Abstract
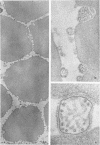
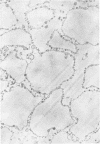
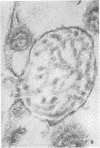
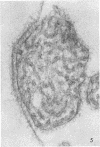
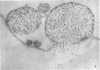
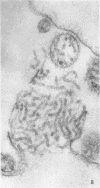
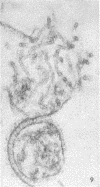
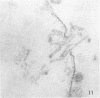
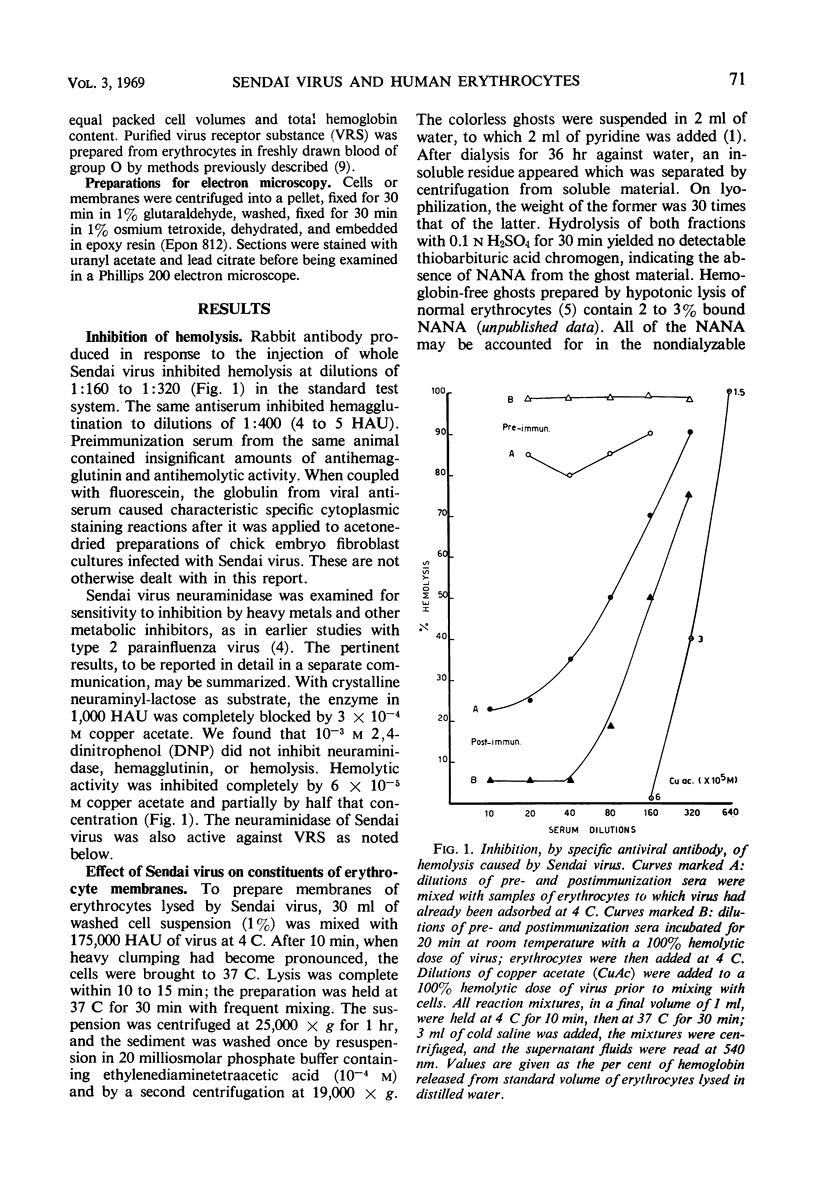
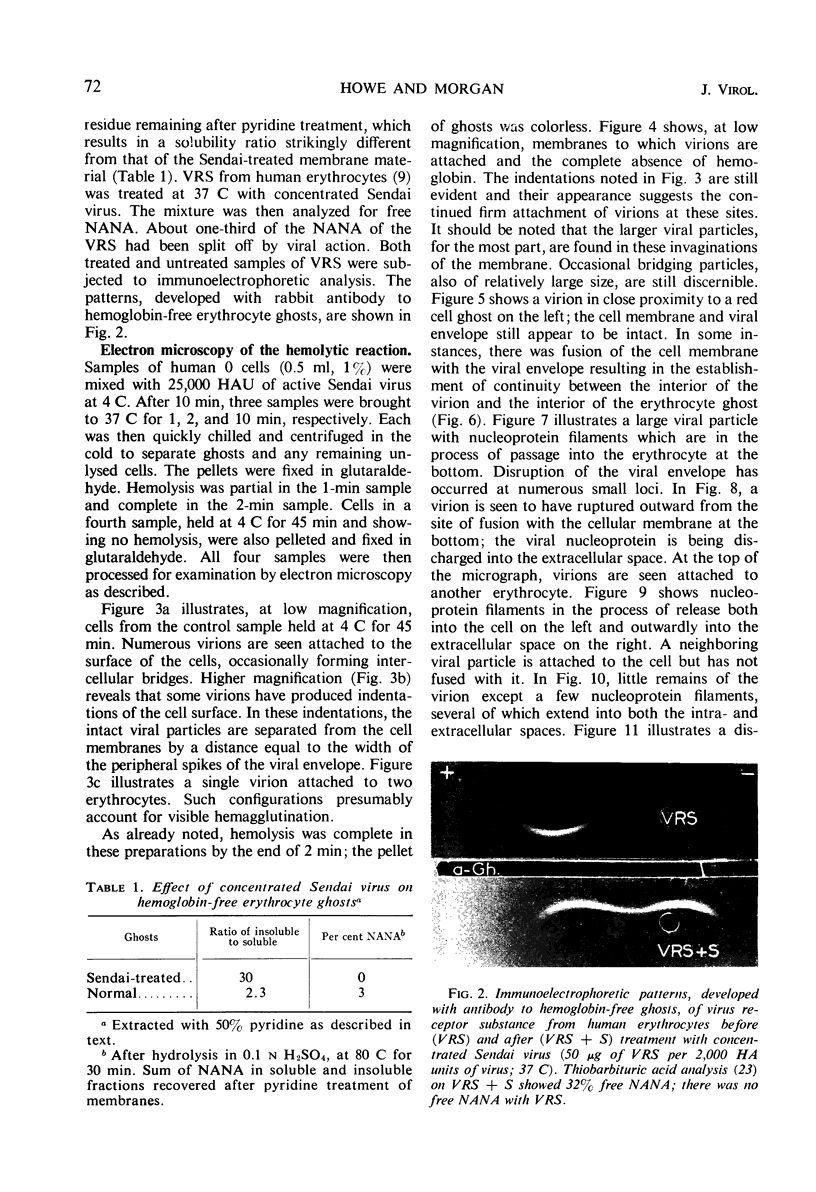
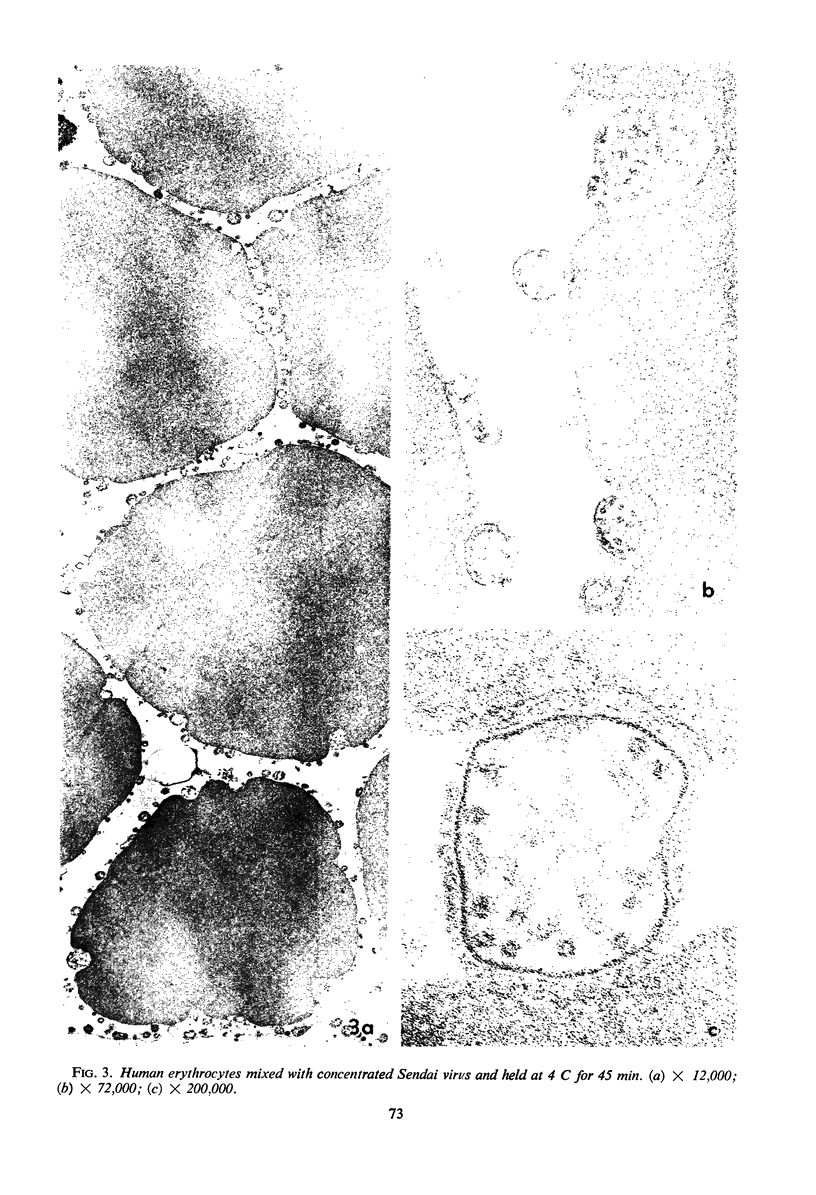
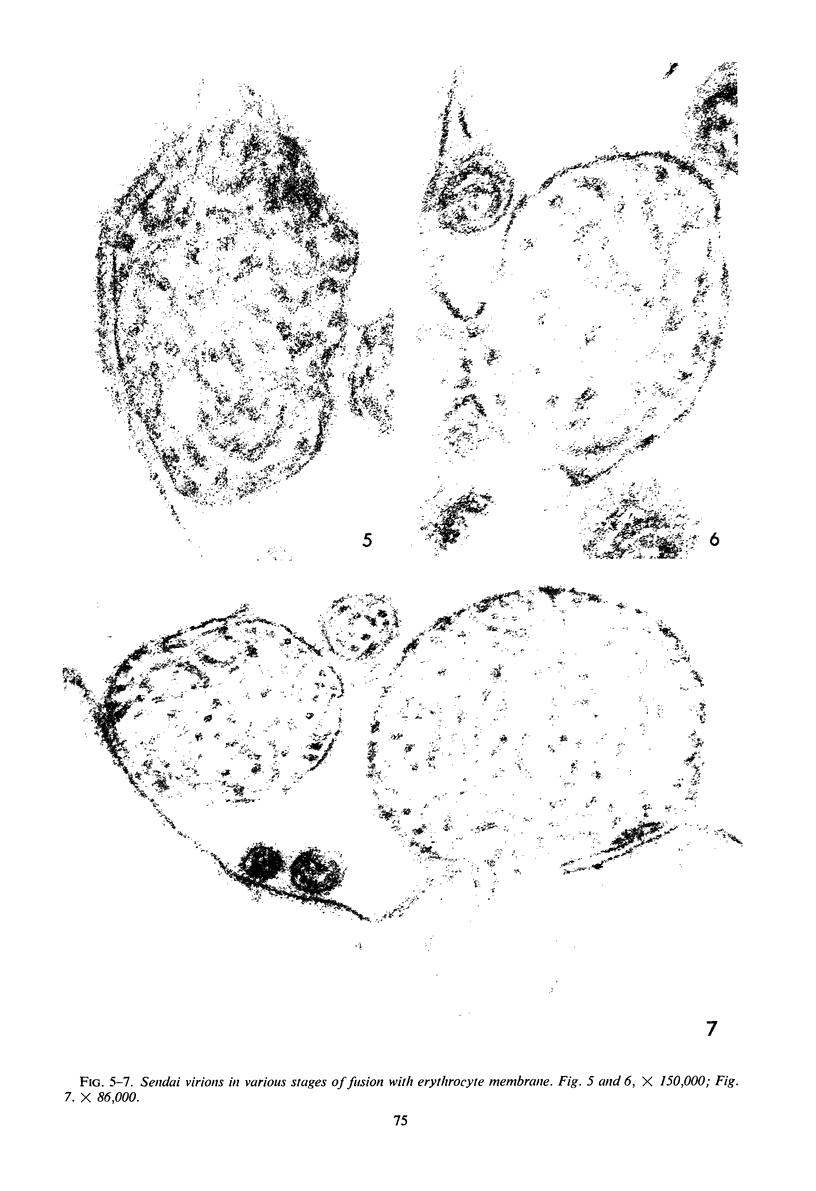
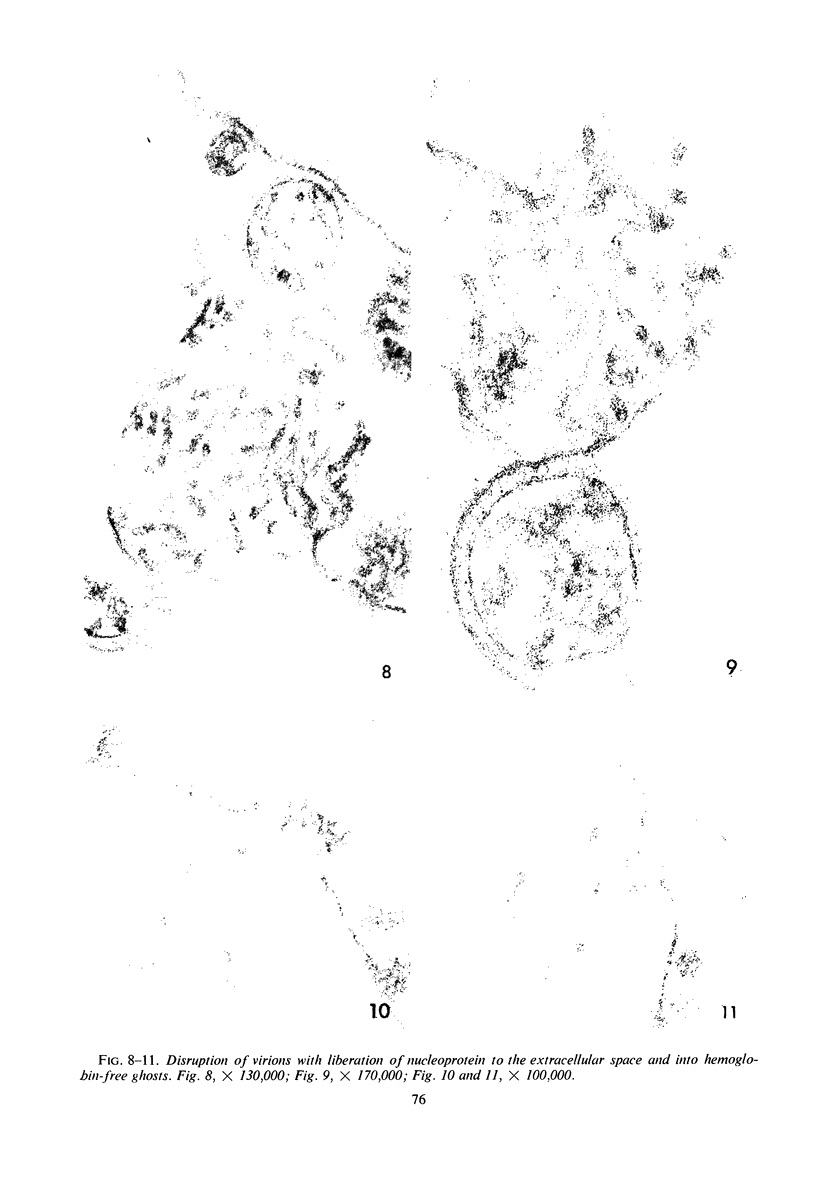
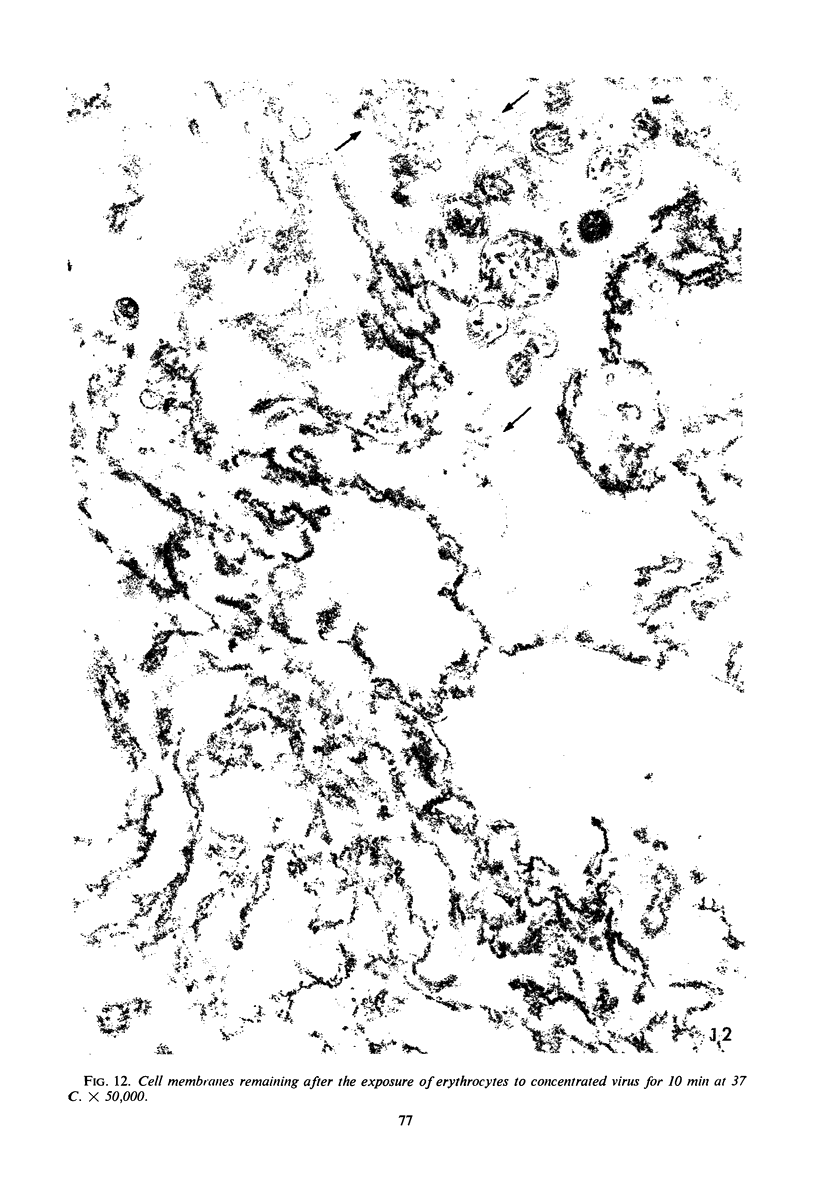
Concentrated Sendai virus, when adsorbed to erythrocytes at 4 C, caused invaginations in the plasma membrane. Following elevation of the temperature to 37 C, the plasma membrane became fused with the viral envelope before dissolution of the virions and rupture of the cells. Cell lysis was accompanied by rapid and total loss of hemoglobin to the extracellular space. Following aqueous pyridine extraction, the hemoglobin-free ghosts remaining were found to be devoid of N-acetylneuraminic acid and to have solubility properties different from those of normal erythrocyte ghosts. By the action of viral neuraminidase, bound N-acetylneuraminic acid was also liberated from purified virus receptor substance whose electrophoretic mobility was thereby substantially reduced. Cu++ selectively inhibited hemolysis and neuraminidase without interfering with hemagglutination and attachment. Neuraminidase appeared to be essential for Sendai virus hemolysis; viral particle size may also be a critical factor in this process.
