Abstract
Objective
The major complaint of Osteoarthritis (OA) patients is pain. However, due to the nature of clinical studies and the limitation of animal studies, few studies have linked function impairment and behavioral changes in OA animal models to cartilage loss and histopathology. Our objective was to study surrogate markers of functional impairment in relation to cartilage loss and pathological changes in a post-traumatic mouse model of OA.
Method
We performed a battery of functional analyses in a mouse model of OA generated by cruciate ligament transection (CLT). The changes in functional analyses were linked to histological changes graded by OARSI standards, histological grading of synovitis, and volumetric changes of the articular cartilage and osteophytes quantified by phase contrast micro-CT.
Results
OA generated by CLT led to decreased time on rotarod, delayed response on hotplate analysis, and altered gait starting from 4 weeks after surgery. Activity in open field analysis did not change at 4, 8, or 12 weeks after CLT. The magnitude of behavioral changes was directly correlated with higher OARSI histological scores of OA, synovitis in the knee joints, cartilage volume loss, and osteophyte formation.
Conclusion
Our findings link functional analyses to histological grading, synovitis, comprehensive 3-dimensional assessment of cartilage volume and osteophyte formation. This serves as a reference for a mouse model in predicting outcomes of OA treatment.
Keywords: Osteoarthritis, functional analysis, pain, motor, gait, synovitis, cartilage volume quantification, osteophyte, animal models
INTRODUCTION
Osteoarthritis (OA) is a joint disease characterized by articular cartilage loss, synovitis, subchondral bone remodeling and osteophyte formation1. Chronic disability imposes motor limitations on about 80% of OA patients and prevents about 25% from performing major activities of daily living2. The functional disability endured by patients with OA of the knee is among the highest, and comparable to heart disease, stroke, and depression3. The major complaint of OA patients is pain, which significantly affects motor function. Clinically, a diagnosis of OA is convincing when pain with joint movement correlates with pathology. However, many patients with pathologic and radiographic evidence of disease may not exhibit pain symptoms4. Mouse models of OA have recapitulated human joint pathology, but methods to assess function changes have not been well characterized.
Various OA mouse models are used to study disease mechanisms, test therapeutics, and implement behavior assays. Genetics, chemical, and surgical models are the main techniques used. The surgical model corresponds with the development of OA in humans after ligamentous knee injury, which makes it the most clinically relevant. The surgical destabilization of the medical meniscus (DMM) is a commonly used surgical model of OA5. Disruption of the medical meniscus leads to aberrant biomechanical loading patterns and a subsequent mild form of knee OA in mice. However, cartilage degeneration is predominantly on the medial condyle of the articular joint. In humans, although cartilage degradation is most apparent in the medial compartment, many combinations of compartments involvement are also present6. The location of disease is unpredictable due to differences in mechanism of injury and individual joint variation. For these reasons, cartilage changes occur in both the medial and lateral compartments7,8. Thus we chose to investigate an alternative cruciate ligament transection (CLT) model as described previously9,10. This model produces cartilage degradation that exhibits characteristics of human OA and disease progression on the microscopic level is well characterized.
Histopathology of the joint is commonly used to assess mouse OA models11. However, this method requires expertise and is prone to variability due to inter-observer subjectivity. More importantly, pain, motor dysfunction, and radiographic changes, and not histopathology, are used to clinically evaluate and diagnose OA in patients1. Recently, we applied novel methodology to image and quantify mouse articular cartilage using phase contrast, ultra-high resolution micro-computed tomography (μCT)9, which combined the sensitivity to visualize the soft tissue of magnetic resonance imaging (MRI) with the resolution required to analyze small rodent models. While this method provided unparalleled quantification of mouse articular cartilage, it could not assess functional impairment.
Functional behavioral tests are frequently used in the neurological field12,13. They provide information about animal activity, learning, memory, sensitivity, pain, and motor function14-22. Here, we use a series of behavioral tests combined with histological and radiologic analysis to assess OA in a CLT mouse model. We show motor dysfunction, hyperalgesia, and altered gait using behavioral tests in a mouse model of OA induced by CLT. When compared to cartilage loss and pathological changes directly, these models would help unveil details about the clinical correlations between OA symptoms and radiographic disease. Taken together, pain, motor function, and imaging findings that are corroborated with histopathology would provide a comprehensive approach to study OA.
MATERIAL AND METHODS
Animals
FVB/N mice were purchased from Jackson Laboratories (Bar Harbor, ME). All studies were performed with approval from the Baylor College of Medicine Institutional Animal Care and Use Committee (IACUC). All mice were housed under pathogen-free conditions. To avoid anxiety and possible behavior changes, every cage contained 4-5 mice. Mice had free access to feed and water. To avoid the effect of potential post-menopausal subchondral bone loss and possible differences in weight and activity, we used male mice in all our experiments. To avoid differences between different litters, we tested littermates in all experiments. And mice in different experimental groups are housed together.
Cruciate ligament transection
Transection procedures are as previously described9. 8-week old male FVB/N mice were anesthetized and aseptically prepared for surgery. In the CLT group (N=17-23 for each time point, 3 time points), the transection of the anterior and posterior cruciate ligament was made bilaterally instead of unilaterally as previously described. We chose to transect the limbs bilaterally because of 2 reasons: 1) From a local injury standpoint, the development of OA in a joint is similar regardless of a bilateral or unilateral transection and 2) it would be easier to interpret a functional change when mice were transected bilaterally. The success of the transection could be tested by valgus and varus laxity of the knee. In the sham group (N=17-23 for each time point, 3 time points), no transection was made to either knee. Mice were allowed free cage activity immediately following recovery from surgery. Sutures were removed 2 weeks after surgery. Animals were randomly chosen from each group to perform the analyses.
