Summary
The formation of a barrier between epithelial cells is a fundamental determinant of cellular homeostasis, protecting underlying cells against pathogens, dehydration and damage. Assembly of the tight junction barrier is dependent upon neighboring epithelial cells binding to one another and forming adherens junctions, but the mechanism for how these processes are linked is poorly understood. Using a knockdown and substitution system, we studied whether ZO-1 binding to α-catenin is required for coupling tight junction assembly to the formation of adherens junctions. We found that preventing ZO-1 binding to α-catenin did not appear to affect adherens junctions. Rather the assembly and maintenance of the epithelial barrier were disrupted. This disruption was accompanied by alterations in the mobility of ZO-1 and the organization of the actin cytoskeleton. Thus, our study identifies α-catenin binding to ZO-1 as a new mechanism for coupling the assembly of the epithelial barrier to cell-to-cell adhesion.
Key words: ZO-1, α-Catenin, Tight junction, Adherens junction
Introduction
In order to assemble and maintain tissues, neighboring epithelial cells must tightly adhere to one another and form a barrier to ions and macromolecules. This assembly of cell–cell junctions is highly coordinated both spatially and functionally, with the adhesions between cells forming first and the barrier forming second. In addition to occuring in a sequential fashion, the assembly of the barrier is completely dependent upon the adhesion between cells (Gumbiner and Simons, 1987; Gumbiner, 1988). How the adhesion and barrier machineries are linked or coupled is not well understood and is critical for understanding not only how mammals maintain homeostasis but also the numerous diseases that arise from barrier defects.
Strong cell-to-cell adhesion is mediated by the adherens junctions. The adherens junctions are composed of two major complexes: the nectin-based adhesions and cadherin-based adhesions. The nectin-based adhesions assemble when the extracellular domain of nectins dimerize with nectins on neighboring cells (Takahashi et al., 1999). On the inside of the cell, the nectins bind to numerous cytoplasmic proteins, including afadin (Takahashi et al., 1999). Afadin is an actin binding protein that is necessary for the assembly of cadherin-based adhesion (Ikeda et al., 1999). Afadin also binds α-catenin, ponsin and zonula occludens-1 (ZO-1) (Mandai et al., 1997; Mandai et al., 1999; Tachibana et al., 2000; Pokutta et al., 2002; Yamada et al., 2006).
The tethering of neighboring cells by the nectin-based adhesions allows E-cadherin, the major cell-surface adhesion receptor in epithelial cells, to mediate cell-to-cell adhesion by binding cadherins on neighboring cells (Gumbiner et al., 1988; Ikeda et al., 1999). The cadherin cytoplasmic tail binds β-catenin which in turn binds α-catenin (Aberle et al., 1994; Huber et al., 1997). Like the nectins, the cadherin-based adhesions are highly integrated with other junctional complexes with some of the components of the cadherin adhesion complex binding to constituents of the nectin-based adhesions. For example, α-catenin binds afadin directly, and this interaction is necessary for strong cell-to-cell adhesion (Asakura et al., 1999; Tachibana et al., 2000; Pokutta et al., 2002).
Soon after the adherens junctions begin to form, the tight junctions assemble. The tight junctions serve as a fence separating apical and basal proteins and establishing cell polarity, and as a barrier to limit the movement of ions and macromolecules across the paracellular space [(Goodenough and Revel, 1970; Mandel et al., 1993), reviewed by Shen et al. (Shen et al., 2011)]. Tight junctions are composed of occludin and members of the claudin family, transmembrane proteins that dimerize across cells (McCarthy et al., 1996; Inai et al., 1999). Another critical component of the tight junctions is ZO-1. ZO-1 is a cytoplasmic actin binding protein that is required for tight junction assembly, organization and maintenance (Stevenson et al., 1986; Furuse et al., 1994; Fanning et al., 1998; Itoh et al., 1999).
While the individual components of the adherens and tight junctions have emerged, an appreciation of how these components are coupled to give rise to a coordinated assembly process is not understood. Two apparent contradictory models have been proposed. In one model, afadin binding to ZO-1 prior to the assembly of cell–cell junctions is essential for linking tight junctions to adherens junctions. Consistent with this notion, cells expressing an afadin deletion mutant lacking an intact ZO-1 binding region have intact adherens junctions but impaired tight junctions (Ooshio et al., 2010). However, this model does not take into account a role for the cadherin based adhesions which other work suggests is critical (Capaldo and Macara, 2007). α-Catenin associates with ZO-1 suggesting this interaction might be important in coupling the assembly of tight junctions to adherens junctions (Rajasekaran et al., 1996; Van Itallie et al., 2013). In this study we examined the role of α-catenin binding to ZO-1 in linking the assembly of tight junctions to adherens junction formation.
Results
Tight junctions, but not adherens junctions, are disrupted in cells lacking the α-catenin C-terminus
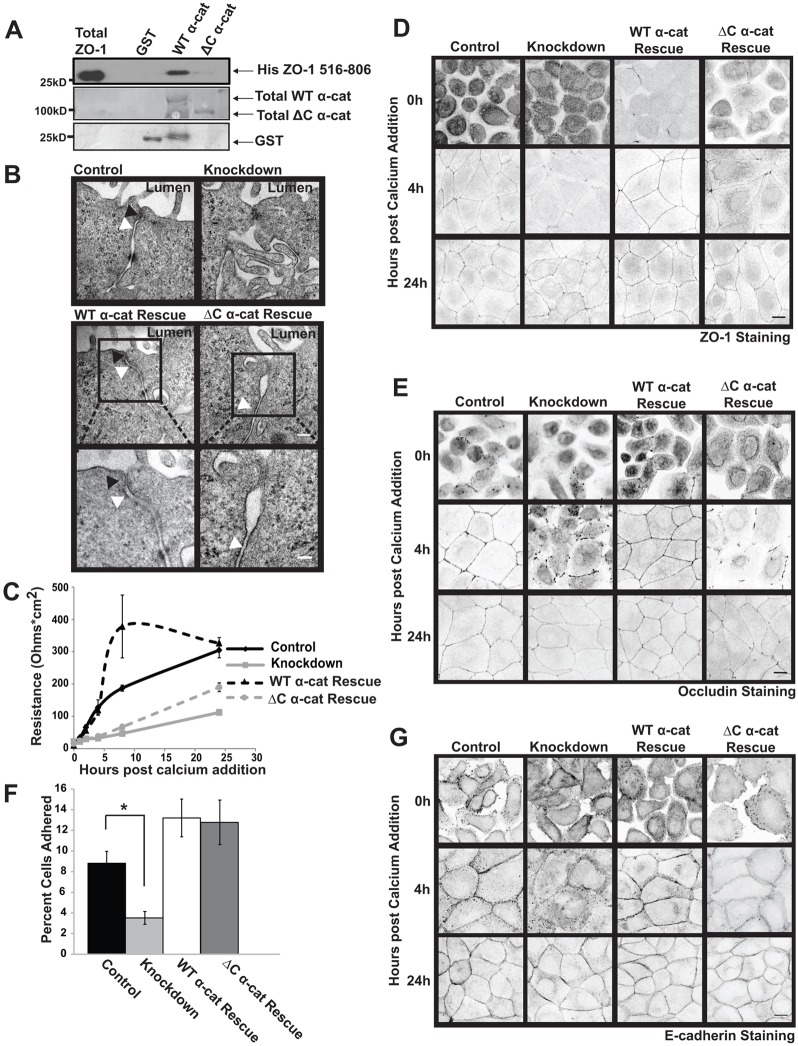
At the outset of this work we were motivated to identify the consequence, if any, that α-catenin binding to ZO-1 had on coupling the assembly of tight junctions to adherens junction formation. The precise binding site on α-catenin that ZO-1 binds was not known so we began our studies by testing the effect that loss of the α-catenin C-terminus, a region previous implicated in binding and recruiting ZO-1, had on junctional assembly. We generated a truncation mutant lacking the C-terminal 209 residues of α-catenin (ΔC) and tested whether this mutant protein bound to purified ZO-1. Unlike wild-type (WT) α-catenin, which bound quite well, little, if any, ZO-1 bound to the truncated protein suggesting that this region is critical (Fig. 1A).
Fig. 1.
