Abstract
Purpose
This study sought to characterize transformation incidence and outcome for patients with follicular lymphoma (FL) in a prospective observational series begun after diffusion of rituximab use.
Patients and Methods
Patients with newly diagnosed FL were prospectively enrolled onto the University of Iowa/Mayo Clinic Specialized Program of Research Excellence Molecular Epidemiology Resource from 2002 to 2009. Patients were actively followed for re-treatment, clinical or pathologic transformation, and death. Risk of transformation was analyzed via time to transformation by using death as a competing risk.
Results
In all, there were 631 patients with newly diagnosed grade 1 to 3a FL who had a median age at enrollment of 60 years. At a median follow-up of 60 months (range, 11 to 110 months), 79 patients had died, and 60 patients developed transformed lymphoma, of which 51 were biopsy proven. The overall transformation rate at 5 years was 10.7%, with an estimated rate of 2% per year. Increased lactate dehydrogenase was associated with increased risk of transformation. Transformation rate at 5 years was highest in patients who were initially observed and lowest in patients who initially received rituximab monotherapy (14.4% v 3.2%; P = .021). Median overall survival following transformation was 50 months and was superior in patients with transformation greater than 18 months after FL diagnosis compared with patients with earlier transformation (5-year overall survival, 66% v 22%; P < .001).
Conclusion
Follicular transformation rates in the immunochemotherapy era are similar to risk of death without transformation and may be lower than reported in older series. Post-transformation prognosis is substantially better than described in older series. Initial management strategies may influence the risk of transformation.
INTRODUCTION
Follicular lymphoma (FL) is an incurable disease without a defined optimal management strategy. Priorities in goals of care include avoiding symptoms, transformation to aggressive subtypes, and death. Rituximab has changed the expected outcomes for patients with FL.1–5 Retrospective series that include patients diagnosed before use of rituximab became prevalent describe diverse rates of transformation with consensus of 3% per year.6–12 Transformation is largely considered a catastrophic event on the basis of these historical series with a median post-transformation survival of less than 2 years.6,7,9,11–13
There are several reasons why observations from older series on transformation may no longer predict the clinical course of current patients. Diagnostic techniques for lymphoma are subtly but perhaps meaningfully different with the availability of technology-aided but smaller biopsy samples, and greater availability of deeper samples at time of relapse.14–16 Initial treatment strategies for FL have changed substantially over 20 years with the availability of nucleoside analogs, increasing use of alkylator-based over anthracycline-including regimens as initial therapy, and monoclonal antibody as part of early treatment choices.17 Indeed, one recent series of patients diagnosed from 1979 to 2007 found a significantly higher risk of transformation in patients diagnosed before 1990.18 Similarly, treatment options for management of transformed lymphoma have expanded over this time frame.
We initiated a prospective observational study in 2002 that enrolled patients with newly diagnosed lymphoma, and we used a protocol-specified methodology for capturing baseline clinical, laboratory, and pathology data, initial therapy, and active follow-up of all patients for clinical events including re-treatments, relapse/progression, transformation, and death, regardless of where the follow-up clinical care occurred. The goal of this analysis was to characterize the rate of transformation in the current era of using immunochemotherapy in early treatment of FL. The clinical features associated with risk of transformation and the risk of transformation in the context of the competing risk of death without transformation were evaluated along with post-transformation outcomes.
PATIENTS AND METHODS
Study Population
This study was approved by institutional review boards at the University of Iowa and Mayo Clinic. Written informed consent was obtained from all participants. This study used the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence, which has been previously reported.19 Briefly, since September 2002, we offered enrollment to consecutive patients with newly diagnosed lymphoma (within 9 months) who were evaluated at the University of Iowa or Mayo Clinic Rochester, were age 18 years or older, had no history of HIV, and were residents of the United States. All diagnoses were confirmed by study hematopathologists (W.R.M., S.I.S.). Baseline clinical, laboratory, and treatment data were abstracted from medical records by using a standard protocol. All participants were systematically contacted every 6 months for the first 3 years and then annually thereafter. Disease progression, re-treatment, transformation, and death were verified through review of pathology and medical records. Cause of death was obtained from death certificates and review of medical records. Inclusion criteria for this analysis were initial diagnosis of grade 1 to 3a FL and enrollment from September 1, 2002, to December 31, 2009. Patients with a composite diagnosis, FL grade 3b, or evidence of clinical or pathologic transformation at the time of initial FL diagnosis were excluded. Patients with de novo diffuse large B-cell lymphoma (DLBCL) enrolled from the same time period were included as a comparison cohort for clinical outcome after transformation.