Histology
Mice were euthanized and whole knee joints were fixed with 4% paraformaldehyde (Sigma-Aldrich) overnight in 4 °C on a shaker. Whole knee joints were decalcified in 14% EDTA for 5 days in 4 °C on a shaker. After dehydration by gradient alcohol and infiltration by xylene and paraffin, samples were embedded in paraffin. Paraffin-embedded joints were sectioned at 6μm on a sagittal plane. Samples were stained with safranin O and fast green using standard protocols. N=8 in each group.
Phase contrast μCT
Samples were prepared and analyzed as previously described9. Whole joint knees were dissected and fixed with ruthenium hexamine tricholoride, glutaraldehyde, cacodylic acid, and osmium tetroxide based fixative buffers. The samples were then dehydrated and embedded in paraffin. Samples were scanned by Xradia (Xradia, California, US) microxCT. Data reconstruction was performed using Xradia software and articular cartilage and osteophytes were analyzed using TriBON software (RATOC, Tokyo, Japan). N=5-6 in each group.
Hotplate Nociception Analysis
Mice were transferred to the room of analysis at least 30 minutes before experiment. Then, mouse was placed on the hotplate at 55°C one at a time (Columbus Instruments, Columbus, OH) (Fig. 1a). The latency period for hind limb response (e.g. shaking, jumping, or licking) was recorded as response time. Each trial has a maximum time of 45 seconds. The mouse was removed from the hotplate immediately after a response was observed. N=12-14 in each group.
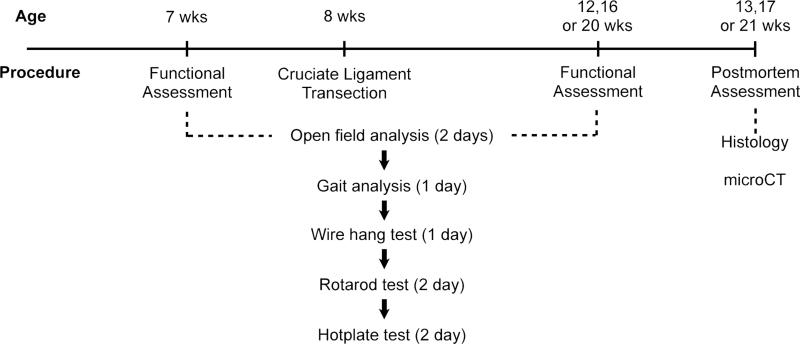
Fig. 1. Experiment scheme of functional change evaluation.
We performed functional evaluation when the mice were 7 months old. This was followed by cruciate ligament transection and sham surgery. In different batches of mice, we waited 4, 8 or 12 weeks before another functional assessment. We performed postmortem assessment after the second and final functional analysis.
Open field analysis
Open field activity was measured by the VersaMax Animal Activity Monitoring System (AccuScan Instruments, Columbus, OH). Mice were placed into the center of a clear chamber of 40cm×40cm×30cm to allow free exploration. Mice were under 800 lm of illumination and 55dB of white noise. The experiments were performed for 30 minutes on each of the 2 consecutive days. Disturbance was avoided during the experiment. Total travel distance, total travel time, total rest time, rearing time and center distance: total distance ratio was calculated. N=10 in each group.
Rotarod Analysis
Mice were placed onto an accelerating rotarod (UGO Basile, Varese, Italy). The first failure to stay atop the rod was referred to as first ride-around time. To rule out differences in learning skills between the two groups of mice, each group was assessed over three trials per day for 2 consecutive days (trials 1 to 6) before surgery. Then, mice were randomly assigned into different groups. Another 6 trials were performed using the same condition at different time points after the surgery. Mice were given a 30 minutes inter-trial rest interval. Each trial had a maximum time of 5 minutes. N=17-23 in each group.
Wire-hang analysis and reverse
Mice were placed on the wire bars (10×18cm area 1mm in diameter and spaced 1 cm apart) and turned upside down. The latency to fall was measured. Each trial had a maximum time of 60 seconds. N=10 in each group.