The α-catenin C-terminus is required for the assembly of tight junctions, but not adherens junctions. (A) The α-catenin C-terminus is necessary for ZO-1 binding. GST or GST fused to either a full-length α-catenin or a C-terminal-truncated form of α-catenin were purified, bound to glutathione–Sepharose beads and incubated with a purified fragment of ZO-1 containing the predicted α-catenin-binding site (residues 516–806). The proteins were recovered, resolved using SDS-PAGE and immunoblotted with an antibody that recognizes the His tag on ZO-1 (upper panel). The immunoblot was stained with Coomassie Blue to show the levels of the GST proteins employed in the assay (lower panels). (B) Tight junctions and adherens junctions display no gross structural alterations in ΔC α-cat Rescue cells. MDCK II cells were infected with GFP-tagged α-catenin (WT α-cat Rescue) or the C-terminal truncation mutant fused to GFP (ΔC α-cat Rescue) and infected a second time with viruses encoding shRNAs targeting canine α-catenin (Knockdown). Cells expressing GFP and an empty shRNA targeting vector were used as a control (Control). The cell–cell junctions were visualized using TEM 4 hours after the assembly of cell–cell junctions was initiated by restoring Ca2+ to Ca2+-starved cells. The black arrows denote tight junctions and white arrows denote adherens junctions. Insets of the boxed areas within WT α-cat Rescue and ΔC α-cat Rescue micrographs are located below the original images. Scale bars: 0.2 µm for top 4 images (shown in the middle row), 0.1 µm for insets. (C) The permeability of the epithelial monolayer is disrupted during junction assembly in ΔC α-cat Rescue cells. The transelectrical epithelial resistance (TER) in confluent cultures of cells was measured using a voltometer at 0, 1, 2, 4, 6, 8 and 24 hours after junctional assembly was initiated by restoring Ca2+ to serum-starved cells. The data in the graph represent the mean±s.e.m. for four independent experiments. (D,E) The integrity of the tight junctions is altered in ΔC α-cat Rescue cells. ZO-1 (D) and occludin (E) localization was examined by immunofluorescence in Control, Knockdown, WT α-cat Rescue and ΔC α-cat Rescue cells that had been incubated in Ca2+-depleted medium (0 h) 4 or 24 hours after Ca2+ restoration. Representative confocal images are shown in inverted grayscale so that the differences between WT α-cat Rescue and ΔC α-cat Rescue cells can be readily visualized. Scale bar: 10 µm. (F) Cadherin-mediated adhesion is maintained in ΔC α-cat Rescue cells. Control, Knockdown, WT α-cat Rescue and ΔC α-cat Rescue cell lines were plated on surfaces coated with cadherin extracellular domains, washed, and the adherent cells were counted. The data presented in the graph are the mean±s.e.m. from four independent experiments. *P≤0.01 (G) ΔC α-cat Rescue cells display proper localization of E-cadherin. E-cadherin localization was examined by immunofluorescence in Control, Knockdown, WT α-cat Rescue or ΔC α-cat Rescue cells incubated overnight in Ca2+-depleted medium (0 h), 4 or 24 hours after Ca2+ was restored to the cell cultures. Representative pictures are shown in inverted grayscale. Scale bar: 10 µm.
We wanted to generate a system whereby we could study the effects of this introduced α-catenin mutant protein in cells. Consequently, we established a knockdown/addback approach for studying α-catenin function in MDCK II cells. To this end, endogenous α-catenin levels were stably silenced using a shRNA targeting canine α-catenin (Knockdown). These cells were rescued with either GFP-fused full length human α-catenin (WT α-cat Rescue) or a GFP-fused mutant α-catenin lacking the C-terminal 209 amino acids (ΔC α-cat Rescue). Cells expressing an empty shRNA targeting vector and GFP alone were used as a control (Control). Expression of endogenous α-catenin and/or the re-expressed proteins were examined by immunoblotting (supplementary material Fig. S1A). α-Catenin levels were reduced by 88±1% in Knockdown cells. To determine if the C-terminal truncation disrupted α-catenin localization during junction assembly, we analyzed α-catenin deposition at cell–cell contacts 4 hours after induction of junction assembly. Both WT α-catenin and the C-terminal truncation localized to regions of cell–cell contact, indicating that the C-terminal truncation does not affect localization of α-catenin (supplementary material Fig. S1B).
We hypothesized that if ZO-1 binding to α-catenin was critical for coupling the assembly of tight junctions to adherens junctions, cells expressing the truncation mutant should not be able to assemble tight junctions despite forming adherens junctions to wildtype levels. To analyze the role of the α-catenin C-terminus on the assembly of adherens and tight junctions, we disassembled cell–cell junctions by incubating the cells in medium lacking Ca2+ and monitored their formation by transmission electron microscopy (TEM) 4 hours after the cells were returned to growth medium. Control cells exhibited typical tight and adherens junctions as evidenced by close apposition of plasma membranes and apparent occlusion of the paracellular space in the most apical aspect of the lateral membrane (Fig. 1B). Adherens junctions were visible as a rigidly consistent space between the plasma membranes of adjacent cells. In contrast, cell–cell junctions were disrupted in Knockdown cells as evidenced by finger-like protrusions that failed to adhere to one another. This effect could be rescued by re-expression of WT α-catenin (Fig. 1B). Interestingly, tight junctions did form, but they were much less discrete. Also a gap between adherens junctions and tight junctions in ΔC α-cat Rescue cells were observed.
The α-catenin C-terminus is required for coupling the assembly of tight junctions to adherens junctions
To examine if loss of the α-catenin C-terminus disrupted the tight junctions, we examined the function of the barrier in cells expressing the mutant form of α-catenin with the C-terminus deleted. For this, cells were grown to confluence on transwell filters and incubated in medium lacking Ca2+ overnight. Resistance across the cell monolayer was measured using a voltometer at 0, 1, 2, 4, 8 and 24 hours post Ca2+ addition (Fig. 1C). At 4, 8 and 24 hours post Ca2+ addition, both the Knockdown and ΔC α-cat Rescue cells exhibited significantly reduced resistance across the cell monolayer. To assess if the disruption of permeability was a result of a loss in the integrity of the tight junctions, we monitored ZO-1 and occludin localization at 0, 4 and 24 hours after Ca2+ was restored. ZO-1 and occludin deposition at cell–cell contacts were disrupted 4 hours after junction assembly was initiated in the Knockdown and ΔC α-cat Rescue cells (Fig. 1D,E). At 24 hours, ZO-1 and occludin localization were restored in both Knockdown and ΔC α-cat Rescue cells. Thus, the α-catenin C-terminus plays a role in the proper assembly of tight junctions.
To determine if these effects were specific to the tight junctions, we examined whether loss of the α-catenin C-terminus affected adherens junction assembly. To test the functionality of the adherens junctions, the ability of the various cell lines to adhere to immobilized cadherin extracellular domains was assessed. Knockdown cells exhibited a 60% decrease in cadherin-mediated adhesion compared to Control cells (P<0.005). WT α-cat Rescue and ΔC α-cat Rescue cells bound ∼1.5 times better than Control cells respectively (Fig. 1F). This increase of adhesion in comparison to Control cells is consistently observed with proteins that are slightly overexpressed (Peng et al., 2010). As another measure of adherens junction integrity, E-cadherin localization was analyzed at 0, 4 and 24 hours after the assembly of cell–cell junctions was initiated. Similar to previous work (Capaldo and Macara, 2007), depletion of α-catenin disrupted the assembly of adherens junctions in Knockdown cells as evident through disrupted and punctate staining of E-cadherin at the cell periphery 4 hours post-Ca2+ addition, though this phenotype became less pronounced at 24 hrs. In both the WT α-cat Rescue and ΔC α-cat Rescue cells, E-cadherin localized to cell–cell junctions (Fig. 1G). We did note some differences in the morphology of the ΔC α-cat Rescue cells, but this was not due to an alteration in the ability of cadherins to form homophilic interactions (Fig. 1F). Furthermore, these effects were not the result of altered expression of tight junctional and adherens junctional proteins as expression of ZO-1, occludin, afadin, E-cadherin and actin were unaltered (supplementary material Fig. S1C). Taken together, these data indicate that the tight junctions are disrupted in cells lacking the α-catenin C-terminus and suggest that the α-catenin C-terminus is required to couple the assembly of tight junctions to the adherens junctions.
Disruption of ZO-1 binding to α-catenin via proline insertion
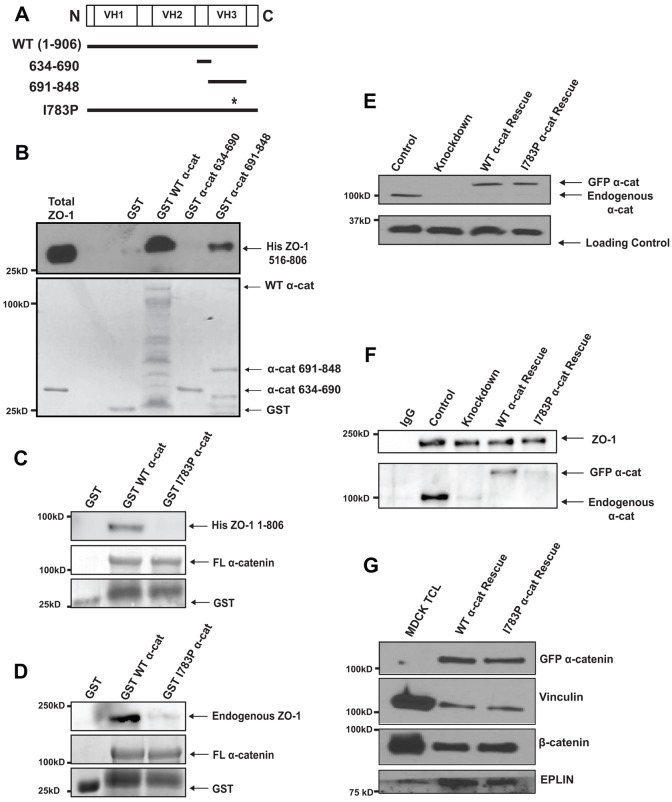
Given that ZO-1 is a key regulator of tight junctions, we considered the possibility that its binding to α-catenin was the critical interaction. To test if ZO-1 binding to the α-catenin C-terminus is required for coupling the assembly of tight junctions to adherens junctions, we considered generating a mutant form of α-catenin that specifically ablates ZO-1 binding. We mapped the ZO-1 binding site on α-catenin using α-catenin fragments expressed as GST fusion proteins (Fig. 2A). Fragments that contained residues 691–848 bound ZO-1 (Fig. 2B). To define further which amino acids within this sequence were important for binding of ZO-1, we made point mutations in evolutionarily conserved residues in this region. An isoleucine residue at 783 is located in the middle of a putative α-helix within this α-catenin region. We substituted a proline for this isoleucine (I783P α-cat) and tested its ability to bind ZO-1. We found that full length α-catenin harboring the I783P substitution consistently did not bind to a fragment of ZO-1; in contrast, the wildtype α-catenin protein bound well (Fig. 2C). To ensure that endogenous, full length ZO-1 behaved similarly as the fragments of ZO-1 utilized in Fig. 2B,C, we measured the ability of GST, GST WT α-catenin, or I783P α-catenin to recover full length ZO-1 from cell lysates. ZO-1 bound WT α-catenin well but failed to co-purify with I783P α-catenin (Fig. 2D). Thus, the I783P substitution in α-catenin blocks binding to both purified and endogenous ZO-1.
