Summary
Entry into mitosis or meiosis relies on the coordinated action of kinases and phosphatases that ultimately leads to the activation of Cyclin-B–Cdk1, also known as MPF for M-phase promoting factor. Vertebrate oocytes are blocked in prophase of the first meiotic division, an arrest that is tightly controlled by high PKA activity. Re-entry into meiosis depends on activation of Cdk1, which obeys a two-step mechanism: a catalytic amount of Cdk1 is generated in a PKA and protein-synthesis-dependent manner; then a regulatory network known as the MPF auto-amplification loop is initiated. This second step is independent of PKA and protein synthesis. However, none of the molecular components of the auto-amplification loop identified so far act independently of PKA. Therefore, the protein rendering this process independent of PKA in oocytes remains unknown. Using a physiologically intact cell system, the Xenopus oocyte, we show that the phosphorylation of ARPP19 at S67 by the Greatwall kinase promotes its binding to the PP2A-B55δ phosphatase, thus inhibiting its activity. This process is controlled by Cdk1 and has an essential role within the Cdk1 auto-amplification loop for entry into the first meiotic division. Moreover, once phosphorylated by Greatwall, ARPP19 escapes the negative regulation exerted by PKA. It also promotes activation of MPF independently of protein synthesis, provided that a small amount of Mos is present. Taken together, these findings reveal that PP2A-B55δ, Greatwall and ARPP19 are not only required for entry into meiotic divisions, but are also pivotal effectors within the Cdk1 auto-regulatory loop responsible for its independence with respect to the PKA-negative control.
Key words: ARPP19, Cdk1, Greatwall, Meiotic division, PP2A, PKA
Introduction
Eukaryotic cells reproduce by means of the mitotic cell cycle and require the activity of a universal Serine/Threonine (S/T) kinase, MPF (M-phase Promoting Factor). MPF is a complex between the kinase Cdk1 and its regulatory subunit, Cyclin B. Throughout G2-phase, Cyclin B is synthesized and associates with Cdk1, which is kept catalytically inactive by its phosphorylation on T14 and Y15 by the kinases Wee1 and Myt1. The G2-M phase transition is induced by the dephosphorylation of those residues, catalyzed by the phosphatase Cdc25 (Hunt and Nasmyth, 1997). Once activated, MPF orchestrates the ordered structural changes required for a cell to divide and to progress through M-phase by phosphorylating a specific set of protein substrates (Nigg, 1993).
It is well established that Cdk1 activation obeys a complex biochemical mechanism called ‘MPF auto-amplification’ (Masui and Markert, 1971; Morgan, 1995). This mechanism is initiated by the generation of a threshold level of activated MPF and relies on the property of Cdk1 to phosphorylate its own regulatory enzymes, Cdc25 and Myt1/Wee1. Once a starter amount of active MPF is present, it phosphorylates Wee1/Myt1 and Cdc25 promoting their respective inhibition and activation. This positive feedback mechanism amplifies Cdk1 activity and ensures commitment to a mitotic state contributing to the abrupt transition from G2- to M-phase. Other molecular players themselves controlled by Cyclin-B–Cdk1 have been shown to be part of this feedback loop by regulating Cdc25 and/or Wee1/Myt1 activities, complicating the simple tripartite core based on Cdk1-Cdc25-Wee1/Myt1 (Hunt and Nasmyth, 1997; Morgan, 1995). To decipher the molecular network of the auto-amplification loop, we took advantage of the Xenopus oocyte system, a historically major model that has contributed to considerable advances in our understanding of the regulation of MPF activation.
Amphibian oocytes are stably arrested in prophase of meiosis I and contain an inactive form of MPF, called pre-MPF, where Cdk1 is associated with Cyclin B. This complex is held inactive by inhibitory phosphorylations of Cdk1 on T14 and Y15. In response to progesterone, MPF is activated and induces the entry into meiotic M-phase characterized by the breakdown of the oocyte nucleus (or GVBD for Germinal Vesicle BreakDown) (Haccard and Jessus, 2006a). Two biochemical events are required for this initial activation of MPF in response to progesterone: a drop in cAMP-dependent protein kinase (PKA) activity and the synthesis of new proteins (Haccard and Jessus, 2006a). The rapid suppression of PKA activity several hours before MPF activation is a prominent biochemical event, both necessary and sufficient for meiosis resumption. PKA substrates are then predicted to directly or indirectly regulate the translation of mRNAs essential for meiotic resumption. Among newly synthesized proteins are Cyclin B1 and Mos, a S/T kinase that directly activates MEK, which in turn activates MAP kinase (MAPK) and then p90Rsk (Frank-Vaillant et al., 1999; Posada et al., 1993). Cyclin B1 synthesis is of particular importance since it can form new complexes with monomeric stockpiled Cdk1 to form the threshold level of Cdk1 activity responsible for the triggering of the auto-amplification loop (Gaffré et al., 2011; Haccard and Jessus, 2006b; Nebreda et al., 1995).
This initial step generating the first molecules of active MPF therefore depends on protein synthesis and is negatively controlled by PKA activity. In contrast, the MPF auto-amplification loop works independently of these upstream events. Indeed, a cytoplasm ‘MPF transfer’ from a metaphase II-arrested oocyte into a prophase oocyte induces MPF activation and GVBD in the absence of protein synthesis (Wasserman and Masui, 1975) or when PKA activity is maintained high (Daar et al., 1993). Therefore, all components of the auto-amplification loop should formally share the following specific and common features: their activation should depend on Cdk1; the microinjection of their active form in the oocyte should induce MPF activation and meiotic maturation independently of both protein synthesis and high PKA activity, as it is the case for MPF cytoplasmic transfer.
Oocyte studies have revealed that the Cdk1 auto-amplification loop includes several players, as Cyclin B, the homologue of the Drosophila polo kinase Plx1 (Abrieu et al., 1998; Karaïskou et al., 1999), members of the Mos/MAPK cascade (Haccard et al., 1995; Palmer et al., 1998; Peter et al., 2002; Sagata et al., 1989) and the Greatwall (Gwl) kinase (Hara et al., 2012; Zhao et al., 2008). As expected for participants within the MPF auto-amplification loop, they are regulated by Cdk1 itself and contribute either to Myt1 downregulation (Wee1 is not expressed during meiosis I in Xenopus oocytes) (Palmer et al., 1998; Peter et al., 2002) or Cdc25 activation (Inoue and Sagata, 2005; Zhao et al., 2008). Paradoxically, although the auto-amplification loop itself is independent of PKA, none of its identified players is capable of inducing M-phase entry and Cdk1 activation in a context of high PKA activity (Daar et al., 1993; Duckworth et al., 2002; Eyers et al., 2005; Matten et al., 1994; Rime et al., 1992; Rime et al., 1994). Therefore, a key actor of the loop, working independently of PKA, remains unknown.
Another crucial issue regards the mechanism by which phosphatases would counteract the phosphorylation events implicated in the MPF feedback loop. Recent results have shed light on the involvement of the phosphatase PP2A in regulating mitotic progression. PP2A enzymes typically exist as heterotrimers comprising catalytic C-, scaffolding A- and different regulatory B-type subunits (Janssens et al., 2008). PP2A associated with the B55δ subunit appears to be a major enzyme that dephosphorylates Cdk1 targets (Castilho et al., 2009; Mochida et al., 2009), hence controlling the downstream events of MPF activation: mitotic progression and mitotic exit. The regulation of PP2A-B55δ depends on the kinase Gwl (Castilho et al., 2009; Vigneron et al., 2009). Once activated, Gwl directly phosphorylates two small related proteins, α-endosulfine or ARPP19, both of which then become able to interact with and inhibit PP2A-B55δ (Gharbi-Ayachi et al., 2010; Mochida et al., 2010; Rangone et al., 2011). As a consequence, Cdk1 activity is no longer antagonized by PP2A-B55δ phosphatase, resulting in an increased and stable level of phosphorylation of the mitotic substrates responsible for cell division (Glover, 2012; Haccard and Jessus, 2011; Lorca and Castro, 2013). Thus, without the contribution of Gwl inhibiting PP2A-B55δ through ARPP19/α-endosulfine, active Cyclin-B–Cdk1 can neither promote nor maintain the phosphorylation of its M-phase substrates. This new vision led to the proposal that MPF is not solely identical to Cyclin-B–Cdk1 but would also include Gwl (Hara et al., 2012).
These studies highlight the role of the Gwl/ARPP19-α-endosulfine/PP2A system towards the downstream target proteins of Cdk1, but they do not provide information regarding the role of this pathway in MPF auto-amplification. Several studies have reported that a phosphatase sensitive to okadaic acid (OA), probably PP2A, is responsible for Cdc25 and Myt1 dephosphorylation, hence playing a role in MPF activation (Abrieu et al., 1998; Félix et al., 1990; Karaïskou et al., 1998; Margolis et al., 2006). In Xenopus oocytes, injection of okadaic acid promotes meiotic maturation (Goris et al., 1989). Since OA is not a specific inhibitor of PP2A activity, essential questions regarding the physiological involvement and the regulation of endogenous PP2A during meiotic resumption are still pending. Whether or not PP2A is downregulated and how this downregulation is achieved in intact oocytes remain unknown. Interestingly, the microinjection of active Gwl into oocytes activates MPF (Yamamoto et al., 2011; Zhao et al., 2008). However, the physiological implication of endogenous Gwl and of its downstream substrates has not been yet determined and deserves to be studied.
Here, we investigate for the first time the detailed role of the Gwl/ARPP19/PP2A module in a physiological cellular process, namely meiotic maturation of Xenopus oocytes. We show that ARPP19 is expressed in prophase-arrested oocytes and is in vivo phosphorylated by Gwl at S67 during meiotic resumption. We reveal that the Gwl/ARPP19/PP2A system plays an essential role during meiotic M-phase entry, not only by counteracting Cdk1 substrate phosphorylation but also by promoting Cdk1 activation. Once activated, it behaves as a major component of the MPF auto-amplification loop, rendering this process independent of PKA activity and independent of protein synthesis provided that a small amount Mos is present. Gwl/ARPP19/PP2A therefore not only regulates the events required for M-phase progression and exit, downstream of MPF, but also MPF activation per se and meiotic M-phase entry.