Gait analysis
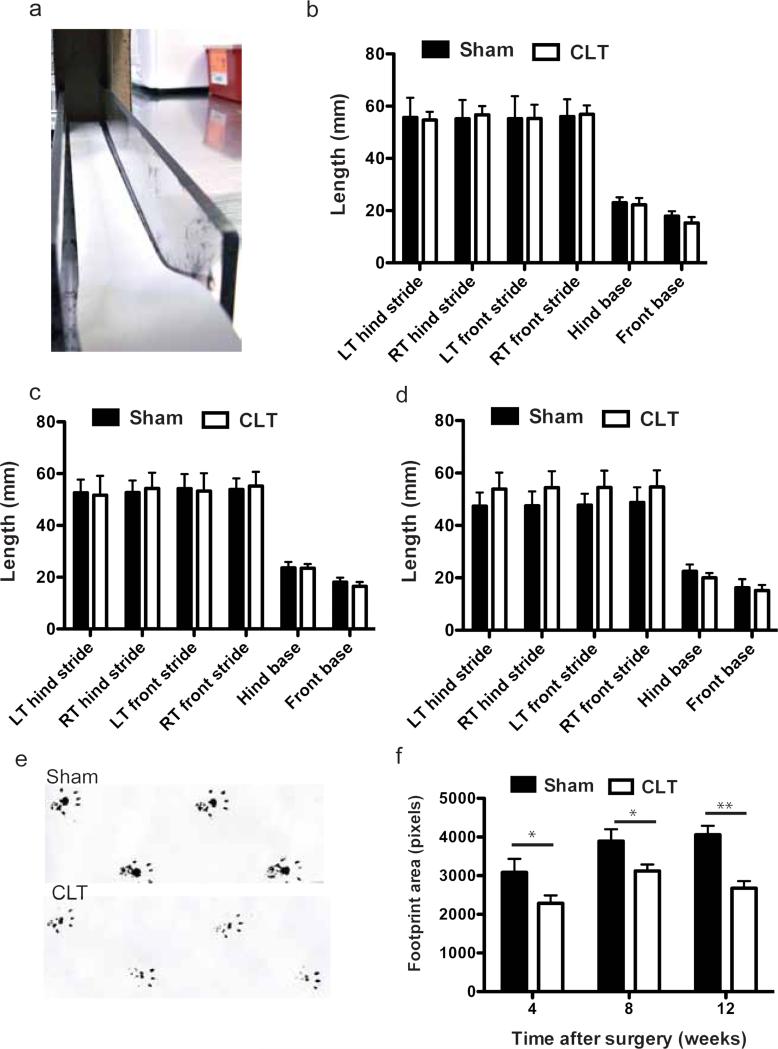
The paws of the mice were brushed with ink by an examiner blinded to the surgical procedures. Front paws and hind paws were colored with ink of different color. Immediately after ink was applied, the mice were allowed to run on a 60cm long, 6cm wide Plexiglas track with white paper on the bottom. A dark chamber was present in the end of the track to entice the mice. Upon completion of the test, paper was scanned at 300dpi. The distance of the same paw between two steps were defined as stride. And the horizontal distance between the left and right paw were defined as base23. The areas of third to the fifth set of footsteps were quantified by image J. N=17-19 in each group
Timeline of experiments
We performed a pilot experiment on a small batch of animals to determine which analyses to use. In the final experiment from which we collected data, the behavioral and functional analyses were performed in the order of least stress (least likely to affect activity on the following day) to most stress (most likely to affect activity on the following day) for the animals. To account for habituation formation, all mice were tested 2 times: before and after surgery at specific time points. The scheme of the study is illustrated in Fig. 1.
Statistical Analysis
Statistical significance comparing two groups with parametric data was assessed by Student's t test. Statistical analysis comparing multiple groups with parametric data was performed by one-way ANOVA followed by tukey's post-hoc. Rotarod analysis was tested by one-way ANOVA with repeated measures followed by tukey's post-hoc. Histological grades were compared by Wilcoxon rank sum test. Correlation analyses were performed by nonparametric correlation (Spearman) test between OARSI score and functional changes with pooled time points and experimental groups. All assumptions were fulfilled for the statistical tests used. All analyses were performed by Sigma Plot software. A P value of <0.05 was considered statistically significant.
RESULTS
CLT mice showed increased response time on hotplate analysis
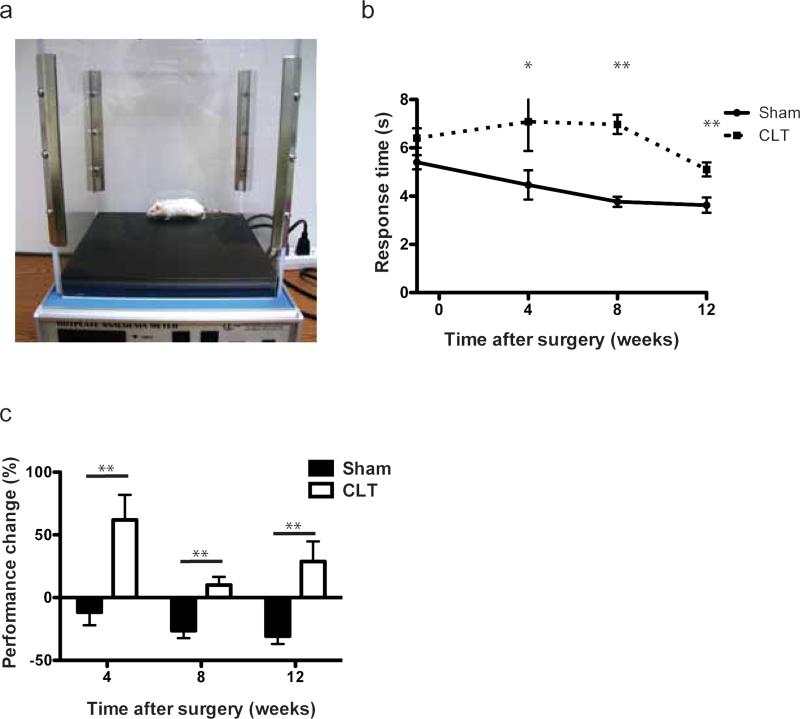
Multiple studies have reported changes in pain sensitivity in OA patients24,25. Therefore, we evaluated sensitivity in mice using hotplate nociception analysis (Fig. 2a). The hotplate analysis mainly provides an assessment of sensitivity and reaction to pain stimulus. Importantly, surgical transection of both hind limbs did not alter body weight compared to sham-operated mice as this may confound subsequent behavioral assessments (Supplementary Fig. 1a). Mice in the CLT group responded slower compared to mice in the sham group (Fig. 2b). We normalized the performance of an individual mouse after the surgery to its own performance before the surgery by calculating performance change (performance change = (response time after surgery-response time before surgery)*100%/response time before surgery) to account for possible variation between mice neurological function. After normalization, the CLT group showed delayed responses on hotplate after surgery, while the sham group responded faster (Fig. 2c). Since hotplate also indirectly assesses the mobility and gait of the animal, the significant change in response time on the hotplate prompted us to further investigate its cause.