Fig. 2.
α-Catenin harboring an I783P substitution does not bind ZO-1 in vitro or in MDCKII cells. (A) A linear schematic of α-catenin and the fragments of α-catenin used in this study (GST–α-catenin). (B,C) In vitro binding of ZO-1 to various GST–α-catenin proteins. The indicated α-catenin proteins were purified, attached to beads and incubated with purified His-tagged ZO-1 (516–806 for B; 1–806 for C). The levels of GST-tagged α-catenin proteins employed were similar, as represented by the Coomassie-stained blot in the lower panels. Note that in C, ZO-1 fails to bind α-catenin I783P. We consistently obtained some GST in the preparations of the full-length (FL) α-catenin proteins. (D) Endogenous ZO-1 binds WT α-catenin but not I783P α-catenin. GST, GST-tagged WT α-catenin or GST-tagged I783P α-catenin were prebound to glutathione beads and incubated with MDCKII cell lysates. The GST-tagged proteins were recovered and separated using SDS-PAGE. The co-precipitating levels of ZO-1 were examined by immunoblotting. This figure shows that endogenous, full-length ZO-1 binds to WT α-catenin, but fails to bind I783P α-catenin. (E) Expression levels of I783P α-catenin in MDCKII cells. MDCKII cells expressing an shRNA against canine α-catenin were infected a second time with retroviruses encoding GFP–α-catenin with the I783P substitution (I783P α-cat Rescue). Lysates were harvested from cell lines stably expressing these proteins or Control, Knockdown or WT α-cat Rescue cells. Immunoblot analysis was performed using antibodies against α-catenin or the p34-Arc subunit of the Arp2/3 complex as a loading control. (F) ZO-1 fails to co-immunoprecipitate with α-catenin I783P. Confluent monolayers of the indicated cell lines were Ca2+-starved overnight and lysed 1 hour post Ca2+ addition. ZO-1 was immunoprecipitated, and the bound proteins were washed, resolved using SDS-PAGE, and immunoblotted with an antibody against ZO-1 or α-catenin. (G) Substitution of I783P does not impair α-catenin binding to other ligands. WT α-cat Rescue or I783P α-cat Rescue cells were grown to confluence, lysed and full-length α-catenin and α-catenin I783P were immunoprecipitated using an antibody against GFP. Proteins were recovered, resolved using SDS-PAGE, and immunoblotted with antibodies against vinculin, β-catenin or EPLIN to show the levels of each protein recovered, or GFP to show the levels of each α-catenin protein recovered.
To determine if the I783P substitution disrupts ZO-1 binding in cells, we generated a GFP-tagged full length mutant α-catenin with the I783P substitution and used it to rescue the α-catenin knockdown cells (I783P α-cat Rescue). This mutant α-catenin was expressed to similar levels as the wildtype protein in the MDCKII cells (i.e 129±40% compared to 120±24% of the level of endogenous α-catenin, Fig. 2E). The α-catenin point mutant localized to regions of cell–cell contact as well as the wild-type protein (supplementary material Fig. S2A). Also, there appeared to be few differences between WT α-cat Rescue and I783P α-cat Rescue cells when examined by TEM (supplementary material Fig. S2B). We tested the ability of the I783P mutant to co-immunoprecipitate with ZO-1. ZO-1 bound to α-catenin in Control and WT α-catenin Rescue cells but did not bind I783P α-catenin or the residual α-catenin present in the Knockdown cells (Fig. 2F). Finally, to ensure that this substitution did not interfere with α-catenin binding to other proteins, we examined β-catenin, vinculin and EPLIN binding to the mutant α-catenin. β-Catenin binds to the N-terminus of α-catenin, vinculin has been shown to interact with the VH2 domain of α-catenin, whereas EPLIN is known to bind to the C-terminus of α-catenin (Huber et al., 1997; Abe and Takeichi, 2008; Peng et al., 2010; Yonemura et al., 2010). The I783P substitution did not affect recruitment of any of these proteins to α-catenin (Fig. 2G). Together, this data shows that substitution of I783P in α-catenin specifically blocks ZO-1 binding while leaving both its binding to other proteins and its subcellular localization unperturbed.
Tight junction assembly and function are altered by I783P substitution
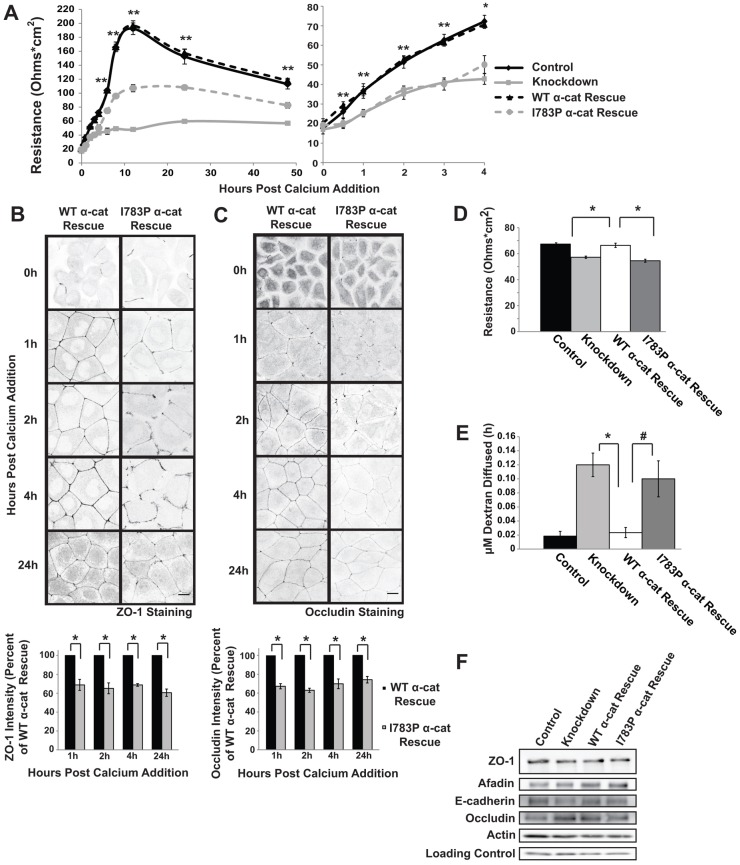
To determine if ZO-1 binding to α-catenin is responsible for the tight junction alterations in cells expressing ΔC α-catenin, we examined if I783P α-cat Rescue cells could establish a paracellular barrier by measuring the transelectrical epithelial resistance across confluent monolayers of the epithelial cell lines. The WT α-cat Rescue and Control cells exhibited a rapid increase in resistance upon Ca2+ readdition, reaching a peak around 12 hours and approaching basal levels over 24–48 hours, whereas Knockdown cells displayed only a gradual increase in resistance and maintained a relatively low resistance up to 48 hours after Ca2+ readdition, suggesting that tight junction assembly is disrupted (Fig. 3A). Similarly, resistance was disrupted in I783P α-cat Rescue cells during both early assembly (0.5–4 hours) (Fig. 3A, right panel) and at later times (6–48 hours) (Fig. 3A, left panel). It appeared from these initial studies that both the early establishment and the later maintenance of the solute barrier were disrupted (Fig. 3A). We explored both possibilities further. We examined the various cell lines by immunofluorescence at early time points after junctional assembly and found dramatic differences in ZO-1 (Fig. 3B) and occludin (Fig. 3C) deposition in regions of cell–cell contact. Specifically, the I783P α-cat Rescue cells showed that ZO-1 deposition in junctions was reduced to 69% (at 1 hr), 65% (at 2 hr), 69% (at 4 hr) and 61% (at 24 h) in comparison to the wildtype expressing cells (Fig. 3B, bottom panel). Similarly, occludin localization was decreased to 67% (at 1 hr), 63% (at 2 hr), 70% (at 4 hr) and 75% (at 24 hr, Fig. 3C, bottom panel). There were also stark differences in the continuity of the staining patterns in the I783P α-cat Rescue cells as numerous breaks were observed (Fig. 3B,C). These data support the notion that loss of ZO-1 binding to α-catenin disrupts recruitment of ZO-1 into a continuous band at the apical junction complex, which likely accounts for the altered kinetics of barrier assembly.