Definition of Transformation
Transformation was defined as refractory/recurrent disease with either clinical or pathologic diagnosis of transformed lymphoma. Medical records and clinical pathology reports were reviewed to verify all transformations. Pathologically defined transformation entailed a biopsy confirmed subtype of FL 3b, DLBCL, unclassifiable B-cell lymphoma with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma, high-grade B-cell lymphoma (including Burkitt), or evidence of transformation per pathologist report.8,11 We did not establish clonality on the basis of molecular studies. Clinical transformation was defined by using a previously published clinical indication of transformation (sudden increase in lactate dehydrogenase [LDH], rapid discordant localized nodal growth, new involvement of unusual extranodal sites, new “B” symptoms or hypercalcemia), or statement in the medical record that the treating physician was clinically treating the patient as a transformation at the time of recurrence.7,8,20
Statistical Analysis
The cumulative incidence of transformation was determined by using death as a competing risk.21,22 Time to transformation was defined as the date of initial FL diagnosis to date of transformation.
Incidence of transformation, as well as differences in transformation incidence by initial treatment, was estimated and displayed graphically by using the cmprsk package in R.23,24 Stratified Cox proportional hazards models as described by Therneau and Grambsch25 were used to assess associations between clinical variables and transformation.
Overall survival (OS) from diagnosis was defined as the date of diagnosis to date of death (any cause) or last known follow-up for patients still alive. OS from transformation was defined as the date of transformation to date of death (any cause) or last known follow-up for patients still alive. In exploratory analyses, we evaluated early versus late transformations by using an a priori defined cut point of 18 months.
RESULTS
Patient Characteristics
In all, 631 patients with newly diagnosed grade 1 to 3a FL who met the study eligibility criteria were enrolled onto the MER from 2002 to 2009. The median age at enrollment was 60 years (range, 23 to 93 years), and 54% were male (Table 1). The most common types of initial therapy were observation (33%), rituximab monotherapy (12%), alkylator-based chemotherapy with or without rituximab (22%), and anthracycline-based chemotherapy with or without rituximab (20%). At a median follow-up of 60 months (range, 11 to 110 months), 79 patients died, 311 patients had an event (death, progression, or re-treatment), and 60 patients (9.5%) developed transformed lymphoma (31 as a first event, 29 as a second or later event).
Table 1.
Patient Characteristics (N = 631)
| Characteristic | No. | % |
|---|---|---|
| Age, years | ||
| Median | 60 | |
| Range | 23-93 | |
| > 60 | 296 | 46.9 |
| Male | 338 | 53.6 |
| Performance status ≥ 2 | 29 | 4.6 |
| Ann Arbor stage III/IV | 428 | 68.8 |
| Two or more extranodal sites | 23 | 3.7 |
| LDH | ||
| Median | 177 | |
| Range | 57-1,273 | |
| > ULN | 118 | 21.1 |
| B symptoms (any) | 46 | 7.3 |
| Bone marrow involvement | 234 | 42.1 |
| Hemoglobin, g/dL | ||
| Median | 13.9 | |
| Range | 5.9-18.6 | |
| < 12 | 60 | 10.1 |
| Grade | ||
| 1-2 | 535 | 84.8 |
| 3a | 96 | 15.2 |
| More than four nodal groups | 210 | 33.8 |
| FLIPI | ||
| 0-1 | 264 | 41.8 |
| 2 | 217 | 34.4 |
| 3-5 | 150 | 23.8 |
| IPI | ||
| 0-1 | 361 | 57.2 |
| 2 | 205 | 32.5 |
| 3 | 55 | 8.7 |
| 4-5 | 10 | 1.6 |
| Initial therapy | ||
| Observation | 208 | 33.0 |
| Rituximab monotherapy | 78 | 12.4 |
| Alkylator-based chemotherapy ± rituximab | 137 | 21.7 |
| Anthracycline-based chemotherapy ± rituximab | 127 | 20.1 |
| Radiation therapy only | 49 | 7.8 |