Fig. 2. Pain sensitivity and mobility measured by hotplate analysis.
(a) Experiment setup and device of hotplate analysis; (b) Response time of the mice before and at different time points after surgery; (c) Performance change on hotplate normalized to individual mice. Performance change = [(time after – time before)/ time before] × 100%. N=12-14. All error bars represent s.e.m. *p<0.05, **p<0.01.
CLT mice showed no change in open field analysis and wire hang test
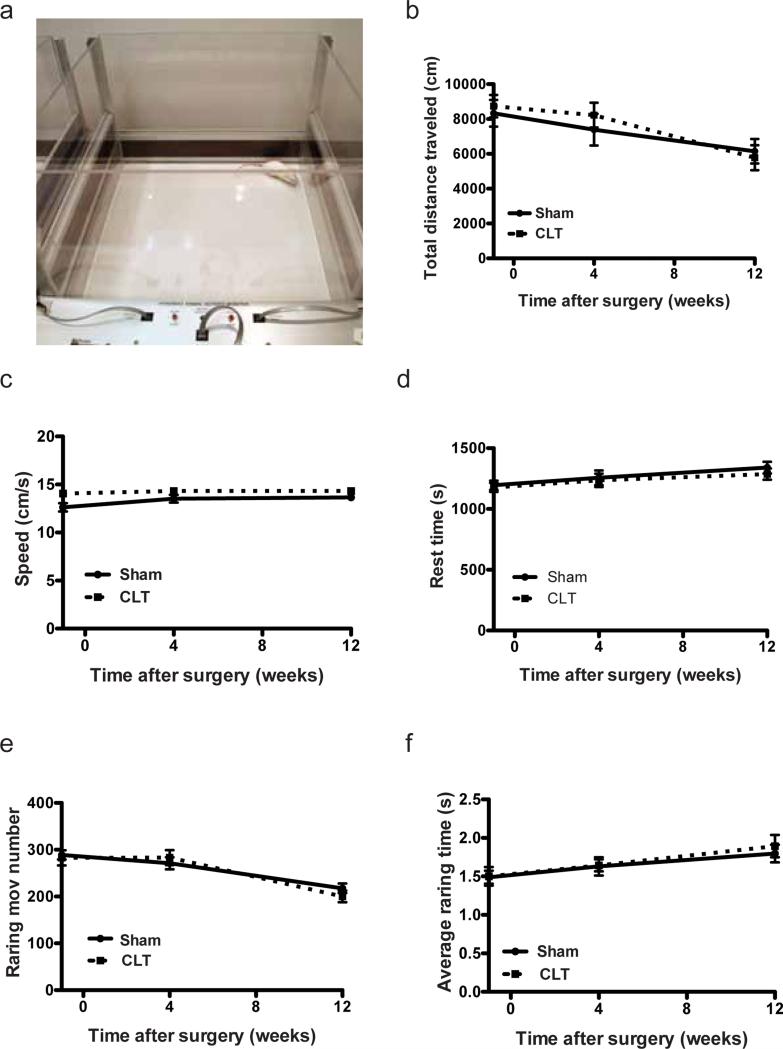
To test the general parameters of mice activity with minimal pressure and stress on the knee joint, we performed the open field analysis (Fig. 3a). Sensors in the open field arena detect movement and record the position of the mice. In the 30 minutes when mice were observed, no differences were detected between the sham group and the CLT group in movement and position of the mice, suggesting that CLT did not alter moving patterns and thigmotaxis (Fig. 3b-f). Because the surgery to induce OA was performed on the hind limbs of the mice, we further focused on the activity dominated by hind limbs by including rearing movement number and average rearing time. Neither of the two parameters changed between the CLT group and the sham group (Fig. 3e,f). In addition, all the mice tested performed at maximum performance in the wire hang analysis designed to test the gripping ability of the mice (Supplementary Fig. 1b). These observations suggested that provided the lab environment, the daily activity of the OA mice including feeding and grooming would not be compromised by CLT.
Fig. 3. Movement of mice measured by open field analysis (OFA).
(a) Arena of the open field analysis; (b) Total distance travelled (during testing period); (c) Average walking speed of the mice in the open field (speed=total distance travelled/total moving time); (d) Total rest time; (e) Number of rearing movement; (f) Average time of each rearing event. N=10. All error bars represent s.e.m.