Fig. 3.
Tight junctions are altered in cells expressing α-catenin with the I783P substitution. (A) The establishment of an epithelial barrier is disrupted in cells expressing I783P α-catenin. Confluent cultures of the indicated cells lines were incubated overnight in Ca2+-free medium (0 h). Ca2+ was restored to the cultures for the indicated times and resistance was measured using a voltometer on quadruplicate filters. The graph displays the mean±s.d. expressed in Ohms*cm2. The right panel shows the early time points (0–4 h) so that the initial delay in barrier establishment can be visualized. *P≤0.01 and **P≤0.001 compared with control. (B,C) The integrity of the tight junction is altered in cells expressing the α-catenin I783P point mutant that doesn't bind ZO-1. WT α-cat Rescue and I783P α-cat Rescue cells were examined using immunofluorescence and antibodies against ZO-1 (B) or occludin (C). Representative confocal images are shown in inverted grayscale. Scale bars: 10 µm. The intensity of ZO-1 or occludin at cell–cell contacts was determined using ImageJ and is shown below the images as the percentage intensity compared with WT α-cat Rescue. *P≤0.01. (D,E) The maintenance of the epithelial barrier is disrupted in I783P-expressing cells. (D) The indicated cell lines were grown to confluence, and resistance across the cell monolayer was measured daily until a consistent reading was made for three consecutive days. The graphed data represents the mean±s.e.m. for four independent experiments. The paracellular flux of 3 kDa FITC-conjugated dextran in the same cultures was measured (E) and the graph represents the mean±s.e.m. for four independent experiments. #P≤0.05; *P≤0.01. (F) Expression of tight and adherens junction proteins are unaltered in I783P α-cat Rescue cells. Lysates from the indicated cell lines were harvested and immunoblotting was performed to analyze the expression levels of ZO-1, occludin, actin, afadin and E-cadherin. p34-Arc serves as a loading control.
To determine if the small differences in resistance that we observed at later time points during junction assembly translated into long-term defects in tight junction permeability, we examined the resistance and flux of solutes in cultures that had been maintained at confluence for several days. We found that under steady state conditions the resistance showed similar defects to those observed at 24 and 48 hours post-assembly (Fig. 3D; Fig. 3A). Since the flux of uncharged solutes is much more sensitive to ZO-1 levels than resistance measurements, we also monitored the movement of 3 kD FITC-conjugated dextran across the cell monolayer at steady-state conditions (Van Itallie et al., 2009; Fanning et al., 2012). Small amounts of dextran migrated across monolayers of Control or WT α-cat Rescue cells (Fig. 3E). In contrast, approximately four to five times as much dextran migrated through the monolayers of Knockdown and 1783P α-cat Rescue cells (Fig. 3E). Moreover, these findings are not the result of alterations in expression of the junctional proteins as occludin, ZO-1, E-cadherin, afadin and actin were all expressed to similar levels in the I783P α-cat and WT α-cat Rescue cells (Fig. 3F). Taken together these findings indicate that preventing ZO-1 binding to α-catenin alters the assembly of the paracellular barrier, and this effect is propagated to long term effects on solute permeability.
The I783P α-catenin mutant disrupts ZO-1 mobility and the actin cytoskeleton
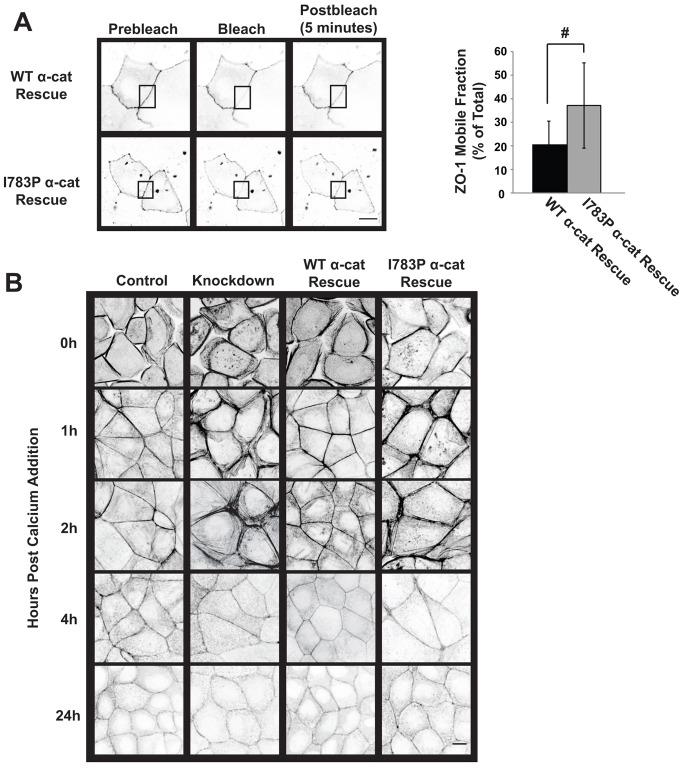
Changes in tight junction permeability are often associated with differential mobility of junctional components (Shen et al., 2008; Yu et al., 2010) and actin cytoskeletal changes (Ivanov et al., 2004; Yu et al., 2010). To determine if ZO-1 mobility at cell–cell junctions is altered in I783P α-cat Rescue cells, we monitored the reappearance of ZO-1 in cell–cell junctions five minutes after photobleaching using fluorescence recovery after photobleaching (FRAP). For these studies, Control, WT α-cat Rescue and I783P α-cat Rescue cells were transfected with a plasmid encoding mCherry ZO-1. Cell–cell junctions between two adjacent transfected cells were photobleached. We found that the mobile fraction of ZO-1 was increased in I783P α-cat Rescue cells compared to WT α-cat Rescue cells (37.1% vs 20.4%, P<0.05) (Fig. 4A). The amount of ZO-1 in the mobile fraction is slightly less than has been observed in MDCK I and Caco-2 cells (Shen et al., 2008; Yu et al., 2010). This difference may be accounted for by the fact that MDCK II cells have additional tight junction components that are not present in MDCK I cells (Dukes et al., 2011).
Fig. 4.
ZO-1 mobility and actin organization is altered by proline substitution at I783. (A) ZO-1 mobility is increased in I783P α-cat Rescue cells. ZO-1 mobility at cell–cell contacts was examined using FRAP. Cells were transfected with mCherry-tagged ZO-1 and FRAP was performed on adjacent transfected cells. The images presented are a representation of cells prior to bleaching (Prebleach), immediately after bleaching (Bleach) and 5 minutes after bleaching (Postbleach). The box indicates the bleached area. The amount of ZO-1 that recovered was quantified and the mobile fraction of ZO-1 was calculated and is shown in the right panel. (B) Actin organization is disrupted in Knockdown and I783P α-cat Rescue cells. Cells were grown to confluence and incubated overnight in Ca2+-free medium. Ca2+ was restored for the indicated times and actin organization was analyzed using immunofluorescence. Representative images are presented in inverted grayscale. Scale bars: 10 µm.
Another key determinant of the tight junction barrier function is the integrity of the actin cytoskeleton. ZO-1 and/or α-catenin organize the cytoskeleton at the cell–cell junctions (Drees et al., 2005; Hartsock and Nelson, 2008; Fanning et al., 2012; Desai et al., 2013). To determine if loss of ZO-1 binding to α-catenin disrupts actin organization during the formation of cell–cell junctions, actin localization was analyzed using immunofluorescence at 0, 1, 2, 4 and 24 hours after assembly was initiated (Fig. 4B). In the Control and WT α-cat Rescue cells, actin was discretely localized in a tight band in cell–cell junctions. In contrast, the organization of actin in Knockdown and I783P α-cat Rescue cells was disrupted. At 1 and 2 hours, diffuse actin structures were observed with several stray actin bundles extending into the cell interior (Fig. 4B). These phenotypes were not as pronounced at 4 hours and were indistinguishable from wild-type re-expressing cells at 24 hours. These less compacted actin phenotypes share some similarities with the actin cytoskeletal defects in cells lacking ZO-1, ZO-2 and Ephrin A4 (Yamazaki et al., 2008). No differences were found in the intensity of actin staining at the cell–cell junctions among the cell lines (supplementary material Fig. S2C). Collectively, these findings suggest that α-catenin binding to ZO-1 is required for the organization of the actin cytoskeleton and anchoring of ZO-1 at areas of cell–cell contact and provide a possible explanation for how severing this interaction gives rise to altered epithelial permeability.