Results
ARPP19 is phosphorylated at S67 during meiotic maturation in Xenopus oocytes
Since ARPP19 was identified in the two independent biochemical screens designed to identify the Gwl substrates in Xenopus (Gharbi-Ayachi et al., 2010; Mochida et al., 2010), we used this protein as a reference to design the tools used in this study. The phosphorylation status of ARPP19 at S67 was monitored during meiotic maturation by a specific antibody directed against the phosphorylated S67 residue of the Xenopus ARPP19. Prophase oocytes were stimulated by progesterone and collected at various times during meiotic maturation (Fig. 1A; supplementary material Fig. S1). Meiosis resumption was ascertained by GVBD (Germinal Vesicle BreakDown) and by western blotting oocyte lysates using antibodies directed against either the phosphorylated form of MAPK or total Gwl, the later undergoing an electrophoretic mobility shift upon its phosphorylation/activation (Yu et al., 2006). During meiotic maturation, MAPK and Gwl became phosphorylated just before or at early GVBD, which corresponds to the first pigment rearrangements at the animal pole of the cell (Fig. 1A). They remained phosphorylated until metaphase II (MII) (supplementary material Fig. S1). The ARPP19 protein level was stable during meiotic maturation as revealed with an anti-ARPP19 antibody (Fig. 1A; supplementary material Fig. S1). ARPP19 was slightly phosphorylated at S67 in prophase-arrested oocytes and this phosphorylation was strongly increased starting from early GVBD (Fig. 1A). Similarly to Gwl, ARPP19 remained phosphorylated at S67 until MII (supplementary material Fig. S1). We further noticed that in order to preserve the physiological unphosphorylated state of ARPP19 in prophase, the lysis buffer should not contain OA (supplementary material Fig. S2).
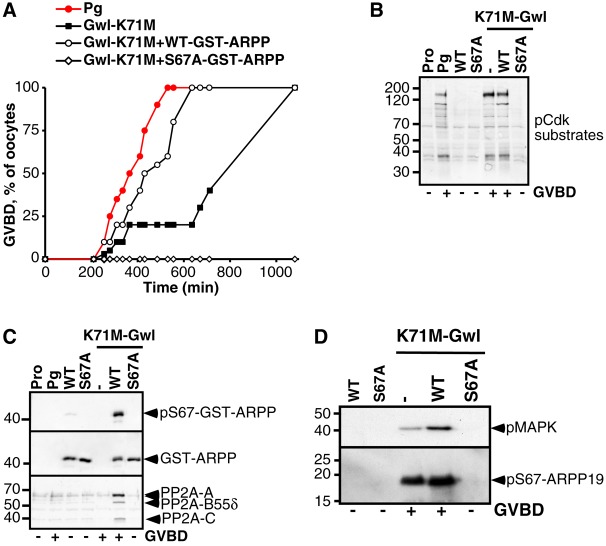
Fig. 1.
Phosphorylation of ARPP19 at S67 is required for resumption of meiosis and mediates binding of ARPP19 to PP2A-B55δ. (A) Prophase oocytes were stimulated with progesterone and collected at the indicated times following addition of progesterone. Oocyte lysates were analyzed by western blot for Gwl, phosphorylated MAPK (pMAPK), endogenous S67-phosphorylated ARPP19 (pS67-ARPP19) and total ARPP19. eGVBD: early GVBD, corresponding to the very first pigment rearrangements at the animal pole of the oocyte. GVBD: well-defined white spot at the animal pole. Lanes ‘no GVBD’ and ‘eGVBD’ correspond to oocytes collected at 150 minutes, either without pigment modifications or at eGVBD. (B) Meiotic maturation time-course of oocytes stimulated by progesterone (Pg), and those injected with 155 ng of either WT-GST-ARPP or S67A-GST-ARPP. (C) Prophase-arrested oocytes (Pro) were either injected with WT-GST-ARPP (WT) or S67A-GST-ARPP (S67A) then stimulated or not by progesterone (Pg). Oocytes were collected at GVBD and WT- or S67A-GST-ARPP were pulled down from oocyte lysates. GST pull-down fractions were then immunoblotted for S67-phosphorylated GST-ARPP (pS67-GST-ARPP), PP2A-A, PP2A-C, PP2A-B55δ and GST (GST-ARPP).
Meiotic M-phase entry in Xenopus oocytes requires the Gwl-dependent phosphorylation of ARPP19
To investigate whether ARPP19 phosphorylation at S67 plays a role during progesterone-induced meiotic maturation, we generated an ARPP19 mutant, S67A-GST-ARPP, harboring an A residue at position 67 that cannot be phosphorylated. Wild-type Xenopus GST-ARPP19 (WT-GST-ARPP) and S67A-GST-ARPP were injected in oocytes and progesterone was added. WT-GST-ARPP injection did not significantly alter the time-course of GVBD induced by progesterone (Fig. 1B). In contrast, S67A-GST-ARPP injection strongly delayed GVBD induced by progesterone, with 80% of oocytes being unable to complete GVBD (Fig. 1B) even after 16 hours. The phosphorylation of GST-ARPP at S67 residue was ascertained by western blot on GST-pull-down fractions. As expected, pulled-down WT-GST-ARPP was phosphorylated at S67 at GVBD whereas S67A-GST-ARPP was not (Fig. 1C). In egg extracts, the phosphorylation of ARPP19 at S67 converts this protein into an inhibitor of PP2A by direct binding (Gharbi-Ayachi et al., 2010; Mochida et al., 2010). Since the phosphorylation of ARPP19 at S67 positively controls meiotic M-phase entry, GST-pull downs fractions were then probed using antibodies directed against PP2A-C, PP2A-A and B55δ subunits (Fig. 1C). WT-GST-ARPP was phosphorylated at S67 and associated with PP2A-B55δ (A-, C- and B55δ-subunits). Importantly, S67A-GST-ARPP did not bind to PP2A-B55δ (Fig. 1C). Since entry into meiotic division is impaired by S67A-GST-ARPP, this strongly suggests that S67A-GST-ARPP acts as a dominant negative and that S67 phosphorylation of ARPP19 positively regulates Xenopus oocyte meiotic maturation by binding and inhibiting PP2A-B55δ.
Active Gwl leads to MPF activation and M-phase entry when microinjected into Xenopus oocytes (Yamamoto et al., 2011; Zhao et al., 2008). To determine whether Gwl is the kinase that phosphorylates ARPP19 at S67 in oocytes and promotes M-phase entry specifically through ARPP19 phosphorylation, we took advantage of a gain-of-function Gwl mutant, termed K71M (Archambault et al., 2007). As previously shown (Yamamoto et al., 2011), injection of K71M-Gwl mRNA induced meiotic maturation independently of progesterone, albeit with a slower rate than observed using progesterone (Fig. 2A), as well as Cdk1 activation ascertained by Cdk1 substrates phosphorylation (Fig. 2B). Injection of WT-GST-ARPP significantly accelerated K71M-Gwl-induced GVBD (Fig. 2A), while injection of recombinant S67A-GST-ARPP protein fully abolished both K71M Gwl-induced GVBD and Cdk1 activation (Fig. 2A,B) even after 16 hours. WT-GST-ARPP was phosphorylated at S67 in response to K71M-Gwl mRNA injection whereas the non-phosphorylable S67A mutant was not (Fig. 2C). The GST-pull down fractions were further immunoblotted for PP2A-B55δ. Phosphorylated WT-GST-ARPP at S67 associated with PP2A-B55δ whereas S67A-GST-ARPP did not (Fig. 2C). Finally, the phosphorylation of endogenous ARPP19 at S67 by K71M-Gwl was prevented by the presence of the S67A-GST-ARPP mutant (Fig. 2D), thus explaining the molecular basis of the dominant negative effect of the S67A mutant protein. Altogether, our results show that meiosis resumption induced by K71M-Gwl relies entirely on S67 phosphorylation of ARPP19. Gwl is the kinase that phosphorylates ARPP19 at S67 in the Xenopus oocyte and S67 phosphorylation of ARPP19 is essential for entry into the first meiotic division of Xenopus oocyte.
Fig. 2.
Phosphorylation of ARPP19 at S67 depends on activation of Gwl during resumption of meiosis. (A) Meiotic maturation time-course of oocytes stimulated by progesterone (Pg) or injected with K71M-Gwl mRNA (Gwl-K71M), with or without 155 ng of either WT- or S67A-GST-ARPP. (B) Prophase-arrested oocytes (Pro) were either injected with WT-GST-ARPP (WT) or S67A-GST-ARPP (S67A), then injected or not with K71M-Gwl mRNA. Control oocytes were treated with progesterone (Pg). Oocytes were collected at GVBD and GST-ARPP was pulled down from oocyte lysates. Immunoblot analysis for phosphorylated Cdk1 substrates (pCdk substrates) was performed on supernatants after GST pull-down. (C) Western blot analysis was performed on GST pull-down fractions corresponding to experiment (B) for S67-phosphorylated GST-ARPP (pS67-GST-ARPP), PP2A-A, PP2A-C, PP2A-B55δ and GST (GST-ARPP). (D) Prophase-arrested oocytes were injected with either WT-GST-ARPP (WT) or S67A-GST-ARPP (S67A), and then injected with K71M-Gwl mRNA. Oocytes were collected at GVBD and immunoblot analysis for phosphorylated MAPK (pMAPK) and S67-phosphorylated ARPP19 (pS67-ARPP19) was performed.
To analyze the interplay between ARPP19-PP2A-B55δ module and Cdk1 activity, oocytes were injected with the Cdk inhibitor p21Cip1, then stimulated by progesterone (Fig. 3). Injecting p21Cip1 allows for the analysis of the early effects triggered by the hormone and occurring before and independently of Cdk1 activation (Frank-Vaillant et al., 1999). As expected, progesterone neither triggered GVBD nor Cdk1 activation in p21Cip1 injected oocytes, as ascertained by the phosphorylation levels of Cdk1 substrates, Y15 residue of Cdk1, Cyclin B2 and MAPK, as well as Cyclin B1 accumulation (Fig. 3A). Importantly, Gwl was not activated in those conditions (Fig. 3A). The injection of WT-GST-ARPP did not allow the dephosphorylation of Cdk1 at Y15 or the activation of the MAPK in the presence of p21Cip1 (Fig. 3A). Moreover, WT-GST-ARPP was neither phosphorylated at S67 nor associated with PP2A-B55δ (Fig. 3B). Therefore, S67 phosphorylation of ARPP19 and its consequent binding to PP2A-B55δ during meiotic maturation relies on the Cdk1-dependent activation of Gwl.
Fig. 3.