CLT mice showed motor dysfunction in rotarod analysis
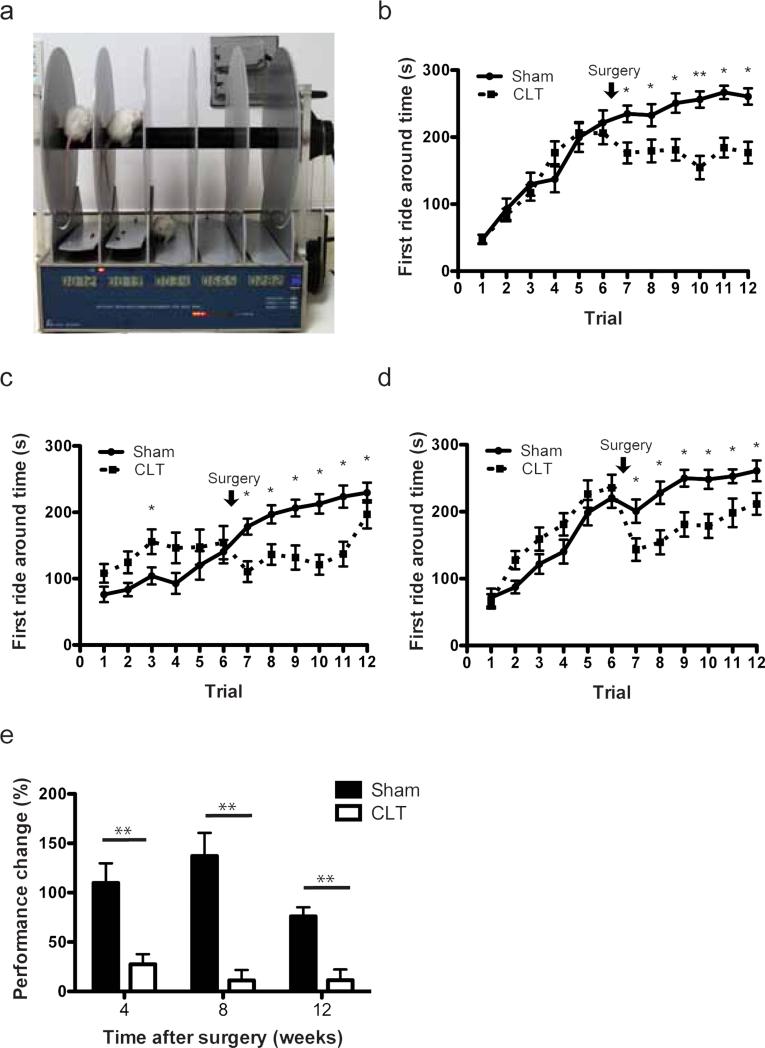
Rotarod analysis provides assessment of motor function with pressure and stress on the knee joint as well as learning and memory skills (Fig. 4a). Because of the effects of learning and memory, we expected the performance of animals to improve in all groups longitudinally. Post-surgery sham groups demonstrated greater improvement than the CLT groups, supporting a motor impairment in the CLT groups (Fig. 4b-d). Similar to hotplate analysis, we calculated performance change of the mice due to the possible neurological variation between individuals by normalizing the average first ride around time of an individual in trial 7-12 to the average first ride around time of itself in trial 1-6. We found that mice in the CLT group showed less improvement compared to mice in the sham group (Fig. 4e).
Fig. 4. Motor function of mice measured by Rotarod analysis.
(a) Experiment setup of Rotarod analysis; (b-d) First ride around time of the mice before the procedure (trials 1-6) and 4 weeks (b, n=20-23), 8 weeks (c, n=19-20) and 12 weeks (d, n-17-18) after either sham or CLT; (e) Performance change of average Rotarod first ride around time of trials performed before and after surgery. Performance change = [(time after – time before)/ time before] × 100%. All error bars represent s.e.m. *p<0.05, **p<0.01.
CLT mice showed altered gait
During the hotplate analysis, we observed that the CLT mice had altered gait. Instead of standing on their feet, mice after CLT tended to stand on their toes only. To test whether delayed response in hotplate was partially caused by altered gait, we performed gait analysis (Fig 5a). While no significant differences in stride and base were detected between the groups (Fig. 5b-d), the CLT group had a smaller hind foot print area compared to the control group (Fig. 5e,f).
Fig. 5. Gait change of mice with osteoarthritis induced by CLT.
(a) Experiment setup of gait analysis; (b-d) Stride and base of the mice 4 weeks (b), 8 weeks (c) and 12 weeks (d) after surgery; (e) Representative hind limb foot prints of Sham and CLT mice on gait analysis; (f) Quantification of hind limb foot print area at different time points after surgery. N=17-19. All error bars represent s.e.m. All the significant comparisons are labeled, *p<0.05, **p<0.01.
