Abstract
Hypoxia has been found in the atherosclerotic plaques of larger mammals, including humans. Whether hypoxia occurs in the plaques of standard mouse models with atherosclerosis has been controversial, given their small size. In this review, we summarize the findings of a recent report demonstrating that direct evidence of hypoxia can indeed be found in the plaques of mice deficient in apolipoprotein E (apoE−/−mice). Furthermore, studies in vitro showed that hypoxia promoted lipid synthesis and reduced cholesterol efflux through the ABCA1 pathway, and that the transcription factor HIF-1α mediated many, but not all, of the effects. These results are discussed in the context of the literature and clinical practice.
Introduction
Animal models remain the most powerful tool in determining the pathophysiology of atherosclerosis. Specifically, knockout mouse models (Apoe and Ldlr) that elevate plasma levels of apoB-lipoproteins, have led to significant discoveries regarding the mechanisms underlying atherosclerosis (Ishibashi et al., 1993; Plump et al., 1992; Zhang et al., 1992). Hueper (1944) proposed the anoxemia theory of atherosclerosis over 60 years ago, suggesting that hypoxia is a key factor for the development and maintenance of atherosclerotic lesions. However, for decades there was no direct evidence that hypoxic areas exist in the arterial wall in vivo. In 1999, Bjornheden used a new hypoxia marker to demonstrate that hypoxic zones occur within atherosclerotic plaques in rabbits. These lesions can form 200–300 μm away from the endothelial surface and are within a distance often observed in humans (Bjornheden et al., 1999). In large animals, such as rabbits and dogs, as well as in humans, it is reasonable to hypothesize that areas of hypoxia in the vessel wall are due to intimal thickening that exceeds the maximum oxygen diffusion distance. Contributing to the susceptibility of arterial cells to hypoxia is diffuse intimal thickening (DIT), which naturally occurs in human coronaries, and is characterized by a thickened intima mainly composed of smooth muscle cells (SMCs), elastin, and proteoglycans, and is devoid of lipid deposition (Nakashima et al., 2007). Due to the small size, in mice, of the intima, even when there is atherosclerosis, the existence in plaques of significant areas of cells that are sufficiently distant from a blood supply to become hypoxic has been controversial. Therefore, it is important to determine the existence of hypoxic areas in murine atherosclerotic plaques, and to study the relationship between hypoxia and atherosclerosis using this small-animal model that is given great importance in the research related to the human disease.
Hypoxia in mouse atherosclerotic plaques
Indirect evidence shows that chronic intermittent hypoxia, a sleep apnea surrogate, increases atherosclerosis in the presence of diet-induced dyslipidemia in mice. Further study showed that stearoyl coenzyme A desaturase 1, a key hepatic enzyme of lipoprotein secretion, is a critical factor in chronic intermittent hypoxia-induced dyslipidemia and atherosclerosis in mice (Savransky et al., 2008). The authors suggested that the plasma lipoprotein profile is the dominant atherogenic influence, as opposed to the effects of hypoxia on plaque cells. Based on the results from our laboratories and others, it is now clear that hypoxia also has direct effects on plaque macrophages in mouse models of atherosclerosis.
In 2011, it was demonstrated that hypoxia in murine atherosclerotic plaques can be detected with [18F]EF5, a specific marker of hypoxia, labeled for use in positron emission tomography (Silvola et al., 2011). This probe has been used in several human studies, as well as in studies investigating the role of hypoxia murine cancer models (Komar et al., 2008; Mahy et al., 2006). Our study provided the first direct evidence of the stabilization of hypoxia responsive factor-1α (HIF-1α) and of the activation of HIF-1α target genes (GLUT-1 and VEGF) in the plaques of apolipoprotein E-deficient mice (Parathath et al., 2011). Not only were these proteins detected in the plaque, they also colocalized with the macrophages (CD68+cells).
Macrophages are a major contributor to the progression of atherosclerosis (Nakashima et al., 2007). Murine plaques are extremely rich in macrophages comprising approximately 30% of the plaque area, a volume 2–4-fold higher than what is found in human plaques (Sluimer and Daemen, 2009). A novel concept from Sluimer and Daemen (2009) suggests that plaque hypoxia seems independent of species and corresponding plaque size, but instead depends on the high metabolic demand of the inflammatory (macrophage-rich) microenvironment. The high oxygen demand of metabolically active inflammatory cells further contributes to hypoxic conditions (Murdoch et al., 2005). We attempted to determine if hypoxia/hypoxic regions play a pathophysiological role or if they are a consequence of advanced atherosclerotic disease.