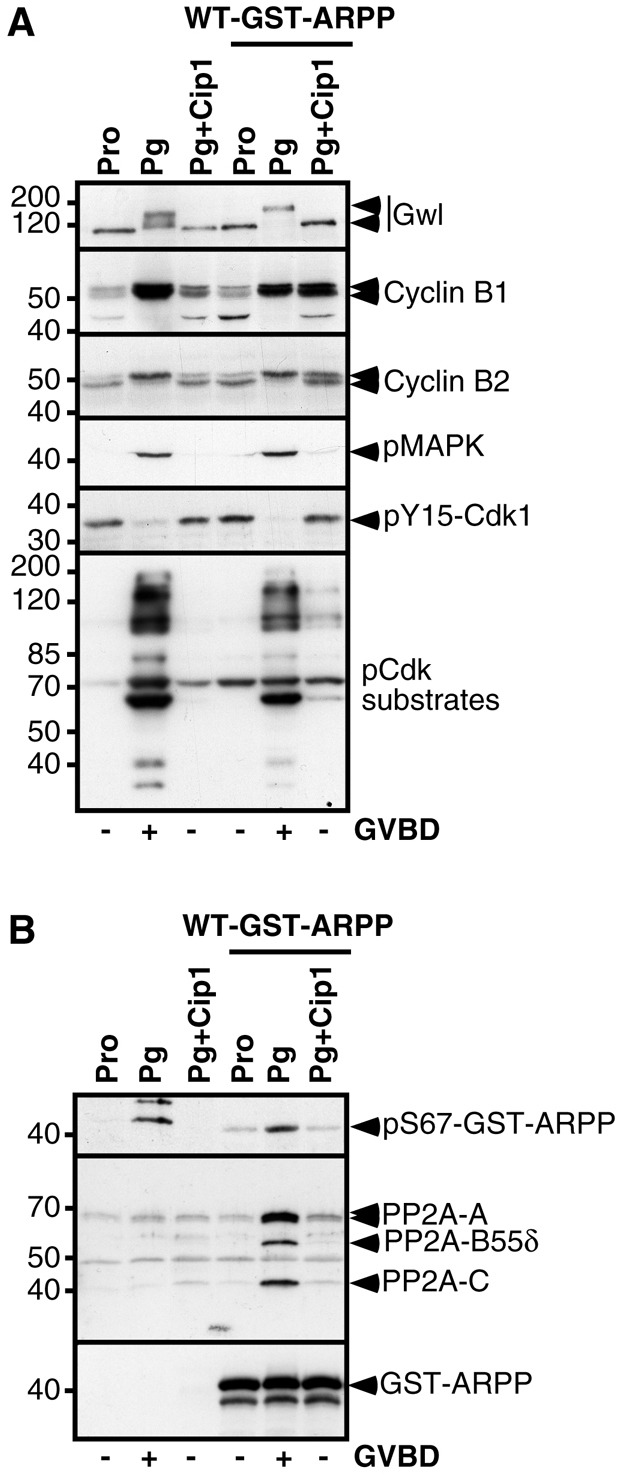
Gwl activation and phosphorylation of ARPP19 at S67 rely on Cdk1 activation. (A) Prophase-arrested oocytes (Pro) were injected or not with p21Cip1 (Cip1), then injected or not with 77.5 ng of WT-GST-ARPP, and where indicated, treated with progesterone (Pg). Oocytes were collected at GVBD and GST-ARPP was pulled down. Supernatants after the pull down were analyzed by western blot for Gwl, Cyclin B1, Cyclin B2, phosphorylated MAPK (pMAPK), Y15-phosphorylated Cdk1 (pY15-Cdk1) and phosphorylated Cdk1 substrates (pCdk substrates). (B) Western blot analysis of GST pull-down fractions corresponding to experiment (A) for S67-phosphorylated WT-GST-ARPP (pS67-GST-ARPP), PP2A-A, PP2A-C, PP2A-B55δ.
S67 phosphorylation of ARPP19 is sufficient to promote Cdk1 auto-amplification
We next investigated whether ARPP19 phosphorylation at S67 could be sufficient to promote meiotic maturation. We generated a S67D mutant in an attempt to mimic constitutive phosphorylation of S67. Injection of this mutant did not induce meiotic resumption. Since this type of mutation does not necessarily mimic phosphorylation, we used an alternative approach and prepared WT-GST-ARPP protein thiophosphorylated at S67 by Gwl in vitro (pS67-WT-GST-ARPP*) (Fig. 4A). Injection of pS67-WT-GST-ARPP* into prophase oocytes triggered GVBD, even faster than upon progesterone addition (Fig. 4B). Cdk1 was activated, as judged by Cyclin B2 and Gwl upshifts as well as MAPK and Cdk1 substrate phosphorylation (Fig. 4C). As expected, pS67-WT-GST-ARPP* interacted with PP2A-B55δ (Fig. 4D). Noteworthy, this association could only be detected in the absence of OA in lysis buffer (supplementary material Fig. S3), showing that OA disrupts PP2A association with ARPP19, as previously reported for the α4 protein (Kloeker et al., 2003).
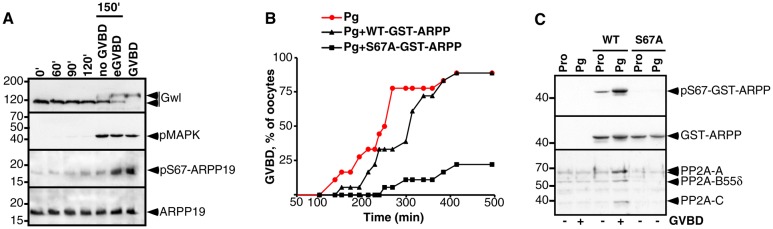
Fig. 4.
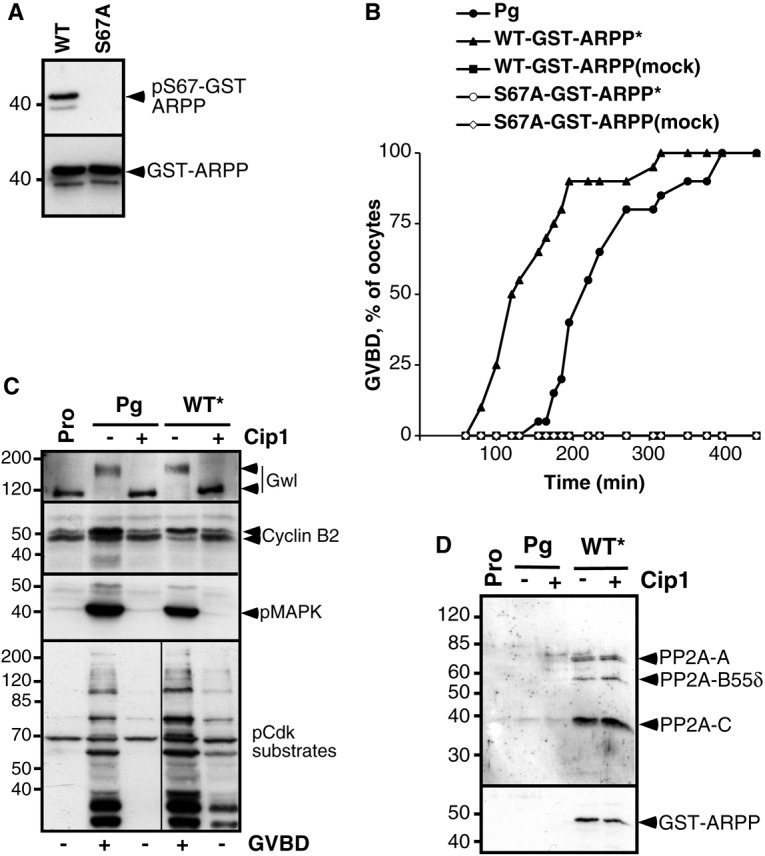
Phosphorylation of ARPP19 at S67 is sufficient to promote its interaction with PP2A and the induction of meiotic maturation. (A) In vitro thiophosphorylation of recombinant GST-ARPP proteins by Gwl. Recombinant WT-GST-ARPP (WT) or S67A-GST-ARPP (S67A) were incubated in the presence of active Gwl bound to agarose beads and γS-ATP, then immunoblot analysis using antibodies directed against S67-phosphorylated ARPP19 (pS67-GST-ARPP) or GST (GST-ARPP) was performed. (B) Meiotic maturation time-course of oocytes treated with progesterone (Pg) or injected with WT-GST-ARPP or S67A-GST-ARPP previously incubated with γS-ATP and Gwl (indicated with *). Mock: no Gwl was added to the reaction. (C) Prophase-arrested oocytes (Pro), injected or not with p21Cip1 (Cip1), were then injected with WT-GST-ARPP thiophosphorylated at S67 (WT*) or treated with progesterone (Pg). Oocytes were collected at the time of GVBD and GST-ARPP was pulled down. Supernatants after GST pull-down were immunoblotted for Gwl, Cyclin B2, phosphorylated MAPK (pMAPK) and phosphorylated Cdk1 substrates (pCdk-substrates). (D) Western blot analysis of PP2A-A, PP2A-C, PP2A-B55δ and GST (GST-ARPP) in GST pull-down fractions corresponding to experiment (C).
To ascertain that the M-phase inducing activity of pS67-WT-GST-ARPP* relies on S67 phosphorylation by Gwl, the S67A-GST-ARPP mutant that cannot be phosphorylated on S67 was preincubated with Gwl and γS-ATP then injected into prophase oocytes. As expected, S67A-GST-ARPP was not phosphorylated in vitro (Fig. 4A) and was unable to trigger GVBD (Fig. 4B). We have shown above that S67 phosphorylation of ARPP19 is required for its binding to PP2A-B55δ (Fig. 1C; Fig. 2C). We therefore wondered whether this phosphorylation was sufficient for promoting ARPP19 interaction with PP2A in oocytes independently of Cdk1 activation. Oocytes were injected with p21Cip1 then with pS67-WT-GST-ARPP*. Under these conditions, pS67-WT-GST-ARPP* was unable to trigger both GVBD and Cdk1 activation, owing to the presence of the Cdk1 inhibitor (Fig. 4C). However, the protein was still able to associate with PP2A-B55δ (Fig. 4D) and to inhibit its activity as judged by a slight increase in the phosphorylation level of the Cdk1 substrates (Fig. 4C). Altogether, these results demonstrate that ARPP19 phosphorylation at S67 is sufficient to promote its interaction with PP2A-B55δ, the induction of meiotic maturation and Cdk1 activation in intact Xenopus oocytes.