Cartilage loss and pathological changes in CLT
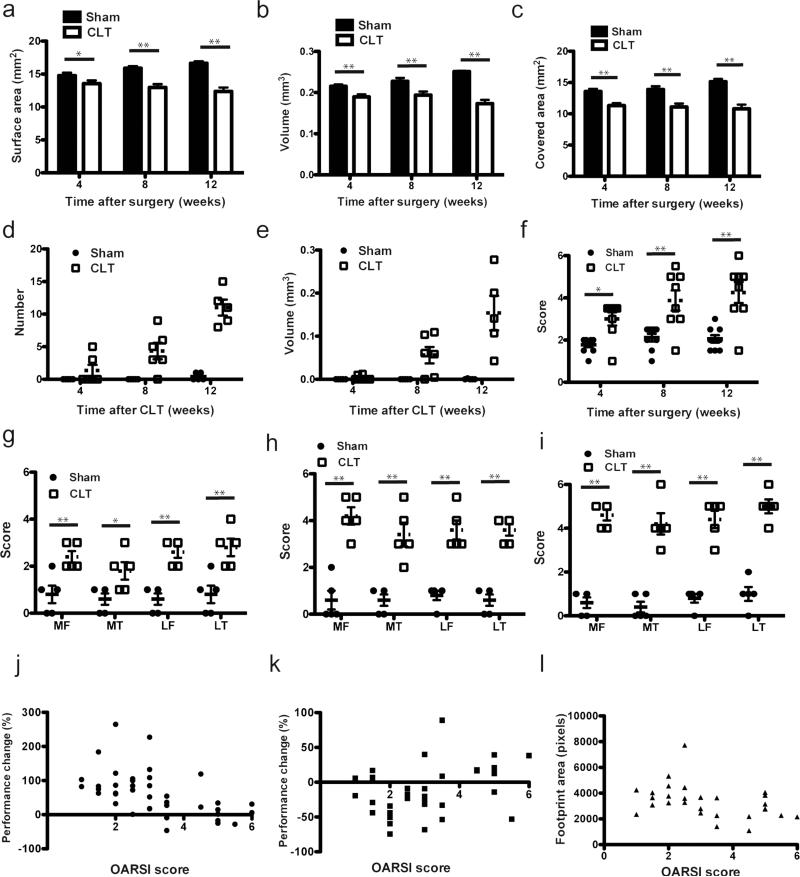
To correlate these functional changes at different time points after CLT to cartilage loss and histopathology, we performed microCT analysis and histology grading of the knee joints of the same mice within 24 hours after the functional analysis. Loss of cartilage surface area, volume and bone area covered by cartilage were around 10% at 4 weeks after CLT and progressed to 30% at 12 weeks after surgery (Fig. 6a-c). Ten percent cartilage loss was significant to change joint function under pressure. Very few osteophytes were detected 4 weeks after surgery and increased with the progression of cartilage loss (Fig. 6d,e). To evaluate the pathological changes in cartilage and subchondral bone, we graded the knee joint using OARSI grading system11 (Fig. 6f). As expected, 4 weeks after CLT, most joints showed mild synovitis as well as loss of proteoglycan and matrix. Erosion started 8 weeks after CLT. At 12 weeks after CLT, most of the remaining cartilage was denuded and undergoing remodeling, compromising its original function and severe synovitis was detected. Since synovitis was suggested to be a cause of pain in OA, we evaluated synovitis in CLT mice using histopathological assessment published by Lewis et al.27 (Fig. 6g-i). We observed increasing severity of synovitis as OA progressed. To test whether correlation exist between microscopic observations and functional changes, we performed bivariate correlation analyses between OARSI score and rotarod analysis, hotplate analysis and hind limb foot print area. We pooled all experimental groups and time points. The histological changes correlate with performance changes on rotarod analysis (Spearman rho = −0.6024, p<0.0001), hotplate analysis (Spearman rho = 0.3585, p<0.05) and the hind limb foot print area (Spearman rho = −0.4586, p<0.05) (Fig. 6j-l).
Fig. 6. Longitudinal evaluation of cartilage loss and pathological changes in CLT mice.
(a) Cartilage surface area, (b) Cartilage volume (c) subchondral bone area covered by cartilage (d) osteophyte number and (e) osteophyte volume in whole hind limb knee joint in mice at different time points after surgery, N=5-6; (f) Maximum OARSI score on sham and CLT whole hind limb joints in mice at different time points after surgery, N=8. (g-i) Synovitis score at 4 weeks (g), 8 weeks (h) and 12 weeks (i) post surgery at medial femur (MF), medial tibia (MT), lateral femur (LF) and lateral tibia (LT), N=5. (j-l) Bivariate correlation between OARSI score and performance changes in rotarod analysis (Spearman rho=−0.6024, p<0.0001, n=39) (j), hotplate analysis (Spearman rho =0.3585, p<0.05, n=34) (k) and hind limb foot print area (Spearman rho=−0.4586, p<0.05, n=29) (l) All error bars represent s.e.m. *p<0.05, **p<0.01.
DISCUSSION
Our findings show that hyperalgesia, motor dysfunction, and altered gait in OA can be assessed using hotplate, rotarod and gait analysis respectively in a CLT mouse model. The open field analysis and wire hang test did not reveal statistically significant differences. However, this variability of motor dysfunction and pain parallels what is observed in patients, where the extent of joint damage may or may not correlate with knee pain4. Nevertheless, our positive findings offer clinical correlation to existing histology and radiographic techniques. Together, these functional changes in OA can be linked with the underlying pathological changes to gain a better understanding of the disease process.
Hyperalgesia to stimuli is a common finding in knee OA patients28,29. We found the CLT group had significantly slower response times to nociception compared to sham. On the contrary, a study in a rat model of MIA-induced OA found decreased response times in the OA group compared to sham30. However, the MIA-induced OA model is generally a pain model and the presence of hyperalgesia may be exaggerated19,31-33. Our findings of a delayed response in the CLT model of OA suggests decreased pain sensitivity and/or a motor dysfunction component. Alternatively, motor disability may be delaying or hindering a response by the animal. Clinically, knee OA patients lose about 18% of knee flexion, which appears to be consistent with the motor dysfunction we observed34. As observed by our gait analysis, mice placed less pressure on the hind foot and more on the toes. This stance creates decreased contact area between the hind foot and hotplate, which could also explain the increased response times. Interestingly, our sham group showed a reduction in latency to response after sham surgery, while the CLT group was increased. This phenomenon of decreased latencies after repeated testing has been reported before35. The abolition of this reduced latency after a second testing post-surgery in the CLT group indicates a true delayed response.
In rotarod analysis, the sham group had greater improvement on rotarod riding time compared to the CLT group, indicating that the CLT group had more motor dysfunction compared to the sham group. Similar findings were observed in another study, though differences were found only at 14 days in a monosodium iodoacetate (MIA) OA model36. We showed that this motor dysfunction remained at 4, 8, and 12 weeks post-surgery, much like what is observed in OA patients. Further, our CLT model closely followed the post-traumatic OA seen in patients, while the MIA model has been questioned for its inability to reproduce OA-like progression and a significant inflammatory component37. Other studies applied the rotarod to assess affected limb use during forced ambulation on the rod14,19. However, this technique involves subjective observation of limb use and does not necessarily measure motor function.
