer-specific quality of life scores: Differences between improvement and worsening. Qual Life Res. 2002;11:207–221. doi: 10.1023/a:1015276414526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.