Adherens junction assembly and cadherin-mediated adhesion are unaffected by the I783P substitution
We hypothesize that if ZO-1 binding to α-catenin was critical for coupling the assembly of tight and adherens junctions, the tight junctional phenotypes should not be the result of alterations in adherens junction function. To examine the integrity of the adherens junctions, cadherin-mediated adhesion was measured by examining the number of cells that adhered to immobilized cadherin extracellular domains. Consistent with previous reports, Knockdown cells adhered poorly when compared to Control cells (Fig. 5A) (Shimoyama et al., 1992; Nagafuchi et al., 1994). WT α-cat Rescue and I783P α-cat Rescue cells adhered to a similar extent (Fig. 5A). As a second measure of the integrity of the cadherin-based adhesions, we examined localization of E-cadherin in cells expressing I783P α-catenin. E-cadherin localization was unaffected by the I783P substitution at 1, 2, 4 and 24 hours after the assembly of cell–cell junctions was initiated (Fig. 5B, quantified in supplementary material Fig. S2D). Hence, while I783P α-catenin disrupts tight junctions, the adherens junctions do not appear to be affected by loss of ZO-1 binding to α-catenin.
Fig. 5.
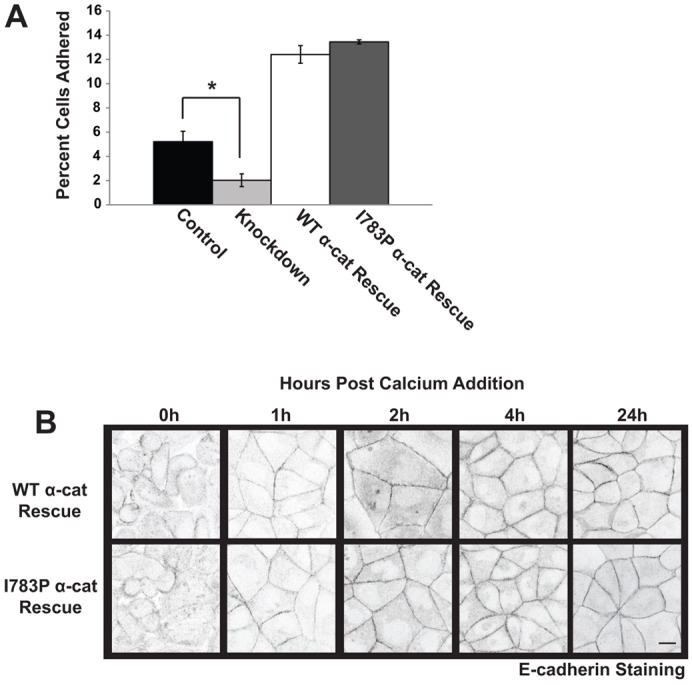
The proline substitution at I783 has no effect on adherens junction assembly or cadherin-mediated adhesion. (A) Cadherin-mediated adhesion is established in the presence of I783P α-catenin. The adhesion of the indicated cell lines to immobilized cadherin extracellular domains was examined as described in Fig. 1F. The data presented in the graph are the mean±s.e.m. cells adhered from four independent experiments. (B) E-cadherin localization is unaffected by I783P substitution. WT α-cat Rescue and I783P α-cat Rescue cells were grown to confluence, incubated in Ca2+-free medium overnight, and placed in Ca2+-containing medium for 0, 1, 2, 4 or 24 hours. The cells were then fixed, and examined by immunofluorescence using a Zeiss 510 confocal microscope. Images are presented in inverted grayscale. Scale bar: 10 µm.
Afadin binding to α-catenin and localization during junction assembly is unaffected by the α-catenin I783P substitution
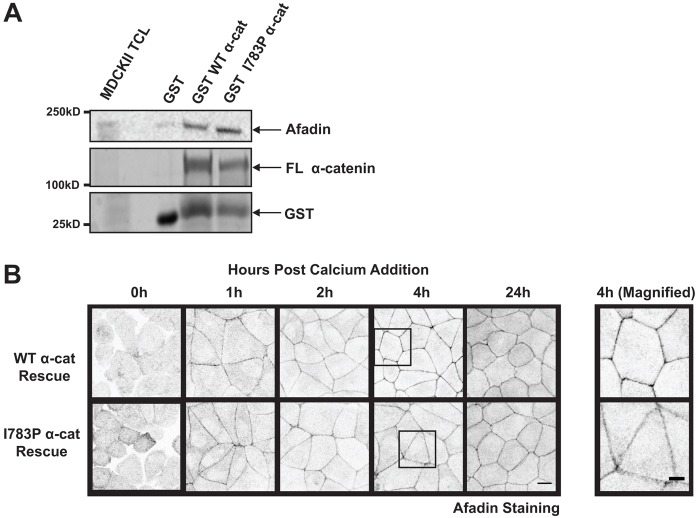
Afadin associates with the nectin-based adhesions before the cadherin-based adhesions and tight junctions begin to form, and this interaction is thought to be important in coupling the assembly of tight junctions to adherens junctions (Tachibana et al., 2000). Afadin binds the VH2 domain of α-catenin. While this region is far removed from the α-catenin point mutation that we made, we examined whether or not afadin bound to I783P α-catenin and if afadin localization to cell–cell junctions was intact. Both WT α-catenin and I783P α-catenin bound to afadin to a similar extent (Fig. 6A). Furthermore, afadin localized to cell–cell contacts as early as 1 hour after the assembly of cell–cell junctions was initiated in both WT α-cat Rescue and I783P α-cat Rescue cells and continued to accumulate to wildtype levels up to 24 hours (Fig. 6B). The amounts of afadin that accumulated in cell–cell junctions were not statistically significant between the two cell types (supplementary material Fig. S2E). However, we did consistently observe that afadin was not as discretely localized in cell–cell junctions in I783P α-cat Rescue cells when compared to WT α-cat Rescue cells at 4 h post-Ca2+ switch (Fig. 6B, right panel). We cannot rule out the possibility that these changes in afadin deposition contribute to the tight junction defects we observed. However a loss of afadin or preventing afadin from localizing to adherens junctions typically produces altered localization of E-cadherin and α-catenin (Tachibana et al., 2000; Sato et al., 2006). Hence we do not believe that these changes in afadin deposition contribute to the tight junction defects. Rather, we believe that these changes might arise from the morphological changes that we observe in the I783P cells.
Fig. 6.
Afadin localization and binding to α-catenin is unaffected by I783P substitution. (A) The I783P substitution has no effect on afadin binding to α-catenin. Purified GST-tagged FL α-catenin or I783P α-catenin were bound to glutathione beads and incubated with MDCKII cell lysates. The beads were washed and the bound proteins were separated using SDS-PAGE. Afadin levels were determined using by immunoblotting and the levels of GST-tagged WT α-catenin and I783P α-catenin loaded were determined by Coomassie staining. (B) Afadin localization to adherens junction during assembly is not affected by expression of I783P α-catenin. Afadin localization to cell–cell junctions was examined before (0 h) or 1, 2, 4 or 24 h after Ca2+ was restored to serum-starved cultures. Images are shown in inverted grayscale. The right panel displays magnified inserts of the boxed areas within the WT α-cat Rescue and I783P α-cat Rescue images at 4 h. Scale bars: 10 µm (B, left panels); 5 µm (B, right panels).
Discussion
In this study we investigated mechanisms for coupling the assembly of tight junctions to adherens junctions. Previous studies implicated the nectin-based adhesion machinery in this process (Ooshio et al., 2010), but this explanation was incomplete as other work indicated a critical role for the cadherin-based adhesion machinery (Capaldo and Macara, 2007). α-Catenin binds ZO-1 once cell–cell junction formation is initiated (Yonemura et al., 1995; Rajasekaran et al., 1996; Itoh et al., 1997). Here, we tested if this interaction is required for coupling the assembly of tight junctions to adherens junctions. To test this possibility, we established a powerful knockdown/addback approach for studying α-catenin function in cells and found that loss of the α-catenin C-terminal tail or substitution of I783P (specifically) prevents ZO-1 binding. Preventing this interaction had no effect on E-cadherin localization and function. Rather it dramatically altered the integrity of the tight junctions and impaired the establishment and maintenance of an epithelial barrier. The mechanism underlying these phenotypes likely involves anchoring a pool of ZO-1 at cell–cell junctions as well as cytoskeletal organization as I783P α-cat Rescue cells display increased mobility of ZO-1 as well as loose actin bundling at the cell periphery. Hence, ZO-1 binding to α-catenin is critical for coupling the assembly of tight junctions to adherens junction formation.
These data suggest that the assembly of apical junction complex may occur in a sequential fashion such that the adherens junctions form prior to the assembly of the tight junctions. In support of this notion, it has been shown that cadherin-based adhesions are disrupted when nectin-based adhesions are disrupted. Furthermore, tight junctions do not form in the absence of afadin and only weak cadherin-based adhesions assemble (Sato et al., 2006). In addition, we show that binding of ZO-1 to α-catenin is a later step in the assembly process as preventing this interaction does not affect afadin binding to α-catenin or its enrichment into cell–cell junctions (Fig. 6A,B). Finally, occludin and ZO-1 colocalization at cell–cell junctions follows that of E-cadherin and ZO-1 (Ando-Akatsuka et al., 1999).
What our data does not explain is whether or not the same ZO-1 molecule is shuttled from afadin to α-catenin and then to occludin or whether different ZO-1 populations bind each. The binding sites for afadin and α-catenin on ZO-1 are not overlapping suggesting that a tripartite interaction could exist (Müller et al., 2005; Ooshio et al., 2010). However, α-catenin and occludin bind to overlapping sites on ZO-1 indicating that it is likely that binding is mutually exclusive (Muller et al., 2005). These observations raise the intriguing possibility that there is some competition for the different ligands to bind ZO-1. Future work is needed to resolve this issue and identify the signal transduction events that regulate the individual steps in the assembly process.