| Other treatment | 32 | 5.1 |
Abbreviations: FLIPI, Follicular Lymphoma International Prognostic Index; IPI, International Prognostic Index; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Risk of Transformation
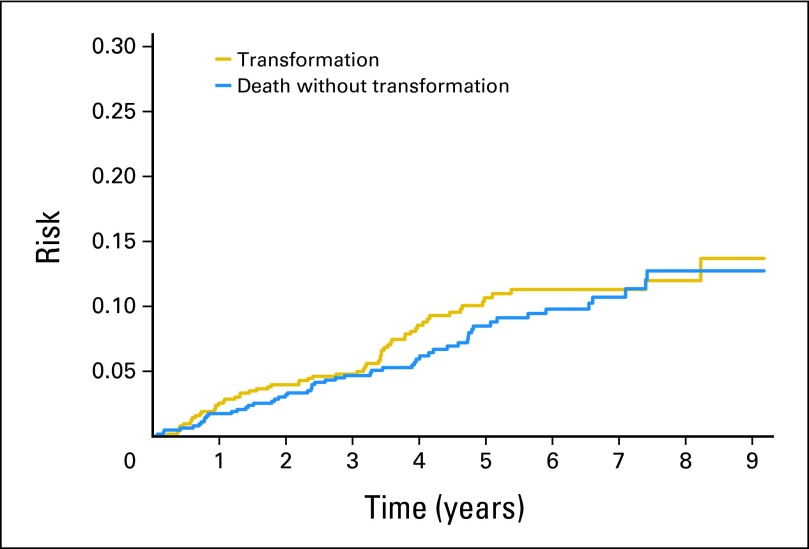
The cumulative risk of transformation and death without transformation (competing risk) increased steadily over time up through 5 years of follow-up (Fig 1) and then appeared to slow, with only four transformations observed beyond 5 years from diagnosis (Fig 1). The overall transformation rate at 5 years (TR5) was 10.7% (95% CI, 8.3% to 13.8%), with an estimated rate of 2% per year over the first 5 years. Transformation was biopsy proven in 51 (85%) of the 60 patients. The lymphoma subtype at the time of transformation was DLBCL in 29 patients with other subtypes of large-cell lymphoma not otherwise specified (n = 17), FL grade 3b (n = 2), and high-grade B-cell lymphoma (n = 3). Criteria invoked to include the nine patients with clinical lymphoma transformation included new involvement of extranodal sites with biopsy too small to characterize histology beyond lymphoma (brain, 1; stomach, 1; bone, 1), rapid discordant localized nodal growth (with necrotic biopsy) with rising and abnormal LDH (n = 4), and rapid discordant growth with B symptoms (n = 1) or hypercalcemia (n = 1).
Fig 1.
Risk of transformation with death as a competing risk.
In univariate analysis (Table 2), the risk of transformation was associated with an increased serum LDH at diagnosis (hazard ratio [HR], 2.50; P = .0013) and inversely associated with hemoglobin less than 12 g/dL at diagnosis (HR, 0.86; P = .040). Increased age trended toward association (per 5-year HR, 1.10; P = .059). A Follicular Lymphoma International Prognostic Index (FLIPI) score ≥ 3 was also associated with increased risk of transformation (P = .0058); no significant associations with other standard clinical characteristics were evaluated.26 In a multivariable model containing the significant variables just mentioned, only increased serum LDH remained significant (P = .018).
Table 2.
Risk of Transformation in FL, Molecular Epidemiology Resource, 2002-2009
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age, years | |||
| Per 5-year increase | 1.10 | 1.00 to 1.21 | .059 |
| > 60 | 1.45 | 0.87 to 2.42 | .15 |
| Male | 0.85 | 0.51 to 1.41 | .52 |
| Performance status 2+ | 0.92 | 0.22 to 3.75 | .90 |
| Ann Arbor stage III to IV | 1.66 | 0.90 to 3.07 | .11 |
| Two or more extranodal sites | 1.89 | 0.59 to 6.05 | .28 |
| LDH | |||
| Continuous, log2 | 2.11 | 1.39 to 3.21 | < .001 |
| > ULN | 2.50 | 1.43 to 4.69 | .0013 |
| B symptoms | 1.48 | 0.64 to 3.45 | .36 |
| Bone marrow involvement | 1.33 | 0.78 to 2.30 | .30 |
| Hemoglobin, g/dL | |||
| Continuous, log2 | 0.33 | 0.11 to 1.03 | .056 |
| < 12 | 0.86 | 0.74 to 0.99 | .040 |
| Grade | |||
| 1-2 | 1.00 | (reference) | |
| 3a | 0.80 | 0.36 to 1.76 | .58 |
| More than four nodal areas | 1.22 | 0.72 to 2.06 | .47 |
| FLIPI | |||
| 0-1 | 1.00 | (reference) | |
| 2 | 1.31 | 0.69 to 2.48 | .41 |
| 3-5 | 2.38 | 1.28 to 4.40 | .0058 |
| IPI | |||
| 0-1 | 1.00 | (reference) | |
| 2 | 1.75 | 1.01 to 3.05 | .048 |
| 3 | 2.84 | 1.33 to 6.07 | .0072 |
| 4-5 | 2.71 | 0.37 to 20.1 | .33 |
| Initial therapy | |||
| Observation | 1.00 | (reference) | |
| Rituximab monotherapy | 0.19 | 0.04 to 0.78 | .021 |
| Alkylator-based chemotherapy ± rituximab | 0.67 | 0.34 to 1.32 | .24 |
| Anthracycline-based chemotherapy ± rituximab | 0.74 | 0.38 to 1.46 | .39 |
| Radiation therapy only | 0.61 | 0.21 to 1.74 | .35 |
| Other treatment | 0.50 | 0.12 to 2.08 | .34 |
Abbreviations: FL, follicular lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; HR, hazard ratio; IPI, International Prognostic Index; LDH, lactate dehydrogenase; ULN, upper limit of normal.