Full text
PDF


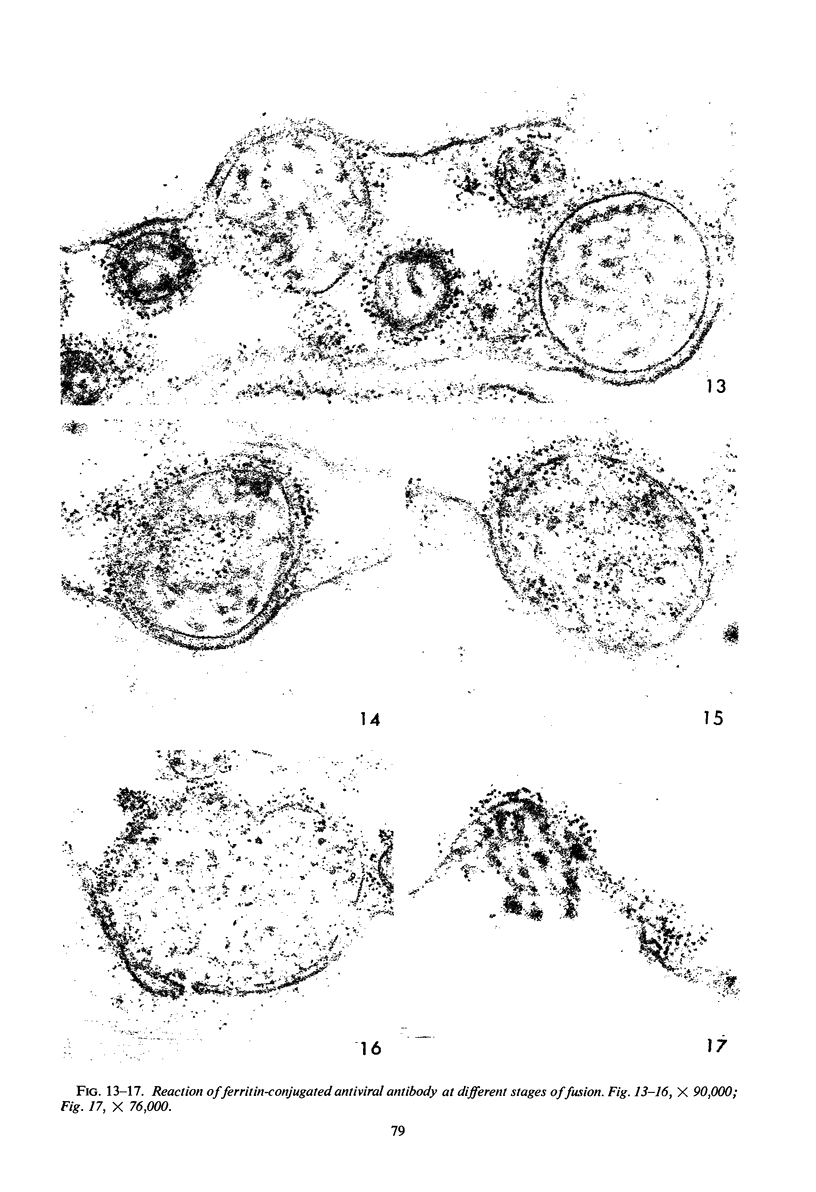


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenfeld O. O. The proteins of the erythrocyte membrane obtained by solubilization with aqueous pyridine solution. Biochem Biophys Res Commun. 1968 Jan 25;30(2):200–205. doi: 10.1016/0006-291x(68)90471-3. [DOI] [PubMed] [Google Scholar]
- DANON D., MARIKOVSKY V. DETERMINATION OF DENSITY DISTRIBUTION OF RED CELL POPULATION. J Lab Clin Med. 1964 Oct;64:668–674. [PubMed] [Google Scholar]
- DARRELL R. W., HOWE C. THE NEURAMINIDASE OF PARAINFLUENZA VIRUS (TYPE 2). Proc Soc Exp Biol Med. 1964 Aug-Sep;116:1091–1094. doi: 10.3181/00379727-116-29461. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- HOWE C., AVRAMEAS S., VAUXSTCYRC DE, GRABAR P., LEE L. T. ANTIGENIC COMPONENTS OF HUMAN ERYTHROCYTES. J Immunol. 1963 Nov;91:683–692. [PubMed] [Google Scholar]
- HOWE C., LEE L. T., ROSE H. M. Collocalia mucoid: a substrate for myxovirus neuraminidase. Arch Biochem Biophys. 1961 Dec;95:512–520. doi: 10.1016/0003-9861(61)90184-9. [DOI] [PubMed] [Google Scholar]
- Hosaka Y., Koshi Y. Electron microscopic study of cell fusion by HVJ virions. Virology. 1968 Mar;34(3):419–434. doi: 10.1016/0042-6822(68)90062-7. [DOI] [PubMed] [Google Scholar]
- KOHN A. POLYKARYOCYTOSIS INDUCED BY NEWCASTLE DISEASE VIRUS IN MONOLAYERS OF ANIMAL CELLS. Virology. 1965 Jun;26:228–245. doi: 10.1016/0042-6822(65)90050-4. [DOI] [PubMed] [Google Scholar]
- MOBERLY M. L., MARINETTI G. V., WITTER R. F., MORGAN H. R. Studies of hemolysis of red blood cells by mumps virus. III. Alterations in lipoproteins of the red blood cell wall. J Exp Med. 1958 Jan 1;107(1):87–94. doi: 10.1084/jem.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
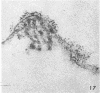
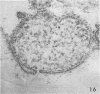