Many studies have evaluated gait biomechanics and kinematics in OA patients to understand compensatory changes, target rehabilitation, and assess the efficacy of treatments. We assessed gait in our model using paw print analysis. Interestingly, differences in hind and front limb stride or base length were not different between the CLT and sham groups. These results are consistent with previous findings of no change in stride length in rats26. Base length is shown to be increased in OA patients, but we did not see a difference38. We did find a significantly lower footprint area in the CLT group compared to sham. These findings of joint load shifting are reported in rats and in humans with unilateral OA26,39. This is a similar phenomenon observed in larger animal models, suggesting that mice with CLT placed less pressure on the hind feet and more pressure on the toes or on the forelimbs26. Taken together, the data suggest that CLT established a clinically relevant OA model in mice using standard histological and mouse behavioral assessments.
Our findings provided a comprehensive method to evaluate OA in mouse model by combining functional analysis, quantitative evaluation of articular cartilage and osteophytes by μCT and traditional joint and synovial pathology9. These can be applied the study of genetic mouse models, disease-modifying drugs and gene therapy.
Supplementary Material
Supplementary Fig. 1. (a) Weight of the mice before and at different times after surgery. n=16-18. All error bars represents s.e.m. No statistical significance was observed between groups. (b) Experiment setup for wire-hang and reverse test
ACKNOWLEDGEMENTS
We thank Dr. Corinne Spencer for training in behavior analysis. The project described was supported by the BCM IDDRC Grant Number 5P30HD024064 from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute Of Child Health & Human Development or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
M.R. performed experiments, analyzed data and prepared the manuscript. R.P. analyzed data and prepared the manuscript. B.D. and M.J. performed experiments and analyzed data. B.L conceived and supervised the project and prepared the manuscript. All authors approve the manuscript submission.
CONFLICT OF INTEREST
There is no conflict of interests.
Contributor Information
Merry ZC Ruan, Molecular and Human Genetics, Baylor College of Medicine, ruan@bcm.edu.
Ronak M Patel, Molecular and Human Genetics, Baylor College of Medicine, Ronak.Patel@bcm.edu.
Brian C Dawson, Molecular and Human Genetics, Baylor College of Medicine, bcd@bcm.edu.
Ming-ming Jiang, Molecular and Human Genetics, Baylor College of Medicine, mjiang@bcm.edu.
Brendan HI Lee, Molecular and Human Genetics, Baylor College of Medicine.
REFERENCE
- 1.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis & Rheumatism. 1986 Aug;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 2.Brooks PM. Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol. 2002 Sep 1;14(5):573. doi: 10.1097/00002281-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Guccione AA, Guccione AA, Felson DT, Felson DT, Anderson JJ, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994 Mar;84(3):351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis and Cartilage. 2007 Sep;15(9):1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Ledingham J, Regan M, Jones A, Doherty M. Radiographic patterns and associations of osteoarthritis of the knee in patients referred to hospital. Annals of the Rheumatic Diseases. 1993 Jul;52(7):520–6. doi: 10.1136/ard.52.7.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicuttini FM, Wluka AE, Stuckey SL. Tibial and femoral cartilage changes in knee osteoarthritis. Annals of the Rheumatic Diseases. 2001 Oct;60(10):977–80. doi: 10.1136/ard.60.10.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cicuttini FM, Wluka AE, Wang Y, Stuckey SL. Longitudinal study of changes in tibial and femoral cartilage in knee osteoarthritis. Arthritis & Rheumatism. 2004 Jan;50(1):94–7. doi: 10.1002/art.11483. [DOI] [PubMed] [Google Scholar]
- 9.Ruan MZC, Dawson B, Jiang M-M, Gannon F, Heggeness M, Lee BHL. Quantitative imaging of murine osteoarthritic cartilage by phase-contrast micro computed tomography. Arthritis & Rheumatism. 2013 Feb;65(2):388–96. doi: 10.1002/art.37766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruan MZC, Erez A, Guse K, Dawson B, Bertin T, Chen Y, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013 Mar 13;5(176):176ra34. doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis and Cartilage. 2010 Oct;18:S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Hamm RJ, Pike BR, O'Dell DM, Lyeth BG, Jenkins LW. The rotarod test: an evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J. Neurotrauma. 1994 Apr;11(2):187–96. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 13.Zausinger S, Hungerhuber E, Baethmann A, Reulen H-J, Schmid-Elsaesser R. Neurological impairment in rats after transient middle cerebral artery occlusion: a comparative study under various treatment paradigms. Brain Research. 2000 Apr;863(1-2):94–105. doi: 10.1016/s0006-8993(00)02100-4. [DOI] [PubMed] [Google Scholar]