Transformation risk was higher with observation than with systemic treatment including rituximab, chemotherapy, or rituximab plus chemotherapy (HR, 1.75; 95% CI, 1.04 to 2.96; P = .036). Risk of transformation varied among the common initial treatment groups (Table 2), with the highest rate in patients who were initially observed (TR5, 14.4%) and lowest rate in patients who initially received rituximab monotherapy (TR5, 3.2%; P = .021 compared with observation; Fig 2). No other specific treatment group was associated with significantly lower transformation risk than observation. Follow-up for all treatment cohorts was the same, and results remained similar after adjustment for increased LDH at diagnosis.
Fig 2.
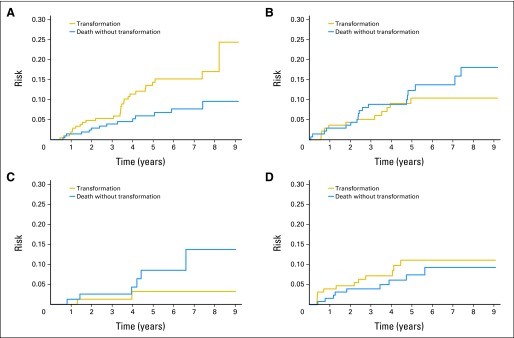
Risk of transformation by initial follicular lymphoma treatment. (A) Observation (n = 208); (B) alkylator (n = 137); (C) rituximab monotherapy (n = 78); (D) anthracycline (n = 127).
Outcome After Transformation
The median OS after transformation was 50 months (95% CI, 17 months to unreached) with 5-year OS (OS5) of 48% (95% CI, 35% to 68%; Fig 3A). There was no difference in survival after transformation between patients with clinical versus pathology- confirmed transformation (P = .39). Survival after transformation was inferior in patients who experienced transformation early (< 18 months) after FL diagnosis (OS5, 22%) compared with later transformation (≥ 18 months OS5, 76%; P < .001; Fig 3B).
Fig 3.
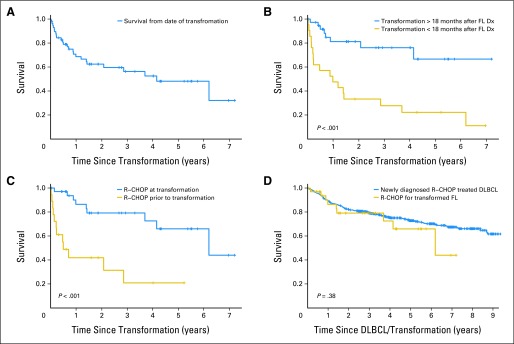
Overall survival after transformation. (A) All transformed patients; (B) comparing early versus late transformation; (C) comparing anthracycline-based immunochemotherapy pre- versus post-transformation; (D) comparing immunochemotherapy in post-transformation follicular lymphoma (FL) versus de novo diffuse large B-cell lymphoma (DLBCL). Dx, diagnosis; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone.
The most common treatment after transformation was R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone) or similar therapy (n = 35; 59%). Patients receiving R-CHOP at transformation had a superior outcome (OS5, 66%) compared with patients who received R-CHOP before transformation (n = 18; OS5, 21%; P < .001; Fig 3C). Notably, the OS of patients treated with R-CHOP following transformed FL was similar to MER patients treated with R-CHOP for de novo DLBCL (OS5, 73%; P = .38; Fig 3D).
Thirteen patients including eight (23%) of the 35 patients receiving R-CHOP after transformation underwent autologous stem-cell transplantation (ASCT) as part of therapy. An additional five patients who received R-CHOP before transformation underwent ASCT (four with rituximab-ifosfamide-carboplatin-etoposide induction). The addition of ASCT to R-CHOP was not significantly associated with improved OS (HR, 0.76; 95% CI, 0.16 TO 3.71), although numbers were small. Median OS was just 25 months in the five ASCT patients who received R-CHOP before transformation.