Protein synthesis is necessary for activation of Cdk1 induced by S67-phosphorylated ARPP19
Protein synthesis is necessary for progesterone-induced meiotic maturation and Cdk1 activation (Wasserman and Masui, 1975). To determine if Cdk1 activation induced by pS67-WT-GST-ARPP* would also depend on newly synthesized proteins, oocytes were pre-incubated with the protein synthesis inhibitor, cycloheximide (CHX), then injected with pS67-WT-GST-ARPP*. Under these conditions, pS67-WT-GST-ARPP* neither induced GVBD nor Cdk1 activation, as judged by the phosphorylation level of Gwl, Cdc27 and Cdk1 substrates (Fig. 5A). Nevertheless, PP2A-B55δ was recovered in association with pS67-WT-GST-ARPP* (Fig. 5B), showing that PP2A-B55δ-ARPP19 interaction does not require protein synthesis. However, the inhibition of PP2A-B55δ mediated by the binding of S67-phosphorylated ARPP19 is not sufficient for meiosis resumption and the synthesis of new proteins remains necessary for Cdk1 activation.
Fig. 5.
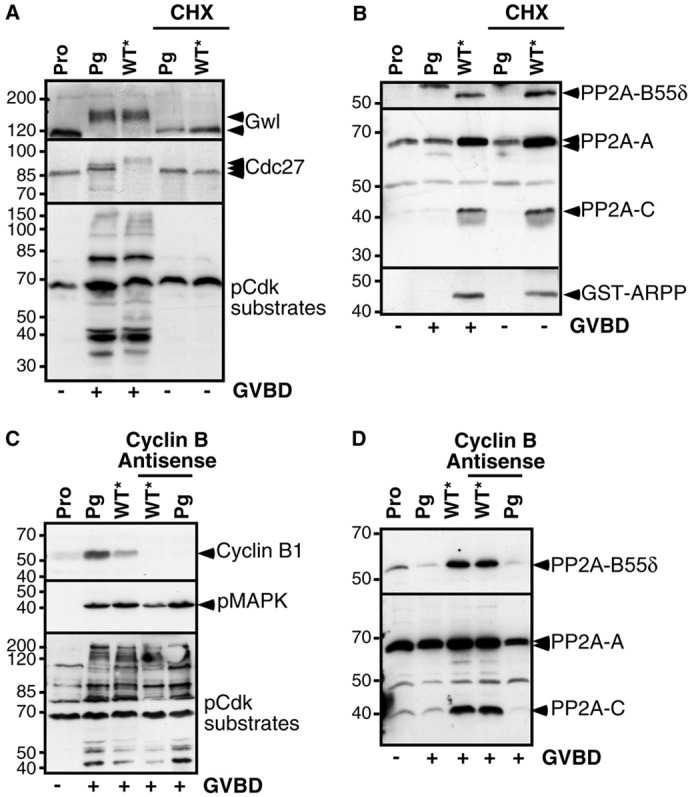
Cdk1 activation induced by S67-thiophosphorylated ARPP19 depends on protein synthesis. (A) Prophase-arrested oocytes (Pro), treated or not with cycloheximide (CHX), were injected with S67-thiophosphorylated WT-GST-ARPP (WT*) or treated with progesterone (Pg). Oocytes were homogenized at the time of GVBD, then western blot analysis for Gwl, Cdc27 and phosphorylated Cdk1 substrates (pCdk-substrates) was performed. (B) GST-ARPP was pulled down from oocyte lysates described in panel (A), and its interaction with PP2A was analyzed by immunoblotting for PP2A-A, PP2A-C, PP2A-B55δ and GST (GST-ARPP). (C) Prophase-arrested oocytes (Pro) were injected with antisense oligonucleotides directed against Cyclin B mRNA, then either treated with progesterone (Pg) or injected with S67-thiophosphorylated WT-GST-ARPP (WT*). Oocytes were collected at the time of GVBD and lysates were analyzed by western blot with antibodies directed against Cyclin B1, phosphorylated MAPK (pMAPK) and phosphorylated Cdk1 substrates (pCdk substrates). (D) GST-ARPP was pulled down from oocyte lysates described in panel (C), and western blot analysis was performed for PP2A-A, PP2A-C and PP2A-B55δ on pull-down fractions.
We next looked for the identity of the synthesized proteins needed for the action of ARPP19. In response to progesterone, two synthesized proteins play a critical role for Cdk1 activation: Cyclin B and Mos, the latter being responsible for MAPK activation (Haccard and Jessus, 2006b). We first tested whether S67-phosphorylated ARPP19 could promote Cdk1 activation in the absence of Cyclin B synthesis by injecting oocytes with antisense oligonucleotides directed against Cyclin B mRNA, then with pS67-WT-GST-ARPP* (Fig. 5C). In the absence of new Cyclin B synthesis, pS67-WT-GST-ARPP* was still able to promote Cdk1 activation, as seen by MAPK and Cdk1 substrate phosphorylation (Fig. 5C), as well as to bind PP2A-B55δ (Fig. 5D).
We then analyzed whether S67-phosphorylated ARPP19 could promote Cdk1 activation in the absence of an active Mos/MAPK pathway. The activation of the Mos/MAPK pathway was prevented in oocytes by adding the MEK inhibitor U0126 to the external medium, as previously described (Dupré et al., 2002; Gross et al., 2000). In the absence of MAPK activity, progesterone was able to induce GVBD and Cdk1 activation, as judged by Y15 dephosphorylation of Cdk1, Cyclin B1 accumulation and Gwl activation (Fig. 6A). Similarly, Cdk1 was activated by pS67-WT-GST-ARPP* in the absence of any detectable MAPK activity (Fig. 6A). Identical results were obtained when the Mos/MAPK pathway was impaired by injecting antisense Mos morpholinos (supplementary material Fig. S4). Therefore, neither the inhibition of the expression of all Cyclin B isotypes nor the inhibition of the Mos/MAPK cascade prevent MPF activation triggered by S67-phosphorylated ARPP19. As already shown (Haccard and Jessus, 2006b), when both pathways were simultaneously inhibited by U0126 and antisense oligonucleotides against Cyclin B, progesterone no longer triggered maturation (Fig. 6A). Similarly, injection of pS67-WT-GST-ARPP* in the absence of both Cyclin B and Mos synthesis was unable to promote both GVBD and Cdk1 activation (Fig. 6A), showing that S67-phosphorylated ARPP19 requires the synthesis of either Cyclin B or Mos to promote MPF activation.
Fig. 6.
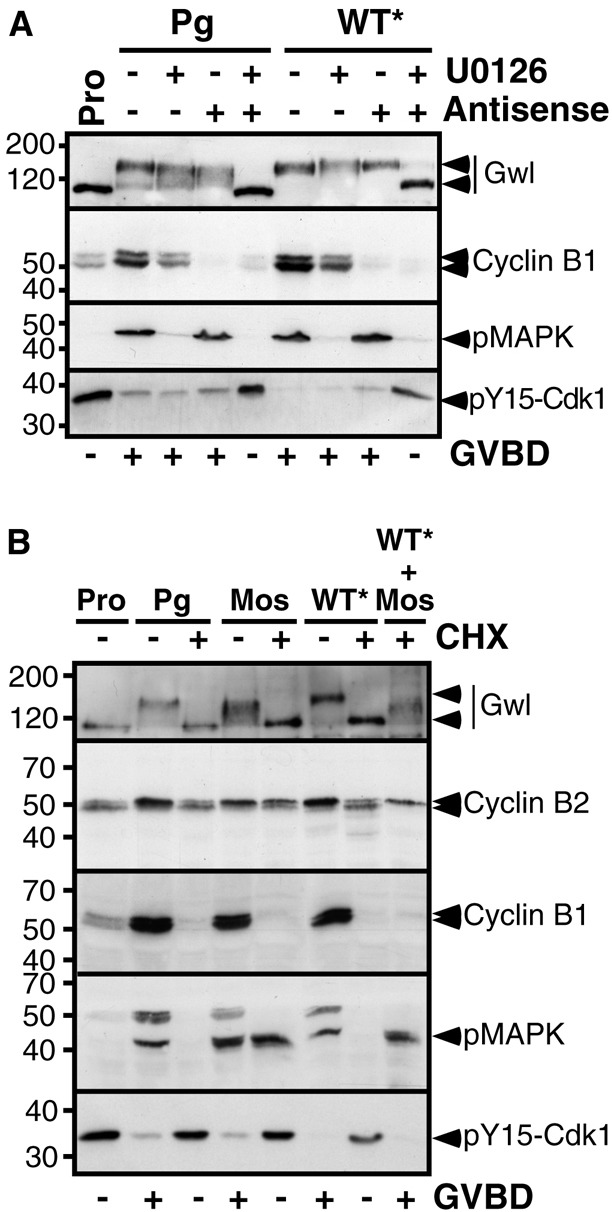
S67-thiophosphorylated ARPP19 requires the synthesis of either Cyclin B or Mos to promote meiotic resumption. (A) Prophase-arrested oocytes (Pro) were either injected with antisense oligonucleotides directed against Cyclin B mRNA or incubated with U0126, or simultaneously subjected to both treatments. Oocytes were then either treated with progesterone (Pg) or injected with S67-thiophosphorylated WT-GST-ARPP (WT*). Oocytes were collected at the time of GVBD and lysates were western blotted for Gwl, Cyclin B1, phosphorylated MAPK (pMAPK) and Y15-phosphorylated Cdk1 (pY15-Cdk1). (B) Prophase-arrested oocytes (Pro) were incubated or not with cycloheximide (CHX) then induced to mature by adding progesterone (Pg) or by injecting either Mos or S67-thiophosphorylated WT-GST-ARPP (WT*) or both. Oocytes were collected at the time of GVBD and lysates were analyzed by western blot for Gwl, Cyclin B2, Cyclin B1, phosphorylated MAPK (pMAPK) and Y15-phosphorylated Cdk1 (pY15-Cdk1).