Hypoxia and macrophage lipid content
We performed several experiments using multiple sources of macrophages [J774, Raw 264.7 cell lines and bone marrow derived primary macrophages (BMDM)] to directly determine the role of hypoxia (1% O2) in their lipid metabolism. Our in vitro data showed that under hypoxic conditions, cholesterol and triglyceride (TG) content were significantly increased by 50% and 120%, respectively. The ability of hypoxic human macrophages to increase TG was previously demonstrated by Bostrom et al. (2006), but they did not observe the cholesterol effect, probably because of the use of a medium with a high concentration of glucose, which blunts some of the effects of hypoxia.
To extend the cell culture results, we used laser capture microdissection (LCM) to isolate macrophage RNA from hypoxic (CD68+ and VEGF/GLUT-1 stained) and non-hypoxic (CD68+ only) regions of the atherosclerotic plaque. In hypoxic macrophages, our molecular analysis showed statistically significant increases in VEGF and GLUT-1, along with increasing HMG CoA reductase in primary and cultured macrophages. Using our in vitro system, we tested 2 different macrophage cell lines to determine the effect of hypoxia on these genes. As expected, significant increases in VEGF and GLUT-1 were seen. Hmgcr mRNA also increased significantly. Consistent with this, there are several HIF responsive elements in the upstream of the start site of HMG CoA reductase promoter (Pallottini et al., 2008).
We followed-up these observations by performing cholesterol synthesis assays using radiolabeled acetate, which resulted in a statistically significant increase of 500% of cholesterol synthesis under hypoxic conditions. We also noted that the increased cholesterol content contained a high percentage of unesterified cholesterol (UC) or free cholesterol. This was surprising, considering that excess UC can be toxic (Feng et al., 2003; Tabas, 1997), particularly to human macrophages (Liu et al., 2007), and ordinarily macrophages can easily esterify UC to cholesteryl esters (CE) and store them as lipid droplets. In hypoxic bone marrow derived primary macrophages (BMDM), CE formation was found to be statistically significantly decreased by 30%. It is also theoretically possible that hypoxia somehow increased neutral cholesteryl hydrolysis, resulting in increased UC levels, but we did not investigate this.
Hypoxia and macrophage cholesterol efflux
The ability of cells to rid themselves of excess cholesterol is an integral part of cell survival. The majority of cells eliminate excess cholesterol by efflux pathways. Cholesterol efflux requires cholesterol transporters (e.g., ABCA1, ABCG1, and SR-BI) at the plasma membrane, as well as cholesterol acceptors (e.g., apoA1 and/or HDL). When we tested the ability of ABCA1 (apoA1 as acceptor) to efflux UC from hypoxic macrophages, there was an 84% decrease in cholesterol efflux from hypoxic J774 macrophage cells. These results were confirmed in Raw 264.7 macrophages and in BMDM. There was also a 26% diminished ability of HDL3 to accept cholesterol, which is predominantly mediated by ABCG1 and SR-BI. Since the majority of the UC efflux is mediated by ABCA1 in the macrophage (Adorni et al., 2007) we focused on ABCA1 expression from both hypoxic areas of the plaque and cells in culture. At the protein level, ABCA1 was not significantly changed after 24 h in a hypoxic cell culture, but with longer incubation times and in the plaque, protein abundance trended downward. There were also effects of hypoxia on the subcellular distribution of ABCA1. In a series of experiments using confocal microscopy, we found that under hypoxic conditions, ABCA1 was less expressed at the plasma membrane in both J774 and BMDM, suggesting that independent of the effects on the cellular pool of ABCA1, what was present in the cell was in a location unsuitable for efflux. Overall, the results show that several pathways involved in cholesterol metabolism are perturbed by hypoxia.