If α-catenin binding to ZO-1 couples the assembly of tight junctions to adherens junctions as we propose, it should follow then that the phenotypes found in I783P α-cat Rescue cells should be at least partially overlapping with the phenotypes of cells lacking ZO-1 expression. Consistent with this notion we found several similarities. First, occludin recruitment to cell–cell junctions is impaired in the I783P α-cat Rescue cells and in cells with ZO-1 expression suppressed (Fig. 3C) and (Umeda et al., 2004; McNeil et al., 2006; Fanning et al., 2012). Second, the actin cytoskeleton is disrupted in cells lacking ZO-1/2, a phenotype rescued by reexpression of the N-terminal half of ZO-1, which contains the α-catenin-binding site but not the actin-binding region. Third, loss of ZO-1 or preventing ZO-1 binding to α-catenin delays the formation of a barrier to ions [compare supplemental data in Van Itallie et al. (Van Itallie et al., 2009) and Fanning et al. (Fanning et al., 2012) and Fig. 3]. There are two measures of tight junction permeability: ion permeability and solute permeability. Solute permeability is analyzed by measuring the flux of different sized molecules across the monolayer. Unlike the resistance measurements which are instantaneous, flux is measured over a long period of time (typically 2 hours). In cells lacking ZO-1 and in our I783P α-cat Rescue cells, the flux of 3 kDa Dextran across the monolayer is much greater than in Control cells indicating a defect in solute permeability (Fig. 3E) and (Van Itallie et al., 2009; Fanning et al., 2012). On the other hand, ion permeability is measured by examining the instantaneous transepithelial electrical resistance across a monolayer. Both the I783P α-cat Rescue cells and ZO-1 knockdown cells are delayed in formation of a barrier to ions (Fig. 3A) (Van Itallie et al., 2009; Fanning et al., 2012). Hence, there are some striking similarities between our I783P cells and those lacking ZO-1. Interestingly, we did also find some differences in the steady state resistance measurements in the I783P α-cat Rescue cells that were not uncovered in the cells lacking ZO-1 (Fig. 3D). The size of the functional changes we observe here are similar to changes in the barrier that are believed to contribute to inflammatory bowel disease (Shen et al., 2011). With a similar fold decrease in a person, proteins would be flowing out of the serosal space and into lumen of kidney tubules (proteinuria) or into the gastrointestinal tract (Shen et al., 2011). Hence, while these changes appear to be small, they can have a profound effect physiologically and provide insight into the etiology of inflammatory bowel diseases and into the steady state regulation of paracellular permeability.
The mechanism underlying reduced ZO-1 and occludin localization, as well as increased ion and solute permeability is likely to be attributed to slight alterations in the organization of the actin cytoskeleton and altered ZO-1 mobility at cell–cell contacts (Fig. 4A,B). Interesting while we do observe slight changes in the organization of actin bundles (i.e. many loosely packed bundles present), we do not see gross changes in E-cadherin localization or binding capacity suggesting that adherens junctions are unaffected. Moreover, we do not yet know if the defects between altered actin bundling morphologies are linked to ZO-1 mobility. In support of a linkage, a role for actin in mediating ZO-1 mobility at cell–cell junctions has been previously described (Shen et al., 2008; Yu et al., 2010). While actin has been implicated in regulating ZO-1 mobility, the involvement of α-catenin in this process is novel. This raises the question of how these two proteins might cooperate to organize the actin cytoskeleton at cell–cell junctions. Intriguing possibilities are that binding affects the ability of one or both proteins to recruit, stabilize or coordinate the formation of actin networks at the adherens junction complex. This role would be restricted to early events of junctional assembly and not as important later since ZO-1 and α-catenin are segregated in mature junctions. Future work is aimed at addressing these possibilities.
In summary, α-catenin binding to ZO-1 is a new mechanism for coupling the assembly of tight junctions to adherens junctions. This finding increases our understanding of the process of junction assembly at cell–cell contacts and provides a mechanism through which tight junctions and adherens junctions are linked. Future studies will be aimed at understanding if alterations in the recruitment of ZO-1 to α-catenin contribute the development or progression of pathological states that are associated with tight junction defects.
Materials and Methods
Constructs
pLEGFP-C1-FL α-catenin is a full-length human α-catenin cDNA fused to GFP. pGEX4T1-FL α-catenin is full length human α-catenin fused with GST and is previously described (Peng et al., 2010). pGEX4T1-α-catenin fragments were developed through PCR-amplifying corresponding regions of human α-catenin. pET14b-TEV-ZO-1 516–806 is a fragment of human ZO-1 spanning the SH3-GUK domains. pFastBacHTa-ZO-1 is a fragment of ZO-1 spanning residues 1–806 as described previously (Utepbergenov et al., 2006). pLEGFP-C1 α-catenin ΔC-term (spanning residues 1–697), pLEGFP-C1 α-catenin I783P, pGEX4T1-α-catenin ΔC and pGEX4T1-FL α-catenin I783P were constructed using site directed mutagenesis. The α-catenin ΔC constructs were engineered through introduction of a stop codon after nucleotide 2091, truncating the protein. The α-catenin I783P construct was engineered through introduction of a single amino acid substitution in pLEGFP-C1-FL α-catenin or pGEX4T1-FL α-catenin.
Cell lines and infection
MDCKII canine kidney epithelial cells (kindly provided by Charles Yeaman, University of Iowa, Iowa City, IA) were maintained in DMEM medium supplemented with 10% FBS, 1% penicillin and streptomycin and 1% L-glutamine in a 10% CO2 incubator at 37°C. Stable MDCKII cells expressing GFP-tagged proteins were developed through infection of cells with retrovirus (Peng et al., 2010). Retroviruses were harvested and MDCKII cells were infected with viruses encoding pSUPER-RETRO or pSUPER-sh α-catenin and pLEGFP-Cl, pLEGFP-C1 FL α-catenin, pLEGFP-C1 α-catenin 1–697, or pLEGFP-C1 FL α-catenin I783P. Clonal populations of infected cells were selected in 0.5 mg/mL G418 and 2 mg/ml puromycin.
Ca2+ switch and immunofluorescence
A standard Ca2+ switch assay was performed. The cells were incubated in Ca2+-free medium overnight, and then Ca2+-containing medium was restored to the cultures for the indicated times. Typically after the Ca2+ switch, cells were processed for immunofluorescence. To visualize E-cadherin, cells were fixed in 3.7% paraformaldehyde in PBS, permeabilized in 0.5% Triton X-100 in universal buffer (UB: 150 mM NaCl, 50 mM Tris pH 7.6, 0.01% NaN3) for 10 minutes and washed in UB. Cells were blocked in 2% goat serum + 0.2% BSA in UB for 40 minutes at room temperature, incubated with primary antibody for 90 minutes, washed and incubated with a secondary antibody for 45 minutes at room temperature. To visualize occludin, cells were fixed in 1% paraformaldehyde in PBS, permeabilized in 0.25% Triton X-100 in PBS for 10 minutes and washed in PBS. Cells were blocked in 1% BSA in PBS for 40 minutes at 37°C, incubated with a primary antibody overnight at 4°C, washed and incubated with a secondary antibody for 45 minutes at 37°C. To visualize ZO-1 and afadin, cells were fixed in 1% paraformaldehyde in PBS, permeabilized in 0.25% Triton X-100 in PBS for 10 minutes and washed in PBS. Cells were blocked in 1% BSA in PBS for 40 minutes at room temperature, incubated with a primary antibody for 90 minutes at room temperature, washed and incubated with a secondary antibody for 45 minutes at room temperature. To visualize actin, cells were fixed in 3.7% paraformaldehyde in PBS, permeabilized in 0.1% Triton in PBS for 5 minutes and washed in PBS for 10 minutes. Coverslips were incubated with a conjugated primary antibody for 90 minutes at 37°C, washed in PBS and mounted. E-cadherin was visualized using RR-1 (Developmental Studies Hybridoma, University of Iowa, Iowa City, IA) at 1∶50, followed by a Texas-Red-conjugated donkey anti-mouse IgG (H+L) (Jackson ImmunoResearch Laboratories) at 1∶300. Occludin was visualized using mouse anti-occludin (Zymed) at 1∶200 followed by a texas red-conjugated donkey anti-mouse IgG at 1∶300. ZO-1 was visualized using mouse anti-ZO-1 (Invitrogen) followed by a Texas-Red-conjugated donkey anti-mouse IgG at 1∶300. Afadin was visualized using rabbit anti-afadin (Sigma) followed by Texas-Red-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories) at 1∶300. Actin was visualized using Texas Red-X phalloidin (Invitrogen) at 1∶250. With the exception of the cells examined 24 h after Ca2+ restoration to the media, the cells in each experiment were fixed and prepared the same day. Images were captured with a confocal microscope (model LSM 510; Carl Zeiss MicroImaging) using a 63×oil objective (Carl Zeiss MicroImaging) with an NA of 1.4 and the same exposure time and laser intensity. Images were obtained using the LSM Image Browser (Carl Zeiss MicroImaging) as described previously (Peng et al., 2010). Quantification of the images was calculated using ImageJ.