These results suggest that an active kinase is required on top of PP2A-B55δ inhibition in order to induce GVBD, either Cdk1 itself bound to newly synthesized Cyclin B or the members of the Mos/MAPK pathway. Injection of Cyclin B promotes Cdk1 activation independently of protein synthesis (Gotoh et al., 1995; Rime et al., 1992) whereas small amounts of injected Mos (less than 12 ng) depend on protein synthesis to activate Cdk1 (Yew et al., 1992). We therefore tested if S67-phosphorylated ARPP19 could promote M-phase entry in the presence of Mos when protein synthesis is inhibited. For this purpose, pS67-WT-GST-ARPP* was injected together with a small amount of Mos (7 ng) in the presence or in the absence of the protein synthesis inhibitor, CHX. In control oocytes, injection of either Mos or pS67-WT-GST-ARPP* triggered meiotic maturation, as seen by MAPK phosphorylation, Y15 dephosphorylation of Cdk1, Cyclin B2 upshift, Cyclin B1 accumulation and Gwl activation (Fig. 6B). In contrast, injecting either Mos or pS67-WT-GST-ARPP* did not trigger Cdk1 activation when protein synthesis was inhibited (Fig. 6B). Remarkably, pS67-WT-GST-ARPP* injection in oocytes together with Mos led to Cdk1 activation in the absence of protein synthesis, as seen by Cyclin B2 upshift and Y15 dephosphorylation of Cdk1 (Fig. 6B). Notably, the injection of S67A-GST-ARPP was able to block GVBD normally induced by Mos (Fig. 7A), indicating that ARPP19 phosphorylation at S67 is essential for the Mos/MAPK pathway to activate Cdk1. Altogether, our results demonstrate that S67-phosphorylated ARPP19 and the Mos/MAPK pathway cooperate to activate Cdk1 without any further protein synthesis requirement.
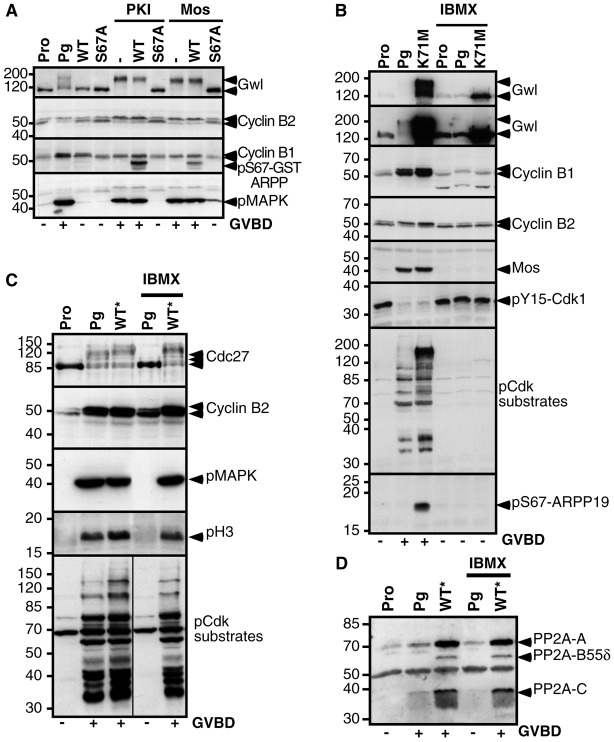
Fig. 7.
S67-phosphorylated ARPP19 bypasses the lock fulfilled by PKA. (A) Prophase-arrested oocytes (Pro) were either injected with WT-GST-ARPP (WT) or S67A-GST-ARPP (S67A), then induced to mature by injecting the PKA inhibitor, PKI or recombinant Mos. Control oocytes were stimulated by progesterone (Pg). Oocytes were collected at the time of GVBD and lysates were analyzed by western blot for Gwl, Cyclin B2, Cyclin B1, S67-phosphorylated GST-ARPP (pS67-GST-ARPP) and phosphorylated MAPK (pMAPK). (B) Prophase-arrested oocytes (Pro) were incubated or not with IBMX. 16 hours later, oocytes were induced to mature either by progesterone (Pg) or by K71M-Gwl mRNA injection (K71M). Oocytes were collected at the time of GVBD and western blot analysis was performed on lysates for Gwl, Cyclin B1, Cyclin B2, Mos, Y15-phosphorylated Cdk1 (pY15-Cdk1), phosphorylated Cdk1 substrates (pCdk substrates) and S67-phosphorylated ARPP19 (pS67-ARPP19). The same Gwl western blot is illustrated at two periods of exposure: a short period (top panel) to reveal exogenously expressed K71M-Gwl and a longer period (second panel) to detect both exogenous K71M-Gwl and endogenous Gwl. (C) Prophase-arrested oocytes (Pro) were incubated or not with IBMX, then treated with progesterone (Pg) or injected with S67-thiophosphorylated WT-GST-ARPP (WT*). GST-ARPP was pulled down. Immunoblot analysis was performed on supernatants after GST pull-down for Cdc27, Cyclin B2, phosphorylated MAPK (pMAPK), S10-phosphorylated histone H3 (pH 3) and phosphorylated Cdk1 substrates (pCdk substrates). (D) Western blot analysis of GST pull-down fractions corresponding to experiment (C) using antibodies directed against PP2A-A, PP2A-C and PP2A-B55δ.
ARPP19 phosphorylation at S67 triggers MPF activation independently of PKA
In vivo, ARPP19 phosphorylation at S67 is controlled by Cdk1. However, when already phosphorylated at S67, ARPP19 triggers Cdk1 activation. These results place this protein at the center of the MPF auto-amplification loop. S67-phosphorylated ARPP19 should therefore act independently of the early signaling steps induced by progesterone. In all vertebrate species, entry into the first meiotic division requires a decrease of cAMP level leading to PKA inhibition. In Xenopus oocytes, PKA inhibition occurs within the first hour following hormonal stimulation, long before GVBD. Inhibiting PKA activity induces MPF activation and GVBD independently of progesterone (Huchon et al., 1981; Maller and Krebs, 1977). We therefore investigated whether S67 phosphorylation of ARPP19 acts downstream of PKA inhibition in response to progesterone. Oocytes were injected with either WT-GST-ARPP or S67A-GST-ARPP then with a specific protein inhibitor of PKA, PKI (Fig. 7A). WT-GST-ARPP did not affect Cdk1 activation and GVBD triggered by PKI injection whereas S67A-GST-ARPP totally prevented meiotic resumption under this condition (Fig. 7A). Therefore, the phosphorylation at S67 of ARPP19 is required downstream PKA downregulation to induce MPF activation.
Only cytoplasm transfer from a mature oocyte to a prophase-arrested oocyte (known as ‘MPF transfer’ and reproducing the MPF auto-amplification) and OA injection are able to promote GVBD in a high PKA activity context (Daar et al., 1993; Rime et al., 1990). Intriguingly, in the presence of high PKA activity, all known players of the amplification loop, like Mos, Cdc25 or Cyclin B, are unable to promote Cdk1 activation or GVBD (Daar et al., 1993; Duckworth et al., 2002; Eyers et al., 2005; Matten et al., 1994; Rime et al., 1992; Rime et al., 1994). This suggests that the main actor of the auto-amplification loop that renders the process independent of PKA, probably a phosphatase inhibitor, remains unidentified. Recently, it has been proposed that Gwl is part of MPF activity in starfish and Xenopus oocytes (Hara et al., 2012) but whether Gwl could induce meiosis resumption independently of ongoing PKA activity remains unknown. To address this question, oocytes were first incubated in the presence of the phosphodiesterase inhibitor IBMX (1-methyl-3-isobutylxanthine) to maintain a high intracellular cAMP concentration and fully active PKA. Oocytes were then induced to mature by either progesterone addition or K71M-Gwl mRNA injection. IBMX prevented Cdk1 activation and meiotic resumption under both conditions (Fig. 7B). Importantly, in the presence of IBMX, endogenous ARPP19 was not phosphorylated by overexpressed K71M-Gwl (Fig. 7B) or in the presence of progesterone (supplementary material Fig. S5 illustrates different exposure periods required to visualize the phosphorylation of the endogenous protein in response to progesterone or to injected K71M-Gwl). Accordingly, the activation of Gwl was also blocked by the presence of IBMX, as seen by the lack of upshift of both the endogenous kinase and the overexpressed K71M mutant (Fig. 7B). This result indicates that, as for Cdc25, Mos and Cyclin (Daar et al., 1993; Duckworth et al., 2002; Eyers et al., 2005; Matten et al., 1994; Rime et al., 1992; Rime et al., 1994), Gwl activation is negatively controlled by PKA.
The injection of OA induces Cdk1 activation in the presence of IBMX in oocytes, possibly by inhibiting PP2A (Rime et al., 1990). Since S67-phosphorylated ARPP19 promotes Cdk1 activation by inhibiting PP2A, we wondered whether S67-phosphorylated ARPP19 could also function independently of PKA. Oocytes were incubated in the presence of IBMX then injected with pS67-WT-GST-ARPP*. Remarkably, although PKA remained fully active, pS67-WT-GST-ARPP* injection was able to induce meiotic maturation and to activate Cdk1, as judged by Cyclin B2 upshift, phosphorylation of Cdc27, histone H3 and Cdk1 substrates, as well as activation of the Mos/MAPK pathway (Fig. 7C). It also interacted with PP2A in spite of IBMX (Fig. 7D). This unique property of S67-phosphorylated ARPP19 to bypass the negative effect of PKA highlights its pivotal role in MPF activation and identifies ARPP19 as the protein that renders the auto-amplification loop independent of PKA.
Discussion
Recently, evidence has emerged of a new regulatory pathway that inhibits the phosphatase PP2A-B55δ, allowing the maintenance of the phosphorylated state of mitotic Cyclin-B–Cdk1 substrates. This pathway relies on the powerful protein inhibitor of PP2A-B55δ, ARPP19, which is activated by its Gwl-dependent phosphorylation. These findings obtained in Xenopus egg extracts (Gharbi-Ayachi et al., 2010; Mochida et al., 2010) were further confirmed by genetic studies in the Drosophila model (Rangone et al., 2011) and highlight the essential role of the Gwl/ARPP19/PP2A module for the maintenance of the mitotic state (Lorca and Castro, 2013). However, the details of the contribution of these molecular players to MPF activation and M-phase entry remain an open and important question. The aim of this paper was to investigate the potential role of ARPP19 in MPF activation. We took advantage of the hormone-stimulated G2/M transition of oocyte that offers powerful features to study in vivo the molecular network governing meiotic divisions and more broadly to elucidate the regulation of M-phase entry. MPF activation is a two-step mechanism, starting with the formation of a threshold level of Cdk1 kinase activity that brings about Cdc25 activation and Myt1 inactivation, hence establishing a positive feedback loop. The molecular players as well as the contribution of PP2A to this mechanism are far from being unraveled. In this study, we found that ARPP19 is phosphorylated in vivo by Gwl at S67 and thereafter binds and inhibits PP2A-B55δ. This step appears to be essential for the MPF auto-amplification loop to function. This is the first demonstration in a living cell that ARPP19 plays a key role for M-phase entry through the downregulation of PP2A and that this process is required for entry into the female meiotic M-phase.