Hypoxia and hypoxia-inducible factor (HIF)
HIF is a major transcription factor regulating hypoxia-mediated pathways. Therefore, we tested the direct role of HIF-1α in cholesterol metabolism using stable J774 cell lines with knocked down HIF-1α or HIF-1α mutants (“M”-HIF-1α) that are resistant to degradation under normoxic conditions (mutation of conserved proline, Pro402→Ala, Pro564→Gly) (Jaakkola et al., 2001; Masson et al., 2001). Overexpression of M-HIF-1α in normoxic conditions resulted in decreased UC efflux. Under hypoxic conditions, overexpression further decreased cholesterol efflux, suggesting that HIF-1α plays an important regulatory role in hypoxia-mediated suppression of cholesterol efflux. Additionally, many of the other changes, increased HMG CoA reductase mRNA, reduced efflux, increased TG, and cholesterol content were blunted in cells with HIF-1α knockdown under hypoxic conditions. Correlative studies by Ugocsai et al. (2010) suggest that HIF-1β may play a significant role in ABCA1 expression in human macrophages.
Although the manipulation of HIF-1α had major effects on macrophage lipid metabolism, the effects of hypoxia could not be entirely explained by this one factor. Remembering that as the cholesterol content of the cell increases (as what happens in a hyperlipidemic environment), the LXR pathway is induced and cholesterol efflux genes are upregulated, other possibilities emerge (Schwartz et al., 2000). A recent study by Plosch et al. (2010) showed a hypoxia-mediated increase in LXR and ABCA1. Although LXR activation can increase cholesterol efflux under normal conditions, it can also increase TG via stimulation of SREBP1C pathways, even in macrophages (Rowe et al., 2003; Na et al., 2011), and potentially contribute to the increase in TG content seen in hypoxic macrophages.
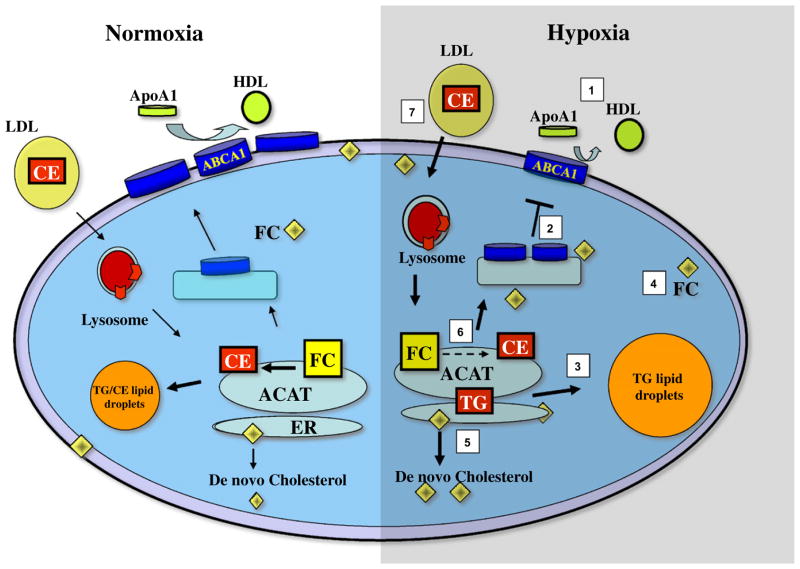
Another potential way to increase lipid content in the macrophage is to increase LDL uptake. Studies by Asplund et al. (2010, 2011) demonstrated that hypoxic macrophages secrete more proteoglycans with increased affinity for LDL. In addition, inducing acidic microenvironments further enhances the binding of apoB-lipoproteins to arterial matrix proteoglycans and also promotes the digestion of these particles by group V secreted phospholipase A2 (Lahdesmaki et al., 2012). It seems likely, then, that besides HIF-1α, there are additional mechanisms by which hypoxia can increase the lipid content of macrophages (Fig. 1).
Fig. 1.
Altered lipid metabolism in hypoxic macrophages: 1—decreased cholesterol efflux to apoA1; 2—decreased ABCA1 expression at the plasma membrane; 3—increased triglycerides content; 4—increased cholesterol content; 5—increased cholesterol synthesis; 6—decreased cholesterol esterification; and 7—increased uptake of LDL-cholesterol. Points 1–6 are based on Parathath et al. (2011) and point 7 on Asplund et al. (2010, 2011) and Lahdesmaki et al. (2012).