- 14.Vermeirsch H, Biermans R, Salmon PL, Meert TF. Evaluation of pain behavior and bone destruction in two arthritic models in guinea pig and rat. Pharmacology Biochemistry and Behavior. 2007 Aug;87(3):349–59. doi: 10.1016/j.pbb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Knights CB, Gentry C, Bevan S. Partial medial meniscectomy produces osteoarthritis pain-related behaviour in female C57BL/6 mice. Pain. 2012 Feb;153(2):281–92. doi: 10.1016/j.pain.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nature Medicine. 2011 Nov 6;17(12):1674–9. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen KD, Griffin TM, Rodriguiz RM, Wetsel WC, Kraus VB, Huebner JL, et al. Decreased physical function and increased pain sensitivity in mice deficient for type IX collagen. Arthritis & Rheumatism. 2009 Sep;60(9):2684–93. doi: 10.1002/art.24783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hielm-Björkman AK, Kuusela E, Liman A, Markkola A, Saarto E, Huttunen P, et al. Evaluation of methods for assessment of pain associated with chronic osteoarthritis in dogs. J. Am. Vet. Med. Assoc. Am Vet Med Assoc. 2003;222(11):1552–8. doi: 10.2460/javma.2003.222.1552. [DOI] [PubMed] [Google Scholar]
- 19.Kalff K-M, Mouedden El M, van Egmond J, Veening J, Joosten L, Scheffer GJ, et al. Pre-treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur. J. Pharmacol. 2010 Sep 1;641(2-3):108–13. doi: 10.1016/j.ejphar.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Mapp PI, Walsh DA, Bowyer J, Maciewicz RA. Effects of a metalloproteinase inhibitor on osteochondral angiogenesis, chondropathy and pain behavior in a rat model of osteoarthritis. Osteoarthritis and Cartilage. 2010 Apr;18(4):593–600. doi: 10.1016/j.joca.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malfait AM, Ritchie J, Gil AS, Austin JS, Hartke J, Qin W, et al. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthritis and Cartilage. 2010 Apr;18(4):572–80. doi: 10.1016/j.joca.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12(4):R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C-H, Tallaksen-Greene S, Chien W-M, Cearley JA, Jackson WS, Crouse AB, et al. Hum. Mol. Genet. 2. Vol. 10. Oxford Univ Press; 2001. Neurological abnormalities in a knock-in mouse model of Huntington's disease. pp. 137–44. [DOI] [PubMed] [Google Scholar]
- 24.Inglis JJ, McNamee KE, Chia S-L, Essex D, Feldmann M, Williams RO, et al. Regulation of pain sensitivity in experimental osteoarthritis by the endogenous peripheral opioid system. Arthritis & Rheumatism [Internet] 2008 Oct;58(10):3110–9. doi: 10.1002/art.23870. Available from: http://doi.wiley.com/10.1002/art.23870. [DOI] [PubMed] [Google Scholar]
- 25.Focht BC, Ewing V, Gauvin L, Rejeski WJ. The unique and transient impact of acute exercise on pain perception in older, overweight, or obese adults with knee osteoarthritis. Annals of Behavioral Medicine. Springer. 2002;24(3):201–10. doi: 10.1207/S15324796ABM2403_05. [DOI] [PubMed] [Google Scholar]
- 26.Clarke KA, Heitmeyer SA, Smith AG, Taiwo YO. Gait analysis in a rat model of osteoarthrosis. Physiology & Behavior. 1997 Nov;62(5):951–4. doi: 10.1016/s0031-9384(97)00022-x. [DOI] [PubMed] [Google Scholar]
- 27.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthr. Cartil. 2011 Jul;19(7):864–73. doi: 10.1016/j.joca.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001 Aug;93(2):107–14. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 29.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS. ScienceDirect.com - PAIN Sensitization in patients with painful knee osteoarthritis. Pain. 2010 doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Silva A, Andersen ML, Tufik S. Sleep pattern in an experimental model of osteoarthritis. Pain. 2008 Dec;140(3):446–55. doi: 10.1016/j.pain.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Beyreuther B, Callizot N. Stöhr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007;9(1):R14. doi: 10.1186/ar2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goranov NV. Clinical changes in sodium monoiodoacetate–induced stifle osteoarthritis model in dogs. Veterinary World [Internet] 2012;5(3):138–44. Dr. Sherasiya Anjum V Available from: http://www.ejmanager.com/mnstemps/2/2-1323941254.pdf. [Google Scholar]
- 33.Fernihough J, Gentry C, Malcangio M, Fox A, Rediske J, Pellas T, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004 Nov;112(1-2):83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Ornetti P, Maillefert J-F, Laroche D, Morisset C, Dougados M, Gossec L. Gait analysis as a quantifiable outcome measure in hip or knee osteoarthritis: A systematic review. Joint Bone Spine. 2010 Oct;77(5):421–5. doi: 10.1016/j.jbspin.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Mogil JS, Wilson SG, Bon K, Eun Lee S, Chung K, Raber P, et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999 Mar;80(1-2):67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 36.Harvey VL, Dickenson AH. Behavioural and electrophysiological characterisation of experimentally induced osteoarthritis and neuropathy in C57Bl/6 mice. Mol Pain. 2009;5(1):18. doi: 10.1186/1744-8069-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Little CB, Smith MM. Animal models of osteoarthritis. Current Rheumatology Reviews. 3. Vol. 4. Bentham Science Publishers; Jun 16, 2008. pp. 175–82. [Google Scholar]
- 38.Bejek Z, Paróczai R, Illyés Á , Kiss RM. The influence of walking speed on gait parameters in healthy people and in patients with osteoarthritis - Springer. Knee Surgery. 2006 doi: 10.1007/s00167-005-0005-6. [DOI] [PubMed] [Google Scholar]
- 39.Messier SP. Osteoarthritis of the knee and associated factors of age and obesity: effects on gait. Med Sci Sports Exerc. 1994 Dec;26(12):1446–52. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. (a) Weight of the mice before and at different times after surgery. n=16-18. All error bars represents s.e.m. No statistical significance was observed between groups. (b) Experiment setup for wire-hang and reverse test