FRAP
WT α-cat Rescue and I783P α-cat Rescue cells were transfected with mCherry-tagged full length ZO-1 using Lipofectamine 2000 (Invitrogen). 24 hours post transfection, cells were placed in HBSS containing CaCl2 and MgCl2 and analyzed at room temperature. FRAP was performed with a LSM 710 microscope (Carl Zeiss MicroImaging) using a 63×immersion lens with a NA of 1.4. Images were obtained with the FRAP module within the Zen 2010 software. Junctions between adjacent cells expressing mCherry-tagged ZO-1 were excited at 561 nm and images were taken at 60-second intervals to examine fluorescence recovery. Once a plateau was achieved, the percent recovery of mCherry-tagged ZO-1 was calculated to determine the mobile fraction of ZO-1 in WT α-cat Rescue and I783P α-cat Rescue cells.
Transmission electron microscopy
TEM was performed as previously described (Peng et al., 2010). Cells were grown to confluence on 0.4 µm Transwell® filters and fixed in 2.5% gluteraldehyde in 0.1M cacodylate buffer. The cells were rinsed in 0.1M cacodylate buffer and processed for transmission electron microscopy by routine procedures. Ultrathin sections were prepared and imaged in a JEOL JEM-1230 transmission electron microscope equipped with a Gatan Ultrascan 2kx2k CCD camera.
Adhesion assays
CHO cells expressing E-cadherin-Fc were generously provided by Chris Stipp (University of Iowa, Iowa City, IA) and E-cadherin-Fc was purified as previously described (Johnson et al., 2009). Adhesion assays were performed as described in (Johnson et al., 2009) with the following changes: 5×105 cells were plated on 10 cm dishes prior to the assay. Following coating and blocking with BSA, 1.07×105 cells were plated on 24-well plates for 30 minutes, washed and counted in triplicate. Percent cells adhered to the coated wells were analyzed using ImageJ.
Transepithelial electrical resistance
Cells were plated on Costar® 0.4 µm Polycarbonate membrane Transwell® 12-well plates and grown to confluence. TER was measured in quadruplicate using a Millipore Voltmeter (MERS 000 01). Results are in Ω*cm2.
Dextran flux assays
FITC–Dextran permeability was measured as previously described (Van Itallie et al., 2009), with the following changes: Only the movement of 3 kDa fluorescein-conjugated dextran (Invitrogen) was measured and the concentration of fluorescent dextran in the lower chamber was quantified using a fluorescent plate reader (Wallac Victor2 1420 Multilabel). Results are in µM diffused/hour.
Protein purification
Recombinant GST, and GST-tagged α-catenin, α-catenin I783P and α-catenin truncations, and His6-tagged ZO-1 516–806 were expressed in and purified from bacteria using affinity chromatography (Fanning et al., 2007; Peng et al., 2012). Alternatively, a His-tagged ZO-1 construct spanning residues 1–806 was expressed in baculovirus, purified and eluted as previously described (Utepbergenov et al., 2006). Following elution, proteins were dialyzed against PBS. Proteins were concentrated using the Amicon Ultra 3,000 or 30,000 MWCO systems (Millipore) and stored at −20°C.
In vitro binding assays
GST-tagged α-catenin proteins (1 µM) were bound to BSA blocked glutathione beads in binding buffer (PBS pH 7.4, 1% Triton X-100, 100 µM CaCl2). His-tagged ZO-1 (1 µM) was then added to the beads and the mixtures were rocked for 90 minutes at 4°C. The proteins were recovered, resolved using SDS-PAGE and immunoblotted using an antibody against the His tag (Covance).
Co-immunoprecipitation and immunoblotting
MDCKII cells were cultured in Ca2+-free medium overnight, after which medium containing Ca2+ was added for 1 hour. The cells were washed and rocked on ice in lysis buffer (20 mM HEPES pH 7.5, 0.5% DOC, 0.2% SDS, 150 mM NaCl, 2 mM EDTA, 1% Triton plus protease inhibitors) for 30 minutes (Gumbiner et al., 1991). The cells were lysed and cleared by centrifugation at 13,000 rpm for 30 minutes. ZO-1 was immunoprecipitated using the ZO-1 antibody R26.4C developed by Barry Gumbiner and obtained from the Developmental Studies Hybridoma Bank (The University of Iowa). The supernatants were incubated at 4°C for 90 minutes, bound to protein G Sepharose beads (Sigma), washed and resuspended in sample buffer. The recovered proteins were separated using SDS-PAGE and immunoblotted for α-catenin using the polyclonal antibody C 2081 (Sigma). The following antibodies and dilutions were used for immunoblotting: E-cadherin (clone rr1 from the Developmental Studies Hybridoma Bank, University of Iowa) was used at a 1:100 dilution, occludin (Invitrogen, clone OC-3F10) was used at 1:500 dilution, p34-Arc (DeMali et al., 2002) was used at 1:1000, vinculin (hVin-1, Sigma) was used at 1:1000, a rabbit polyclonal against β-catenin (Sigma) was used at 1:1000.
Pulldown assays
ZO-1 or afadin binding to GST, and GST-tagged α-catenin or α-catenin I783P was analyzed as described previously (Pokutta et al., 2002) with the following modifications. Cells were grown to 80% confluence before lysing. GST, GST-tagged α-catenin and I783P α-catenin (5 µM) were prebound to glutathione beads and the beads were incubated with MDCKII cell lysates for 2 h at 4°C (Pokutta et al., 2002). The bound proteins were resolved using SDS-PAGE. ZO-1 or afadin were detected by immunoblotting with an antibody against ZO-1 (Invitrogen) or afadin (Sigma).
Supplementary Material
Acknowledgments
We thank Dr Tom Moringer and Dr Jianqiang Shao in the Central Microscopy Facility at the University of Iowa College of Medicine for their help in completing the transmission EM studies, and Drs David Rimm, James Nelson and Charles Yeaman for the generous gift of reagents and equipment. We are grateful to Peter Rubenstein and members of the DeMali laboratory for critical comments on the manuscript. The E-cadherin antibody was developed by Barry Gumbiner and the ZO-1 antibody was developed by D.A. Goodenough. Both were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Author contributions
J.L.M. participated in the execution and design of experiments, data interpretation, and manuscript preparation. X.P. constructed the α-catenin knockdown cell lines and participated in the manuscript preparation. A.S.F. provided key reagents, and aided in the experimental design, interpretation of data and manuscript preparation. K.A.D. participated in the conception and design of experiments, data interpretation and manuscript preparation.