Our study demonstrates that ARPP19 phosphorylation at S67 accounts for the M-phase promoting activity of Gwl, since the non-phosphorylable S67A-ARPP19 mutant inhibits M-phase entry promoted by K71M-Gwl injection. The mechanism leading to Gwl activation is not clear yet. It requires a phosphorylation at its C-terminus together with interactions between N- and C-terminal tails and the possible association to another unknown AGC kinase (Lorca and Castro, 2013; Vigneron et al., 2011). However, all this process depends on Cdk1 activity (Castilho et al., 2009; Yamamoto et al., 2011; Yu et al., 2006; Zhao et al., 2008). In agreement with this finding, we show that S67 phosphorylation of ARPP19 by Gwl in the Xenopus oocyte takes place at GVBD under Cdk1 control. Conversely, S67 phosphorylation of ARPP19 is essential for Cdk1 activation. Indeed, a non-phosphorylatable S67A-ARPP19 mutant prevents Cdk1 activation triggered by progesterone whereas ARPP19 thiophosphorylation at S67 is itself sufficient to promote M-phase entry. Altogether, these observations strongly suggest that the initial threshold level of Cdk1 activity, generated in the oocyte by the synthesis of Cyclin B1, controls Gwl activation followed by ARPP19 phosphorylation at S67 (Fig. 8). Without this starter amount of active Cdk1, ARPP19 cannot be phosphorylated. On the other hand, without Gwl and S67 phosphorylation of ARPP19, the low initial level of Cdk1 activity cannot trigger the MPF auto-amplification loop and M-phase entry (Fig. 8). Gwl and ARPP19 are therefore central players in the process of MPF activation, being at the crossroads between the formation of active Cdk1 and the feedback loop that converts the stockpile of pre-MPF molecules into active MPF (Fig. 8). Surprisingly, although Gwl is an essential component in the MPF assay based on cytoplasm transfer, it is not essential for the meiotic G2/M transition and full activation of Cyclin-B–Cdk1 in starfish oocytes (Hara et al., 2012). In contrast, our study reveals that in frog oocytes, meiotic M-phase entry depends on Gwl and the phosphorylation of its substrate, ARPP19. It will now be important to analyze the involvement of the Gwl-ARPP19-PP2A module in other species to establish if the requirement for ARPP19 in the initiation of meiotic maturation is a common feature of vertebrate oocytes.
Fig. 8.
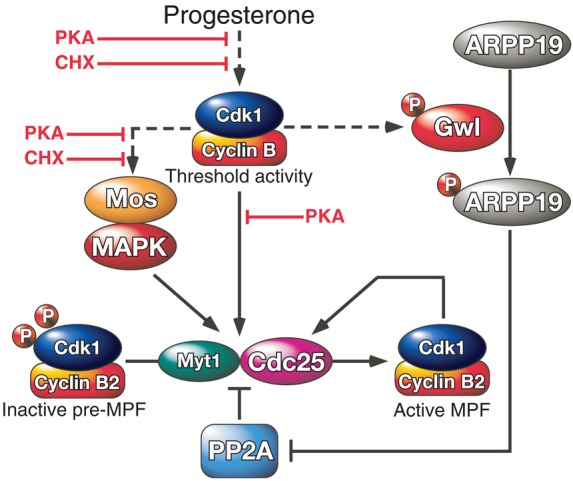
Revisiting MPF activation. MPF activation is initiated by the generation of a threshold level of active Cdk1 in response to progesterone. This step depends on the synthesis of Cyclin B and is negatively regulated by PKA. This starter amount of active Cdk1 phosphorylates its own regulatory enzymes, Cdc25 and Myt1, and induces the accumulation of Mos leading to the activation of the Mos/MAPK pathway. These reactions are under the control of PKA and are counteracted by PP2A. Therefore, the conversion of inactive pre-MPF into MPF cannot take place unless PP2A is inhibited. Gwl is activated by the threshold amount of Cdk1 activity. It phosphorylates ARPP19 at S67, leading to PP2A inhibition independently of PKA. The MPF auto-amplification loop then becomes independent of PKA activity and protein synthesis, irreversibly driving the cell into M-phase.
Interestingly, adding OA to the lysis buffer promotes S67 phosphorylation of ARPP19. This result further suggests that ARPP19 phosphorylation at S67 is actively regulated by an OA-sensitive phosphatase in the prophase oocyte. Presently, the identity of the protein phosphatase required for maintaining the S67 residue of ARPP19 in an unphosphorylated state in prophase oocytes is unknown. PP1 deserves attention as its activity has been shown to be required for the dephosphorylation of mitotic proteins (Wu et al., 2009). Alternatively, it has been reported that Gwl binds active PP2A-B55δ in prophase oocytes, suggesting that one function of PP2A-B55δ is to keep Gwl inactive during the oocyte prophase arrest (Yamamoto et al., 2011). Therefore, PP2A itself could participate in the indirect regulation of S67 phosphorylation of ARPP19, through its actions on Gwl. Alternatively, PP2A could directly act to dephosphorylate S67 of ARPP19 even though S67-phosphorylated ARPP19 has a primary function as a PP2A inhibitor.
Once phosphorylated at S67, ARPP19 binds and inhibits PP2A-B55δ, leading to the full activation of MPF. Our data demonstrate that S67 phosphorylation of ARPP19 is both necessary and sufficient to promote its specific association with PP2A-B55δ. Indeed, S67-thiophosphorylated ARPP19 binds and inhibits PP2A-B55δ in the oocyte independently of Cdk1 activity (in the presence of p21Cip1), of protein synthesis (in the presence of CHX) and of PKA activity (in the presence of IBMX). Conversely, the mutation of S67 into a non-phosphorylable residue renders ARPP19 unable to bind and to regulate PP2A, as well as to promote oocyte M-phase entry. These findings highlight the powerful cell cycle control exerted by this single phosphorylation that proves to be dominant over all other cell events. They also raise the issue of the critical substrates of PP2A-B55δ needed to ensure the auto-amplification feedback loop to function. Among them Cdc25, Polo kinase and Myt1/Wee1 are prime candidates.
The MPF auto-amplification loop works independently of both PKA downregulation and protein synthesis that are only required at earlier steps, for the initial activation of Cdk1 in Xenopus oocytes. Therefore, all components of the auto-amplification loop formally should be able to induce MPF activation independently of protein synthesis and PKA activity. However, the well-known molecular players of the loop do not fulfill these conditions. The Mos/MAPK pathway is activated by Mos synthesis and is one of the important players of Cdk1 activation, by downregulating Myt1 kinase activity (Palmer et al., 1998; Peter et al., 2002; Sagata et al., 1989). Surprisingly however, a low level of Mos is unable to promote MPF activation when protein synthesis is impaired (Yew et al., 1992). Our data show that S67-thiophosphorylated ARPP19 is also unable to trigger MPF activation when protein synthesis is inhibited, indicating that to activate Cdk1 a protein must be synthesized in addition to PP2A inhibition. Small amounts of Mos are sufficient to restore the ability of S67-phosphorylated ARPP19 to promote Cdk1 activation without the requirement of any other protein synthesis. This result demonstrates that the Mos/MAPK pathway and the Gwl/ARPP19/PP2A module play necessary and complementary roles inside the MPF auto-amplification loop, each of them requiring the other one to ensure meiotic M-phase entry (Fig. 8).
Interestingly, none of the well-known molecular players of the loop, like Cyclin B, Cdc25 or Mos, are able to trigger meiotic maturation in the presence of high PKA activity (Daar et al., 1993; Duckworth et al., 2002; Eyers et al., 2005; Matten et al., 1994; Rime et al., 1992; Rime et al., 1994), although MPF auto-amplification, reproduced by the cytoplasm ‘MPF transfer’ experiment, efficiently proceeds in the presence of high PKA activity. Only PP2A inhibition, achieved by OA injection, is able to promote MPF activation independently of PKA (Rime et al., 1990). Strikingly, S67-thiophosphorylated ARPP19 is able to induce meiotic maturation in the presence of high PKA activity, after IBMX treatment. Although ARPP19 is efficiently phosphorylated by PKA in striatal slices and in various cell lines and is proposed to mediate PKA actions in striatum (Dulubova et al., 2001), it clearly escapes PKA regulation under its S67 phosphorylated form in the oocyte. ARPP19 is the first identified protein that can bypass the lock fulfilled by PKA in the oocyte. The demonstration that PP2A downregulation by ARPP19 occurs independently of PKA activity explains how, from the moment where ARPP19 is phosphorylated at S67, the process of MPF activation becomes independent of PKA activity and drives cell cycle into M-phase in an irreversible manner. Therefore our study identifies ARPP19 as a new critical player of meiotic maturation, switched by Gwl to an M-phase inducer by linking Cdk1 activation to PP2A-B55δ.
Materials and Methods
Materials
Xenopus laevis adult females (Xenopus Express, France) were bred and maintained under laboratory conditions. In all figures, each panel illustrates a representative experiment that was reproduced using oocytes from three to four females. Reagents, unless otherwise specified, were from Sigma (Saint Quentin, Fallavier, France).
Preparation and handling of Xenopus oocytes
Fully grown Xenopus prophase oocytes were obtained as described (Haccard and Jessus, 2006b). The usual microinjected volume was 50 nl per oocyte. Progesterone, cycloheximide (CHX), 1-Methyl-3-isobutylxanthine (IBMX) and U0126 (Promega) were used in the external medium at the respective concentrations of 2 µM, 355 µM, 1 mM and 50 µM. Oocytes were microinjected with Cyclin B antisense or Mos antisense morpholino oligonucleotides as described (Haccard and Jessus, 2006b). Oocytes were referred as to GVBD when a white spot was formed at the animal pole. Time courses were extended overnight to confirm final results. Oocytes were homogenized at 4°C in 10 volumes of EB (80 mM β-glycerophosphate pH 7.3, 20 mM EGTA, 15 mM MgCl2) supplemented or not with 1 µM okadaic acid (OA) and centrifuged at 15,000 g for 10 min at 4°C. Supernatants were used for further analysis.