DISCUSSION
In this series describing risk of transformation for patients with FL initially diagnosed in the immunotherapy era for FL, the rate of transformation was 10.7% at 5 years, for an approximate rate of 2% per year. The risk increased through the first 5 years of follow-up, and then appeared to slow; continued follow-up and more patients will establish long-term rates of transformation and whether a plateau exists beyond 5 years. High-risk FLIPI score and increased LDH predicted transformation, and patients receiving rituximab monotherapy for initial treatment had a lower rate of transformation than patients who were observed. Finally, the OS5 rate of 50% following transformation was much better than rates in earlier series and, along with our reported outcomes following R-CHOP therapy, challenges the notion that transformation universally portends a poor prognosis.
Even in the setting of design differences, previous large series of patients diagnosed in the late twentieth century have been fairly consistent in describing clinical plus histologic transformation rates of 3% to 4% per year with 5-year rates of 17% to 21%.7,8,12 There are several possible reasons why the rates in our series are lower. The methodology of competing risk analysis could result in lower estimates; however, an estimate of transformation incidence at 5 years in our cohort was still low at 11.1% by using a Kaplan and Meier approach with patients censored at death, as in previous studies. Modern technology-aided diagnostic techniques may have allowed us to identify more patients at diagnosis who had components of both FL and DLBCL at presentation, thereby reducing the potential for having them characterized as transformed later in their course.14–16 Although the fraction of histologically confirmed transformations (85%) in this series is the highest reported among series that included patients with clinically defined transformation, we systematically applied the well-accepted clinical definition of transformation to all patients with FL with relapses, which should help minimize under-reporting of transformations. In addition, we are unlikely to have missed transformations in patients not followed at our institution since we have been in active contact with all patients, with complete loss to follow-up of 14 (0.3%) and withdrawal of 98 (1.8%) patients of 5,322 patients with lymphoma enrolled to date in the MER. Alternatively, it is possible that incorporation of immunotherapy into early management strategies has lowered the rate of transformation, as has been suggested by the British Columbia series.27 An early report from the National LymphoCare Study was also restricted to patients diagnosed from 2004 to 2007 and described a largely clinical transformation rate of 6% at 37 months (compared with our 4.8% at 36 months), and an update of the British Columbia Cancer Agency (BCCA) experience among immunochemotherapy-treated patients also found transformation rates of just 10% at 5 years.27,28 Other recent series with lower rates of transformation include a Swiss study of patients from 1979 to 2007 with a 5-year transformation rate of 13% but limited the definition of transformation to biopsy proven, and a BCCA report focusing on patients with limited presenting stage treated with radiotherapy with a 5-year risk of transformation of 9%.18,20 The observation that transformation rates and death without transformation at 5 years are similar at approximately 10% will be useful for calculating power in the design of future clinical trials of newly diagnosed FL.
We report provocative differences in the rates of transformation among various initial management strategies, but cautious interpretation is needed because of the uncontrolled nature of treatment selection and delivery. Patients with initial deferral of therapy had the highest rate of FL transformation (similar to the historical rate of 3% per year), although those treated with rituximab as monotherapy had the lowest rate. Yet these two groups were similar in clinical and disease characteristics at diagnosis. No previous large series has looked at transformation rates following rituximab monotherapy, and the only prospective clinical trial of rituximab versus observation at diagnosis did not include transformation events in the initial report.29 An older BCCA series noted lower transformation rates in patients treated on a phase II study with anthracyline combination chemotherapy than in a similar phase II cohort treated with alkylator and purine analog, but found no difference in aggregate between treated and untreated patients, as did a Stanford series.7,10 A St. Barts series of patients were largely treated with single-agent chlorambucil without a clear comparison group, and neither the St. Barts nor the French series had sufficient treatment-deferred patients to make valid statistical comparisons.8,12 Purine analog use has been implicated in increased risk of transformation in patients with chronic lymphatic leukemia.30 We did not observe different rates of transformation between patients treated with anthracycline versus those treated with alkylator-based combinations, and we could not make a meaningful observation on small numbers of purine analog–treated patients.
Perhaps our most unexpected finding was a median survival of nearly 5 years for patients after transformation, which is largely considered a devastating clinical event. The median post-transformation survival was 0.6 years for the Lyon series, 1.2 years for the St. Barts and Barcelona series, and 1.7 years for the BCCA and Stanford series.7–9,12,13 The Stanford authors noted that a subset of 22 patients with no prior chemotherapy exposure had the most favorable prognosis. The Swiss series, which included some patients enrolled after 2000, had a median survival of 2.7 years.18 Survival after transformation was also higher (44% 3-year OS) for the British Columbia patients who transformed after limited-stage FL, 85% of whom were chemotherapy-naive at transformation.20 Our observation of a median survival of nearly 5 years deserves close scrutiny, but extends the observations that anthracycline-naive patients do relatively well as do patients diagnosed in the era of immunotherapy.