Funding
This work is supported by the National Science Foundation [grant number 1120478 to K.A.D.]; the National Institutes of Health [grant number DK061397 to A.S.F.]; and American Heart Association Predoctoral Fellowships [grant numbers 0910127G to X.P. and 9010011 to J.L.M.]. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.126565/-/DC1
References
- Abe K., Takeichi M. (2008). EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc. Natl. Acad. Sci. USA 105, 13–19 10.1073/pnas.0710504105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberle H., Butz S., Stappert J., Weissig H., Kemler R., Hoschuetzky H. (1994). Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J. Cell Sci. 107, 3655–3663 [DOI] [PubMed] [Google Scholar]
- Ando-Akatsuka Y., Yonemura S., Itoh M., Furuse M., Tsukita S. (1999). Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J. Cell. Physiol. 179, 115–125 [DOI] [PubMed] [Google Scholar]
- Asakura T., Nakanishi H., Sakisaka T., Takahashi K., Mandai K., Nishimura M., Sasaki T., Takai Y. (1999). Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 4, 573–581 10.1046/j.1365-2443.1999.00283.x [DOI] [PubMed] [Google Scholar]
- Capaldo C. T., Macara I. G. (2007). Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 18, 189–200 10.1091/mbc.E06-05-0471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali K. A., Barlow C. A., Burridge K. (2002). Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 159, 881–891 10.1083/jcb.200206043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R., Sarpal R., Ishiyama N., Pellikka M., Ikura M., Tepass U. (2013). Monomeric α-catenin links cadherin to the actin cytoskeleton. Nat. Cell Biol. 15, 261–273 10.1038/ncb2685 [DOI] [PubMed] [Google Scholar]
- Drees F., Pokutta S., Yamada S., Nelson W. J., Weis W. I. (2005). Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123, 903–915 10.1016/j.cell.2005.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes J. D., Whitley P., Chalmers A. D. (2011). The MDCK variety pack: choosing the right strain. BMC Cell Biol. 12, 43 10.1186/1471-2121-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A. S., Jameson B. J., Jesaitis L. A., Anderson J. M. (1998). The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 273, 29745–29753 10.1074/jbc.273.45.29745 [DOI] [PubMed] [Google Scholar]
- Fanning A. S., Little B. P., Rahner C., Utepbergenov D., Walther Z., Anderson J. M. (2007). The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol. Biol. Cell 18, 721–731 10.1091/mbc.E06-08-0764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning A. S., Van Itallie C. M., Anderson J. M. (2012). Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol. Biol. Cell 23, 577–590 10.1091/mbc.E11-09-0791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M., Itoh M., Hirase T., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. (1994). Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell Biol. 127, 1617–1626 10.1083/jcb.127.6.1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Revel J. P. (1970). A fine structural analysis of intercellular junctions in the mouse liver. J. Cell Biol. 45, 272–290 10.1083/jcb.45.2.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B. (1988). Cadherins: a family of Ca2+-dependent adhesion molecules. Trends Biochem. Sci. 13, 75–76 10.1016/0968-0004(88)90040-0 [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Simons K. (1987). The role of uvomorulin in the formation of epithelial occluding junctions. Ciba Found. Symp. 125, 168–186 [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Stevenson B., Grimaldi A. (1988). The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J. Cell Biol. 107, 1575–1587 10.1083/jcb.107.4.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B., Lowenkopf T., Apatira D. (1991). Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl. Acad. Sci. USA 88, 3460–3464 10.1073/pnas.88.8.3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsock A., Nelson W. J. (2008). Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778, 660–669 10.1016/j.bbamem.2007.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O., Krohn M., Kemler R. (1997). A specific domain in alpha-catenin mediates binding to beta-catenin or plakoglobin. J. Cell Sci. 110, 1759–1765 [DOI] [PubMed] [Google Scholar]
- Ikeda W., Nakanishi H., Miyoshi J., Mandai K., Ishizaki H., Tanaka M., Togawa A., Takahashi K., Nishioka H., Yoshida H. et al. (1999). Afadin: A key molecule essential for structural organization of cell-cell junctions of polarized epithelia during embryogenesis. J. Cell Biol. 146, 1117–1132 10.1083/jcb.146.5.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inai T., Kobayashi J., Shibata Y. (1999). Claudin-1 contributes to the epithelial barrier function in MDCK cells. Eur. J. Cell Biol. 78, 849–855 10.1016/S0171-9335(99)80086-7 [DOI] [PubMed] [Google Scholar]
- Itoh M., Nagafuchi A., Moroi S., Tsukita S. (1997). Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 138, 181–192 10.1083/jcb.138.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M., Furuse M., Morita K., Kubota K., Saitou M., Tsukita S. (1999). Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 147, 1351–1363 10.1083/jcb.147.6.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A. I., McCall I. C., Parkos C. A., Nusrat A. (2004). Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol. Biol. Cell 15, 2639–2651 10.1091/mbc.E04-02-0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Winterwood N., DeMali K. A., Stipp C. S. (2009). Tetraspanin CD151 regulates RhoA activation and the dynamic stability of carcinoma cell-cell contacts. J. Cell Sci. 122, 2263–2273 10.1242/jcs.045997 [DOI] [PubMed] [Google Scholar]
- Mandai K., Nakanishi H., Satoh A., Obaishi H., Wada M., Nishioka H., Itoh M., Mizoguchi A., Aoki T., Fujimoto T. et al. (1997). Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 139, 517–528 10.1083/jcb.139.2.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K., Nakanishi H., Satoh A., Takahashi K., Satoh K., Nishioka H., Mizoguchi A., Takai Y. (1999). Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J. Cell Biol. 144, 1001–1017 10.1083/jcb.144.5.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel L. J., Bacallao R., Zampighi G. (1993). Uncoupling of the molecular ‘fence’ and paracellular ‘gate’ functions in epithelial tight junctions. Nature 361, 552–555 10.1038/361552a0 [DOI] [PubMed] [Google Scholar]
- McCarthy K. M., Skare I. B., Stankewich M. C., Furuse M., Tsukita S., Rogers R. A., Lynch R. D., Schneeberger E. E. (1996). Occludin is a functional component of the tight junction. J. Cell Sci. 109, 2287–2298 [DOI] [PubMed] [Google Scholar]
- McNeil E., Capaldo C. T., Macara I. G. (2006). Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol. Biol. Cell 17, 1922–1932 10.1091/mbc.E05-07-0650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S. L., Portwich M., Schmidt A., Utepbergenov D. I., Huber O., Blasig I. E., Krause G. (2005). The tight junction protein occludin and the adherens junction protein alpha-catenin share a common interaction mechanism with ZO-1. J. Biol. Chem. 280, 3747–3756 10.1074/jbc.M411365200 [DOI] [PubMed] [Google Scholar]
- Nagafuchi A., Ishihara S., Tsukita S. (1994). The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J. Cell Biol. 127, 235–245 10.1083/jcb.127.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooshio T., Kobayashi R., Ikeda W., Miyata M., Fukumoto Y., Matsuzawa N., Ogita H., Takai Y. (2010). Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 285, 5003–5012 10.1074/jbc.M109.043760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Cuff L. E., Lawton C. D., DeMali K. A. (2010). Vinculin regulates cell-surface E-cadherin expression by binding to beta-catenin. J. Cell Sci. 123, 567–577 10.1242/jcs.056432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X., Maiers J. L., Choudhury D., Craig S. W., DeMali K. A. (2012). α-Catenin uses a novel mechanism to activate vinculin. J. Biol. Chem. 287, 7728–7737 10.1074/jbc.M111.297481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S., Drees F., Takai Y., Nelson W. J., Weis W. I. (2002). Biochemical and structural definition of the l-afadin- and actin-binding sites of alpha-catenin. J. Biol. Chem. 277, 18868–18874 10.1074/jbc.M201463200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekaran A. K., Hojo M., Huima T., Rodriguez-Boulan E. (1996). Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J. Cell Biol. 132, 451–463 10.1083/jcb.132.3.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Fujita N., Yamada A., Ooshio T., Okamoto R., Irie K., Takai Y. (2006). Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 281, 5288–5299 10.1074/jbc.M510070200 [DOI] [PubMed] [Google Scholar]
- Shen L., Weber C. R., Turner J. R. (2008). The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 181, 683–695 10.1083/jcb.200711165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Weber C. R., Raleigh D. R., Yu D., Turner J. R. (2011). Tight junction pore and leak pathways: a dynamic duo. Annu. Rev. Physiol. 73, 283–309 10.1146/annurev-physiol-012110-142150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama Y., Nagafuchi A., Fujita S., Gotoh M., Takeichi M., Tsukita S., Hirohashi S. (1992). Cadherin dysfunction in a human cancer cell line: possible involvement of loss of alpha-catenin expression in reduced cell-cell adhesiveness. Cancer Res. 52, 5770–5774 [PubMed] [Google Scholar]
- Stevenson B. R., Siliciano J. D., Mooseker M. S., Goodenough D. A. (1986). Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 103, 755–766 10.1083/jcb.103.3.755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K., Nakanishi H., Mandai K., Ozaki K., Ikeda W., Yamamoto Y., Nagafuchi A., Tsukita S., Takai Y. (2000). Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150, 1161–1176 10.1083/jcb.150.5.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Nakanishi H., Miyahara M., Mandai K., Satoh K., Satoh A., Nishioka H., Aoki J., Nomoto A., Mizoguchi A. et al. (1999). Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J. Cell Biol. 145, 539–549 10.1083/jcb.145.3.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K., Matsui T., Nakayama M., Furuse K., Sasaki H., Furuse M., Tsukita S. (2004). Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J. Biol. Chem. 279, 44785–44794 10.1074/jbc.M406563200 [DOI] [PubMed] [Google Scholar]
- Utepbergenov D. I., Fanning A. S., Anderson J. M. (2006). Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J. Biol. Chem. 281, 24671–24677 10.1074/jbc.M512820200 [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M., Fanning A. S., Bridges A., Anderson J. M. (2009). ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 20, 3930–3940 10.1091/mbc.E09-04-0320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Itallie C. M., Aponte A., Tietgens A. J., Gucek M., Fredriksson K., Anderson J. M. (2013). The N- and C- termini of ZO-1 are surrounded by distinct proteins and functional protein networks. J. Biol. Chem. 288, 13775–13788 10.1074/jbc.M113.466193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A., Fujita N., Sato T., Okamoto R., Ooshio T., Hirota T., Morimoto K., Irie K., Takai Y. (2006). Requirement of nectin, but not cadherin, for formation of claudin-based tight junctions in annexin II-knockdown MDCK cells. Oncogene 25, 5085–5102 [DOI] [PubMed] [Google Scholar]
- Yamazaki Y., Umeda K., Wada M., Nada S., Okada M., Tsukita S., Tsukita S. (2008). ZO-1- and ZO-2-dependent integration of myosin-2 to epithelial zonula adherens. Mol. Biol. Cell 19, 3801–3811 10.1091/mbc.E08-04-0352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S., Itoh M., Nagafuchi A., Tsukita S. (1995). Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J. Cell Sci. 108, 127–142 [DOI] [PubMed] [Google Scholar]
- Yonemura S., Wada Y., Watanabe T., Nagafuchi A., Shibata M. (2010). alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12, 533–542 10.1038/ncb2055 [DOI] [PubMed] [Google Scholar]
- Yu D., Marchiando A. M., Weber C. R., Raleigh D. R., Wang Y., Shen L., Turner J. R. (2010). MLCK-dependent exchange and actin binding region-dependent anchoring of ZO-1 regulate tight junction barrier function. Proc. Natl. Acad. Sci. USA 107, 8237–8241 10.1073/pnas.0908869107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.