Antibodies and western blot analysis
Oocyte proteins were subjected to SDS gel electrophoresis and then transferred onto nitrocellulose. An equivalent of 0.5 oocyte was loaded on 12% SDS polyacrylamide gels (Laemmli, 1970) and immunoblotted as described (Dupré et al., 2002). To visualize PP2A subunits, the equivalent of two oocytes was loaded on a 10% SDS polyacrylamide gel. To visualize endogenous ARPP19, the equivalent of 1.5 oocyte was loaded on 15% Tris-Tricine gels (Schägger, 2006). Membranes were incubated with the following antibodies: phospho-MAP kinase, pMAPK (Cell Signaling); Cyclin B2 (Abcam); Mos (Santa Cruz biotechnology); Cdc27 (BD transduction laboratory), phospho-histone H3, pHistone H3 (Cell Signaling); phospho-Y15 of Cdk1, pY15-Cdk1 (Cell Signaling); phospho-Cdk1 substrates, pCdk substrates (Cell Signaling) and HRP-conjugated antibody against GST (Sigma). Rabbit B55δ antibody was a kind gift from Dr S. Mochida (Kunamoto University, Japan). Cyclin B1 and ARPP19 antibodies were described respectively by (Frank-Vaillant et al., 1999) and (Dulubova et al., 2001). Antibodies against PP2A-A and PP2A-C subunits were generated as described (Bosch et al., 1995). Gwl antibodies were raised by immunizing rabbits with peptides corresponding to residues 17–31 and 863–876 of Xenopus Gwl (Covalab, France). Specific antibodies directed against phospho-pS67-ARPP19 were raised by immunizing rabbits with the following peptide: CQKGQKYFDpSGDYN, then affinity purified on columns containing the immobilized peptide (Covalab, France). Appropriate horseradish peroxidase-labeled secondary antibodies (Jackson Immunoresearch, West grove, PA) were revealed by chemiluminescence (Pierce).
Cloning of Xenopus Gwl and GST-ARPP
cDNA encoding Xenopus full length ARPP19 was purchased from Open biosystem (clone # BC0772115) and was subcloned into pGex4-T1 vector to express WT-GST-ARPP in BL21 bacteria. Xenopus full-length cDNA encoding Histidine-tagged Gwl, a kind gift from Dr D. Goldberg (Cornell Univ., Ithaca, NY), was subcloned in pRN3 vector (a kind gift from Dr J. Moreau, IJM, Paris). S67A-GST-ARPP and K71M-Gwl mutants were generated using the Quick Change site mutagenesis kit (Stratagene) and were verified by DNA sequencing (Eurofins, Germany). mRNA were transcribed using AmpliCap-Max T3 (Epicentre) from a SfiI linearized pRN3 vector.
Expression and purification of recombinant proteins
Recombinant WT-GST-ARPP, S67A-GST-ARPP, MBP-Mos, PKI and GST-p21Cip1 were produced in E. coli by auto-induction (Studier, 2005) and purified as described (Frank-Vaillant et al., 1999; Thomas et al., 1991). Fractions containing purified recombinant proteins were dialyzed overnight against Phosphate Buffered Saline (PBS: pH 7.4, 13.7 mM NaCl, 2.7 mM KCl, 4.3 mM KH2PO4, 1.4 mM Na2HPO4) and stored at −80°C. The concentrations of the purified recombinant WT- and S67A-GST-ARPP were measured using the Bradford assay (Bradford, 1976).
In vitro thiophosphorylation of GST-ARPP by Gwl kinase
Active Gwl kinase was obtained by injecting prophase oocytes with mRNA encoding Histidine-tagged Xenopus Gwl kinase and recovered on nickel beads from matured oocytes. Gwl bound to beads was incubated for 90 min at 30°C in Kinase buffer (20 mM Hepes pH 7.4, 2 mM β–Mercaptoethanol) with WT-GST-ARPP or S67A-GST-ARPP proteins (500 µg) in the presence of 1 mM γS-ATP. S67-thiophosphorylated GST-ARPP was recovered in the supernatant after centrifugation and dialyzed against PBS.
Intra-oocyte concentrations of endogenous ARPP19 and injected GST-ARPP
To estimate the concentration of endogenous ARPP19 in oocytes, different dilutions of purified WT-GST-ARPP and different quantities of oocyte extracts were loaded on a 15% Tris-Tricine gel and immunoblotted with an antibody directed against total ARPP19. The intensity of signals was quantified by the ImageJ software. The concentration of endogenous ARPP19 was estimated at 2.3 µM per oocyte (supplementary material Fig. S6A).
155 ng or 77.5 ng of GST-ARPP protein were injected per oocyte (1 µl), equivalent to 3.9 or 1.95 µM final concentration respectively.
The concentration of injected S67-thiophosphorylated ARPP per oocyte was measured after microinjection; different dilutions of purified WT-GST-ARPP along with different amounts of extracts prepared from oocytes injected with S67-thiophosphorylated ARPP19 were loaded on a 12% Laemmli gel, revealed using an anti-GST antibody and quantified using ImageJ software (supplementary material Fig. S6B). The final concentration of the injected thiophosphorylated protein was estimated at 3 µM.
GST pull-down assay
50 µl of oocyte lysate were incubated for 1 hr at 4°C with 30 µl of GST magnetic beads (Promega). GST beads were recovered, washed in EB and resuspended in an equal volume of loading buffer for western blot analysis.
Supplementary Material
Acknowledgments
We thank all members of our laboratory for helpful discussions. We thank Satoru Mochida for the generous gift of B55δ antibody. We gratefully acknowledge Anna Castro and Thierry Lorca for communicating their results and for valuable discussions.
Footnotes
Author contributions
A.D., C.J. and O.H. conceived the original idea, designed and planned the experiments, analyzed the data and wrote the paper. A.D., E.B., C.R. and O.H. performed experiments and analyzed results. A.C.N. discussed the results, assisted in writing the manuscript, helped design the peptides and produced some antibodies.
Funding
This work was supported by The National Centre for Scientific Research (CNRS), Université Pierre et Marie Curie (UPMC) and grants from UPMC (Emergence Program to A.D.); the National Institute of Drug Abuse (NIDA) [grant number DA10044 to A.C.N.]; and The French National Research Agency (ANR) [grant number ANR-07-BLAN-0059 to C.J.]. E.B. and C.R. were fellows from ANR. Research reported in this article was supported by the National Institute of Drug Abuse of the National Institutes of Health (award number PO1DA10044). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Deposited in PMC for release after 12 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.126599/-/DC1
References
- Abrieu A., Brassac T., Galas S., Fisher D., Labbé J. C., Dorée M. (1998). The Polo-like kinase Plx1 is a component of the MPF amplification loop at the G2/M-phase transition of the cell cycle in Xenopus eggs. J. Cell Sci. 111, 1751–1757 [DOI] [PubMed] [Google Scholar]
- Archambault V., Zhao X., White-Cooper H., Carpenter A. T., Glover D. M. (2007). Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with Polo kinase. PLoS Genet. 3, e200 10.1371/journal.pgen.0030200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Cayla X., Van Hoof C., Hemmings B. A., Ozon R., Merlevede W., Goris J. (1995). The PR55 and PR65 subunits of protein phosphatase 2A from Xenopus laevis. molecular cloning and developmental regulation of expression. Eur. J. Biochem. 230, 1037–1045 10.1111/j.1432-1033.1995.tb20653.x [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 10.1016/0003-2697(76)90527-3 [DOI] [PubMed] [Google Scholar]
- Castilho P. V., Williams B. C., Mochida S., Zhao Y., Goldberg M. L. (2009). The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55delta, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell 20, 4777–4789 10.1091/mbc.E09-07-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar I., Yew N., Vande Woude G. F. (1993). Inhibition of mos-induced oocyte maturation by protein kinase A. J. Cell Biol. 120, 1197–1202 10.1083/jcb.120.5.1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth B. C., Weaver J. S., Ruderman J. V. (2002). G2 arrest in Xenopus oocytes depends on phosphorylation of cdc25 by protein kinase A. Proc. Natl. Acad. Sci. USA 99, 16794–16799 10.1073/pnas.222661299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I., Horiuchi A., Snyder G. L., Girault J. A., Czernik A. J., Shao L., Ramabhadran R., Greengard P., Nairn A. C. (2001). ARPP-16/ARPP-19: a highly conserved family of cAMP-regulated phosphoproteins. J. Neurochem. 77, 229–238 10.1046/j.1471-4159.2001.t01-1-00191.x [DOI] [PubMed] [Google Scholar]
- Dupré A., Jessus C., Ozon R., Haccard O. (2002). Mos is not required for the initiation of meiotic maturation in Xenopus oocytes. EMBO J. 21, 4026–4036 10.1093/emboj/cdf400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyers P. A., Liu J., Hayashi N. R., Lewellyn A. L., Gautier J., Maller J. L. (2005). Regulation of the G(2)/M transition in Xenopus oocytes by the cAMP-dependent protein kinase. J. Biol. Chem. 280, 24339–24346 10.1074/jbc.M412442200 [DOI] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. (1990). Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 9, 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Vaillant M., Jessus C., Ozon R., Maller J. L., Haccard O. (1999). Two distinct mechanisms control the accumulation of cyclin B1 and Mos in Xenopus oocytes in response to progesterone. Mol. Biol. Cell 10, 3279–3288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffré M., Martoriati A., Belhachemi N., Chambon J. P., Houliston E., Jessus C., Karaiskou A. (2011). A critical balance between Cyclin B synthesis and Myt1 activity controls meiosis entry in Xenopus oocytes. Development 138, 3735–3744 10.1242/dev.063974 [DOI] [PubMed] [Google Scholar]
- Gharbi-Ayachi A., Labbé J. C., Burgess A., Vigneron S., Strub J. M., Brioudes E., Van-Dorsselaer A., Castro A., Lorca T. (2010). The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330, 1673–1677 10.1126/science.1197048 [DOI] [PubMed] [Google Scholar]