Another novel observation was that transformation events more than 18 months after diagnosis have a substantially better outcome than early events. The 18-month cut point was arbitrarily chosen on the basis of clinical experience and will need to be replicated. One possible explanation for the differences in clinical behavior between early and late transformations is that undiagnosed composite histology at time of presentation is more common than previously appreciated, and early transformation events are simply the unmasking of underlying aggressive disease, perhaps enhanced by undertreatment of the unrecognized aggressive component. Although we excluded it from this analysis, we have observed a follicular component in 14% of patients enrolled onto the MER with DLBCL. Functional imaging with positron emission tomography with [18F]fluorodeoxyglucose (FDG-PET) scanning at presentation was done with insufficient frequency in our series to speculate on whether that technology would uncover subclinical composite histology, as proposed by other authors.31,32 Early versus late transformations may represent different biologic processes, which will require molecular characterization to further understand.
The role of anthracycline in treatment of transformation is strongly supported by the observation that previously anthracycline-naive patients who receive R-CHOP after transformation have outcomes essentially indistinguishable from patients with de novo DLBCL in our lymphoma MER. The identification of a cohort of transformed patients who do relatively well invites re-examination of the broad recommendation for considering ASCT following transformation.33,34 In our series, there is no clear signal that ASCT adds to the good outcome of previously anthracycline-naive patients or prevents poor outcome for those who transform after anthracycline use, although as in many series, the numbers of patients with transplantations in this series are too small to support firm conclusions.33,35–38 Radioimmunotherapy has been proposed as appropriate primary treatment or consolidation of response after transformation but was not evaluated in our series.39–42
Strengths of this study include the prospective cohort design of consecutively enrolled patients with newly diagnosed lymphoma; central pathology review; systematically collected clinical data; virtually complete follow-up of the cohort for disease progression, transformation, and death; and medical record validation of these events. Our series of more than 600 patients is the largest published to date, and all patients were managed in the current immunochemotherapy era. A comparison group of R-CHOP-treated patients with DLBCL was available from the same underlying population. The major limitations include the observational design and treatment and clinical follow-up based on routine practice without prescribed rebiopsy criteria. Our follow-up was modest (median of 60 months), and continued follow-up will be necessary to understand long-term outcomes. Although the MER is not a population-based sample, the vast majority of our patients are from the local region, and the clinical characteristics of our patients with FL parallel those of population-based data with the exception of few very elderly patients.
In conclusion, FL transformation rates in this modern, large, prospective observational study are similar to risk of death without transformation and were lower at 5 years than in most previous reports, although post-transformation prognosis was substantially better. These observed differences may be a function of the nature of the study design, modern diagnostic and management strategies, or patient selection factors. Observed differences in transformation rates among various treatment groups leads to speculation that initial management may affect transformation risk. The marked survival differences following early versus late transformation may represent different biologic processes and require further confirmation.
Footnotes
Supported in part by Grant No. P50 CA97274 from the National Institutes of Health.
Presented in part at the 54th Annual Meeting of the American Society of Hematology, Atlanta, GA, December 8-11, 2012.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Brian K. Link, Roche/Genentech (C); James R. Cerhan, Roche/Genentech National LymphoCare Study (C) Stock Ownership: None Honoraria: None Research Funding: Brian K. Link, Roche/Genentech Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Brian K. Link, Matthew J. Maurer, Carrie A. Thompson, Thomas M. Habermann, James R. Cerhan
Financial support: Brian K. Link, Thomas E. Witzig, James R. Cerhan
Administrative support: Brian K. Link, Thomas E. Witzig, James R. Cerhan