- Morgan C., Howe C. Structure and development of viruses as observed in the electron microscope. IX. Entry of parainfluenza I (Sendai) virus. J Virol. 1968 Oct;2(10):1122–1132. doi: 10.1128/jvi.2.10.1122-1132.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama F., Okada Y. Effect of calcium on the cell fusion reaction caused by HVJ. Biken J. 1965 Jun;8(2):103–105. [PubMed] [Google Scholar]
- NEURATH A. R. CHANGES IN THE HAEMOLYTIC ACTIVITY OF SENDAI VIRUS AFTER DIFFERENT CHEMICAL AND PHYSICAL TREATMENTS. Acta Virol. 1964 Mar;8:143–153. [PubMed] [Google Scholar]
- NEURATH A. R. EFFECT OF TRYPSIN TREATMENT ON THE HAEMOLYTIC, HAEMAGGLUTINATING, NEURAMINIDASE AND ADENOSINE DIPHOSPHATASE ACTIVITIES OF SENDAI VIRUS. Acta Virol. 1963 Nov;7:490–497. [PubMed] [Google Scholar]
- NEURATH A. R. SEPARATION OF A HAEMOLYSIN FROM MYXOVIRUSES AND ITS POSSIBLE RELATIONSHIP TO NORMAL CHORIOALLANTOIC MEMBRANE CELLS. Acta Virol. 1964 Mar;8:154–162. [PubMed] [Google Scholar]
- Okada Y., Murayama F., Yamada K. Requirement of energy for the cell fusion reaction of Ehrlich ascites tumor cells by HVJ. Virology. 1966 Jan;28(1):115–130. doi: 10.1016/0042-6822(66)90312-6. [DOI] [PubMed] [Google Scholar]
- RAFELSON M. E., Jr, SCHNEIR M., WILSON V. W., Jr STUDIES ON THE NEURAMINIDASE OF INFLUENZA VIRUS. II. ADDITIONAL PROPERTIES OF THE ENZYMES FROM THE ASIAN AND PR 8 STRAINS. Arch Biochem Biophys. 1963 Dec;103:424–430. doi: 10.1016/0003-9861(63)90432-6. [DOI] [PubMed] [Google Scholar]
- REBEL G., FONTANGES R., COLOBERT L. [The lipid nature of substances responsible for the hemolytic activity of Myxo-virus paraninfluenzae I (Sendai virus)]. Ann Inst Pasteur (Paris) 1962 Feb;102:137–152. [PubMed] [Google Scholar]
- SOKOL F., NEURATH A. R. Subunits of Myxoviruses. V. Inactivation of Sendai virus haemolysin by treatment with ether and the role of virus receptors in haemolysis. Acta Virol. 1962 Mar;6:122–126. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]