- Glover D. M. (2012). The overlooked greatwall: a new perspective on mitotic control. Open Biol. 2, 120023 10.1098/rsob.120023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J., Hermann J., Hendrix P., Ozon R., Merlevede W. (1989). Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett. 245, 91–94 10.1016/0014-5793(89)80198-X [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Masuyama N., Dell K., Shirakabe K., Nishida E. (1995). Initiation of Xenopus oocyte maturation by activation of the mitogen-activated protein kinase cascade. J. Biol. Chem. 270, 25898–25904 10.1074/jbc.270.43.25898 [DOI] [PubMed] [Google Scholar]
- Gross S. D., Schwab M. S., Taieb F. E., Lewellyn A. L., Qian Y. W., Maller J. L. (2000). The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90(Rsk). Curr. Biol. 10, 430–438 10.1016/S0960-9822(00)00425-5 [DOI] [PubMed] [Google Scholar]
- Haccard O., Jessus C. (2006a). Oocyte maturation, Mos and cyclins—a matter of synthesis: two functionally redundant ways to induce meiotic maturation. Cell Cycle 5, 1152–1159 10.4161/cc.5.11.2800 [DOI] [PubMed] [Google Scholar]
- Haccard O., Jessus C. (2006b). Redundant pathways for Cdc2 activation in Xenopus oocyte: either cyclin B or Mos synthesis. EMBO Rep. 7, 321–325 10.1038/sj.embor.7400611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haccard O., Jessus C. (2011). Greatwall kinase, ARPP-19 and protein phosphatase 2A: shifting the mitosis paradigm. Cell Cycle in Development, vol. 53 Kubiak J Z, ed219–234Heidelberg: Springer; [DOI] [PubMed] [Google Scholar]
- Haccard O., Lewellyn A., Hartley R. S., Erikson E., Maller J. L. (1995). Induction of Xenopus oocyte meiotic maturation by MAP kinase. Dev. Biol. 168, 677–682 10.1006/dbio.1995.1112 [DOI] [PubMed] [Google Scholar]
- Hara M., Abe Y., Tanaka T., Yamamoto T., Okumura E., Kishimoto T. (2012). Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat. Commun. 3, 1059 10.1038/ncomms2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchon D., Ozon R., Fischer E. H., Demaille J. G. (1981). The pure inhibitor of cAMP-dependent protein kinase initiates Xenopus laevis meiotic maturation. A 4-step scheme for meiotic maturation. Mol. Cell. Endocrinol. 22, 211–222 10.1016/0303-7207(81)90092-7 [DOI] [PubMed] [Google Scholar]
- Hunt T., Nasmyth K. (1997). Cell multiplication. Curr. Opin. Cell Biol. 9, 765–767 10.1016/S0955-0674(97)80075-0 [DOI] [PubMed] [Google Scholar]
- Inoue D., Sagata N. (2005). The Polo-like kinase Plx1 interacts with and inhibits Myt1 after fertilization of Xenopus eggs. EMBO J. 24, 1057–1067 10.1038/sj.emboj.7600567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V., Longin S., Goris J. (2008). PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem. Sci. 33, 113–121 10.1016/j.tibs.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Karaïskou A., Cayla X., Haccard O., Jessus C., Ozon R. (1998). MPF amplification in Xenopus oocyte extracts depends on a two-step activation of cdc25 phosphatase. Exp. Cell Res. 244, 491–500 10.1006/excr.1998.4220 [DOI] [PubMed] [Google Scholar]
- Karaïskou A., Jessus C., Brassac T., Ozon R. (1999). Phosphatase 2A and polo kinase, two antagonistic regulators of cdc25 activation and MPF auto-amplification. J. Cell Sci. 112, 3747–3756 [DOI] [PubMed] [Google Scholar]
- Kloeker S., Reed R., McConnell J. L., Chang D., Tran K., Westphal R. S., Law B. K., Colbran R. J., Kamoun M., Campbell K. S. et al. (2003). Parallel purification of three catalytic subunits of the protein serine/threonine phosphatase 2A family (PP2A(C), PP4(C), and PP6(C)) and analysis of the interaction of PP2A(C) with alpha4 protein. Protein Expr. Purif. 31, 19–33 10.1016/S1046-5928(03)00141-4 [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Lorca T., Castro A. (2012). Deciphering the new role of the Greatwall/PP2a pathway in cell cycle control. Genes Cancer 3, 712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. (1977). Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3′:5′-monophosphate-dependent protein kinase. J. Biol. Chem. 252, 1712–1718 [PubMed] [Google Scholar]
- Margolis S. S., Perry J. A., Forester C. M., Nutt L. K., Guo Y., Jardim M. J., Thomenius M. J., Freel C. D., Darbandi R., Ahn J. H. et al. (2006). Role for the PP2A/B56delta phosphatase in regulating 14-3-3 release from Cdc25 to control mitosis. Cell 127, 759–773 10.1016/j.cell.2006.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. (1971). Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 177, 129–145 10.1002/jez.1401770202 [DOI] [PubMed] [Google Scholar]
- Matten W., Daar I., Vande Woude G. F. (1994). Protein kinase A acts at multiple points to inhibit Xenopus oocyte maturation. Mol. Cell. Biol. 14, 4419–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Ikeo S., Gannon J., Hunt T. (2009). Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 28, 2777–2785 10.1038/emboj.2009.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S., Maslen S. L., Skehel M., Hunt T. (2010). Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330, 1670–1673 10.1126/science.1195689 [DOI] [PubMed] [Google Scholar]
- Morgan D. O. (1995). Principles of CDK regulation. Nature 374, 131–134 10.1038/374131a0 [DOI] [PubMed] [Google Scholar]
- Nebreda A. R., Gannon J. V., Hunt T. (1995). Newly synthesized protein(s) must associate with p34cdc2 to activate MAP kinase and MPF during progesterone-induced maturation of Xenopus oocytes. EMBO J. 14, 5597–5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A. (1993). Targets of cyclin-dependent protein kinases. Curr. Opin. Cell Biol. 5, 187–193 10.1016/0955-0674(93)90101-U [DOI] [PubMed] [Google Scholar]
- Palmer A., Gavin A. C., Nebreda A. R. (1998). A link between MAP kinase and p34(cdc2)/cyclin B during oocyte maturation: p90(rsk) phosphorylates and inactivates the p34(cdc2) inhibitory kinase Myt1. EMBO J. 17, 5037–5047 10.1093/emboj/17.17.5037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M., Labbé J. C., Dorée M., Mandart E. (2002). A new role for Mos in Xenopus oocyte maturation: targeting Myt1 independently of MAPK. Development 129, 2129–2139 [DOI] [PubMed] [Google Scholar]
- Posada J., Yew N., Ahn N. G., Vande Woude G. F., Cooper J. A. (1993). Mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol. Cell. Biol. 13, 2546–2553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangone H., Wegel E., Gatt M. K., Yeung E., Flowers A., Debski J., Dadlez M., Janssens V., Carpenter A. T., Glover D. M. (2011). Suppression of scant identifies Endos as a substrate of greatwall kinase and a negative regulator of protein phosphatase 2A in mitosis. PLoS Genet. 7, e1002225 10.1371/journal.pgen.1002225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rime H., Huchon D., Jessus C., Goris J., Merlevede W., Ozon R. (1990). Characterization of MPF activation by okadaic acid in Xenopus oocyte. Cell Differ Dev 29, 47–58 [DOI] [PubMed] [Google Scholar]
- Rime H., Haccard O., Ozon R. (1992). Activation of p34cdc2 kinase by cyclin is negatively regulated by cyclic amp-dependent protein kinase in Xenopus oocytes. Dev. Biol. 151, 105–110 10.1016/0012-1606(92)90217-5 [DOI] [PubMed] [Google Scholar]
- Rime H., Huchon D., De Smedt V., Thibier C., Galaktionov K., Jessus C., Ozon R. (1994). Microinjection of Cdc25 protein phosphatase into Xenopus prophase oocyte activates MPF and arrests meiosis at metaphase I. Biol. Cell 82, 11–22 10.1016/0248-4900(94)90061-2 [DOI] [PubMed] [Google Scholar]
- Sagata N., Daar I., Oskarsson M., Showalter S. D., Vande Woude G. F. (1989). The product of the mos proto-oncogene as a candidate “initiator” for oocyte maturation. Science 245, 643–646 10.1126/science.2474853 [DOI] [PubMed] [Google Scholar]
- Schägger H. (2006). Tricine-SDS-PAGE. Nat. Protoc. 1, 16–22 10.1038/nprot.2006.4 [DOI] [PubMed] [Google Scholar]
- Studier F. W. (2005). Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41, 207–234 10.1016/j.pep.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Thomas J., Van Patten S. M., Howard P., Day K. H., Mitchell R. D., Sosnick T., Trewhella J., Walsh D. A., Maurer R. A. (1991). Expression in Escherichia coli and characterization of the heat-stable inhibitor of the cAMP-dependent protein kinase. J. Biol. Chem. 266, 10906–10911 [PubMed] [Google Scholar]
- Vigneron S., Brioudes E., Burgess A., Labbé J. C., Lorca T., Castro A. (2009). Greatwall maintains mitosis through regulation of PP2A. EMBO J. 28, 2786–2793 10.1038/emboj.2009.228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneron S., Gharbi-Ayachi A., Raymond A. A., Burgess A., Labbé J. C., Labesse G., Monsarrat B., Lorca T., Castro A. (2011). Characterization of the mechanisms controlling Greatwall activity. Mol. Cell. Biol. 31, 2262–2275 10.1128/MCB.00753-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman W. J., Masui Y. (1975). Effects of cyclohexamide on a cytoplasmic factor initiating meiotic naturation in Xenopus oocytes. Exp. Cell Res. 91, 381–388 10.1016/0014-4827(75)90118-4 [DOI] [PubMed] [Google Scholar]
- Wu J. Q., Guo J. Y., Tang W., Yang C. S., Freel C. D., Chen C., Nairn A. C., Kornbluth S. (2009). PP1-mediated dephosphorylation of phosphoproteins at mitotic exit is controlled by inhibitor-1 and PP1 phosphorylation. Nat. Cell Biol. 11, 644–651 10.1038/ncb1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. M., Blake-Hodek K., Williams B. C., Lewellyn A. L., Goldberg M. L., Maller J. L. (2011). Regulation of Greatwall kinase during Xenopus oocyte maturation. Mol. Biol. Cell 22, 2157–2164 10.1091/mbc.E11-01-0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N., Mellini M. L., Vande Woude G. F. (1992). Meiotic initiation by the mos protein in Xenopus. Nature 355, 649–652 10.1038/355649a0 [DOI] [PubMed] [Google Scholar]
- Yu J., Zhao Y., Li Z., Galas S., Goldberg M. L. (2006). Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell 22, 83–91 10.1016/j.molcel.2006.02.022 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Haccard O., Wang R., Yu J., Kuang J., Jessus C., Goldberg M. L. (2008). Roles of Greatwall kinase in the regulation of cdc25 phosphatase. Mol. Biol. Cell 19, 1317–1327 10.1091/mbc.E07-11-1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.