Provision of study materials or patients: Brian K. Link, Carrie A. Thompson, David J. Inwards, Patrick B. Johnston, Joseph P. Colgan, Thomas E. Witzig, Thomas M. Habermann, James R. Cerhan
Collection and assembly of data: Brian K. Link, Matthew J. Maurer, William R. Macon, Sergei I. Syrbu, Carrie A. Thompson, David J. Inwards, Patrick B. Johnston, Joseph P. Colgan, Thomas E. Witzig, Thomas M. Habermann, James R. Cerhan
Data analysis and interpretation: Brian K. Link, Matthew J. Maurer, Grzegorz S. Nowakowski, Stephen M. Ansell, Sergei I. Syrbu, Susan L. Slager, Carrie A. Thompson, Thomas E. Witzig, Thomas M. Habermann, James R. Cerhan
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Herold M, Haas A, Srock S, et al. Rituximab added to first-line mitoxantrone, chlorambucil, and prednisolone chemotherapy followed by interferon maintenance prolongs survival in patients with advanced follicular lymphoma: An East German Study Group Hematology and Oncology Study. J Clin Oncol. 2007;25:1986–1992. doi: 10.1200/JCO.2006.06.4618. [DOI] [PubMed] [Google Scholar]
- 2.Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 3.Hochster H, Weller E, Gascoyne RD, et al. Maintenance rituximab after cyclophosphamide, vincristine, and prednisone prolongs progression-free survival in advanced indolent lymphoma: Results of the randomized phase III ECOG1496 Study. J Clin Oncol. 2009;27:1607–1614. doi: 10.1200/JCO.2008.17.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 5.Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: Results of the GELA-GOELAMS FL2000 study. Blood. 2008;112:4824–4831. doi: 10.1182/blood-2008-04-153189. [DOI] [PubMed] [Google Scholar]
- 6.Acker B, Hoppe RT, Colby TV, et al. Histologic conversion in the non-Hodgkin's lymphomas. J Clin Oncol. 1983;1:11–16. doi: 10.1200/JCO.1983.1.1.11. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tourah AJ, Gill KK, Chhanabhai M, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:5165–5169. doi: 10.1200/JCO.2008.16.0283. [DOI] [PubMed] [Google Scholar]
- 8.Bastion Y, Sebban C, Berger F, et al. Incidence, predictive factors, and outcome of lymphoma transformation in follicular lymphoma patients. J Clin Oncol. 1997;15:1587–1594. doi: 10.1200/JCO.1997.15.4.1587. [DOI] [PubMed] [Google Scholar]
- 9.Giné E, Montoto S, Bosch F, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histological subtype are the most important factors to predict histological transformation in follicular lymphoma. Ann Oncol. 2006;17:1539–1545. doi: 10.1093/annonc/mdl162. [DOI] [PubMed] [Google Scholar]
- 10.Horning SJ, Rosenberg SA. The natural history of initially untreated low-grade non-Hodgkin's lymphomas. N Engl J Med. 1984;311:1471–1475. doi: 10.1056/NEJM198412063112303. [DOI] [PubMed] [Google Scholar]
- 11.Hubbard SM, Chabner BA, DeVita VT, Jr, et al. Histologic progression in non-Hodgkin's lymphoma. Blood. 1982;59:258–264. [PubMed] [Google Scholar]
- 12.Montoto S, Davies AJ, Matthews J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007;25:2426–2433. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 13.Yuen AR, Kamel OW, Halpern J, et al. Long-term survival after histologic transformation of low-grade follicular lymphoma. J Clin Oncol. 1995;13:1726–1733. doi: 10.1200/JCO.1995.13.7.1726. [DOI] [PubMed] [Google Scholar]
- 14.Agid R, Sklair-Levy M, Bloom AI, et al. CT-guided biopsy with cutting-edge needle for the diagnosis of malignant lymphoma: Experience of 267 biopsies. Clin Radiol. 2003;58:143–147. doi: 10.1053/crad.2002.1061. [DOI] [PubMed] [Google Scholar]
- 15.Quinn SF, Sheley RC, Nelson HA, et al. The role of percutaneous needle biopsies in the original diagnosis of lymphoma: A prospective evaluation. J Vasc Interv Radiol. 1995;6:947–952. doi: 10.1016/s1051-0443(95)71219-2. [DOI] [PubMed] [Google Scholar]
- 16.Vandervelde C, Kamani T, Varghese A, et al. A study to evaluate the efficacy of image-guided core biopsy in the diagnosis and management of lymphoma: Results in 103 biopsies. Eur J Radiol. 2008;66:107–111. doi: 10.1016/j.ejrad.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Friedberg JW, Taylor MD, Cerhan JR, et al. Follicular lymphoma in the United States: First report of the national LymphoCare study. J Clin Oncol. 2009;27:1202–1208. doi: 10.1200/JCO.2008.18.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conconi A, Ponzio C, Lobetti-Bodoni C, et al. Incidence, risk factors and outcome of histological transformation in follicular lymphoma. Br J Haematol. 2012;157:188–196. doi: 10.1111/j.1365-2141.2012.09054.x. [DOI] [PubMed] [Google Scholar]
- 19.Drake MT, Maurer MJ, Link BK, et al. Vitamin D insufficiency and prognosis in non-Hodgkin's lymphoma. J Clin Oncol. 2010;28:4191–4198. doi: 10.1200/JCO.2010.28.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bains P, Al Tourah A, Campbell BA, et al. Incidence of transformation to aggressive lymphoma in limited-stage follicular lymphoma treated with radiotherapy. Ann Oncol. 2012;24:428–432. doi: 10.1093/annonc/mds433. [DOI] [PubMed] [Google Scholar]
- 21.Kim WR, Poterucha JJ, Benson JT, et al. The impact of competing risks on the observed rate of chronic hepatitis C progression. Gastroenterology. 2004;127:749–755. doi: 10.1053/j.gastro.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S. Measures of disease frequency. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven; 1998. pp. 29–46. [Google Scholar]
- 23.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model, Statistics for Biology and Health. New York, NY: Springer; 2000. p. 350. [Google Scholar]
- 26.Solal-Céligny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 27.Al-Tourah AJ, Sehn LH, Moccia AA, et al. Transformation of follicular lymphoma in the era of immunochemotherapy: A population-based study from British Columbia. J Clin Oncol. 2012;30(suppl):522s. abstr 8049. [Google Scholar]
- 28.Wagner-Johnston ND, Link BK, Taylor M, et al. Risk factors for early transformation of follicular lymphoma (FL): Report From the National LymphoCare Study (NLCS) Blood. 2009 doi: 10.1182/blood-2015-01-621375. (abstr 2698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ardeshna KM, Smith P, Qian W, et al. An intergroup randomised trial of rituximab versus a watch and wait strategy in patients with stage II, III, IV, asymptomatic, non-bulky follicular lymphoma (grades 1, 2 and 3a): A preliminary analysis. Presented at the 53rd ASH Annual Meeting; December 10-13, 2010; San Diego, CA. [Google Scholar]
- 30.Thornton PD, Bellas C, Santon A, et al. Richter's transformation of chronic lymphocytic leukemia: The possible role of fludarabine and the Epstein-Barr virus in its pathogenesis. Leuk Res. 2005;29:389–395. doi: 10.1016/j.leukres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Noy A, Schöder H, Gönen M, et al. The majority of transformed lymphomas have high standardized uptake values (SUVs) on positron emission tomography (PET) scanning similar to diffuse large B-cell lymphoma (DLBCL) Ann Oncol. 2009;20:508–512. doi: 10.1093/annonc/mdn657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schöder H, Noy A, Gönen M, et al. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-Hodgkin's lymphoma. J Clin Oncol. 2005;23:4643–4651. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 33.Williams CD, Harrison CN, Lister TA, et al. High-dose therapy and autologous stem-cell support for chemosensitive transformed low-grade follicular non-Hodgkin's lymphoma: A case-matched study from the European Bone Marrow Transplant Registry. J Clin Oncol. 2001;19:727–735. doi: 10.1200/JCO.2001.19.3.727. [DOI] [PubMed] [Google Scholar]
- 34.Zelenetz AD, Abramson JS, Advani RH, et al. Non-Hodgkin's lymphomas. J Natl Compr Canc Netw. 2011;9:484–560. doi: 10.6004/jnccn.2011.0046. [DOI] [PubMed] [Google Scholar]
- 35.Chen CI, Crump M, Tsang R, et al. Autotransplants for histologically transformed follicular non-Hodgkin's lymphoma. Br J Haematol. 2001;113:202–208. doi: 10.1046/j.1365-2141.2001.02705.x. [DOI] [PubMed] [Google Scholar]
- 36.Foran JM, Apostolidis J, Papamichael D, et al. High-dose therapy with autologous haematopoietic support in patients with transformed follicular lymphoma: A study of 27 patients from a single centre. Ann Oncol. 1998;9:865–869. doi: 10.1023/a:1008349427337. [DOI] [PubMed] [Google Scholar]
- 37.Friedberg JW, Neuberg D, Gribben JG, et al. Autologous bone marrow transplantation after histologic transformation of indolent B cell malignancies. Biol Blood Marrow Transplant. 1999;5:262–268. doi: 10.1053/bbmt.1999.v5.pm10465106. [DOI] [PubMed] [Google Scholar]
- 38.Schouten HC, Bierman PJ, Vaughan WP, et al. Autologous bone marrow transplantation in follicular non-Hodgkin's lymphoma before and after histologic transformation. Blood. 1989;74:2579–2584. [PubMed] [Google Scholar]
- 39.Davies AJ, Rohatiner AZ, Howell S, et al. Tositumomab and iodine I 131 tositumomab for recurrent indolent and transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2004;22:1469–1479. doi: 10.1200/JCO.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 40.Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2001;19:3918–3928. doi: 10.1200/JCO.2001.19.19.3918. [DOI] [PubMed] [Google Scholar]
- 41.Vose JM, Wahl RL, Saleh M, et al. Multicenter phase II study of iodine-131 tositumomab for chemotherapy-relapsed/refractory low-grade and transformed low-grade B-cell non-Hodgkin's lymphomas. J Clin Oncol. 2000;18:1316–1323. doi: 10.1200/JCO.2000.18.6.1316. [DOI] [PubMed] [Google Scholar]
- 42.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2002;20:2453–2463. doi: 10.1200/JCO.2002.11.076. [DOI] [PubMed] [Google Scholar]