Other atherogenic mechanisms of hypoxia
Hypoxia may also play a non-lipid role in the development of atherosclerotic lesions by increasing inflammation, depleting ATP, accumulating lactate, and increasing proteolysis and angiogenesis (Hulten and Levin, 2009). The hypoxic induction of inflammatory mediators in macrophages is well documented (Hulten and Levin, 2009; Murdoch et al., 2005). Recruitment of T lymphocytes and proliferation and migration of smooth muscle and endothelial cells are essential for atherosclerotic plaque formation and development. During this process, a number of proinflammatory factors and cytokines, leukotrienes, and chemokines are increased in expression, especially in lipid-loaded foam cells, such as IL8 (Karakurum et al., 1994), tumor necrosis factor α (TNFα), IL-1 (MacNaul et al., 1990), vascular cell adhesion molecule 1 (VCAM-1) (Cybulsky et al., 2001), and 15-lipoxygenase-2 (15-LOX-2) (Rydberg et al., 2004). Moreover, macrophages are trapped in hypoxic areas of the lesion, however the exact mechanisms have yet to be determined. According to our recent findings, CCR7 mRNA levels are decreased in laser-captured cells from the plaque hypoxic regions (Parathath et al., 2011), suggesting that hypoxia may block macrophage emigration from necrotic cores, as a CCR7-dependent emigration process can play a pivotal role in atherosclerosis regression (Trogan et al., 2006). Alternatively, hypoxia may induce macrophage migration inhibitory factor (MIF). MIF plays a critical role in the progression of atherosclerosis by several different functions. MIF triggered arrest and chemotaxis of monocytes and T cells through its receptors CXCR2/4 (Bernhagen et al., 2007). Further, in vivo studies showed that blockade of MIF in mice with advanced atherosclerosis led to plaque regression and reduced monocyte and T-cell content (Bernhagen et al., 2007). Additionally, the neuronal signaling molecule Netrin-1 was recently shown to play an important role in macrophage retention in atherosclerotic plaques (van Gils et al., 2012). Notably, netrin-1 expression has been shown to be regulated by hypoxia, but this may be tissue or disease specific (Dakouane-Giudicelli et al., 2011; Rosenberger et al., 2009). Combined, these studies suggest that hypoxia interferes with multiple steps of an emigration pathway for plaque macrophages.
Future directions
It is clear that hypoxia plays a significant role in mouse models of atherosclerosis, thereby increasing their relevance to the human disease. Future research could compare molecular signatures and pathway analysis of macrophages from hypoxic vs non-hypoxic plaques in both mice and human samples. Micro-RNAs play a significant role in metabolic pathways, including the hypoxia response (Kulshreshtha et al., 2007), but the direct connection of hypoxia to specific micro-RNAs in atherosclerosis remains a potentially promising area to explore. Many other questions remain to be addressed. For example, what role does hypoxia play in other cells of the atherosclerotic plaque, such as, endothelial cells, lymphocytes, dendritic cells, and smooth muscle cells? Cholesterol-loading has been shown to transdifferentiate smooth muscle cells to macrophage-like cells (Rong et al., 2003); therefore, would hypoxia, which increases cholesterol content, catalyze this phenotypic switching? Significant research is needed to tackle these and other fundamental questions and to gain further insight to atherosclerosis plaque biology. In terms of clinical practice, while it may not be possible to prevent hypoxia completely, given that there is considerable diffuse intimal thickening and plaque progression relatively early in life before aggressive preventive measures are normally started, knowing the pathways adversely targeted by hypoxia could direct therapies to reverse the effects.
Acknowledgments
These studies were supported by NIH post-doctoral fellowship and research project awards (Grant nos. F32HL087627, R01 HL084312 and P01 HL098055).
References
- Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, et al. The roles of different pathways in the release of cholesterol from macrophages. Journal of Lipid Research. 2007;48:2453–62. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- Asplund A, Friden V, Stillemark-Billton P, Camejo G, Bondjers G. Macrophages exposed to hypoxia secrete proteoglycans for which LDL has higher affinity. Atherosclerosis. 2011;215:77–81. doi: 10.1016/j.atherosclerosis.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Asplund A, Stillemark-Billton P, Larsson E, Rydberg EK, Moses J, Hulten LM, et al. Hypoxic regulation of secreted proteoglycans in macrophages. Glycobiology. 2010;20:33–40. doi: 10.1093/glycob/cwp139. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nature Medicine. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- Bjornheden T, Levin M, Evaldsson M, Wiklund O. Evidence of hypoxic areas within the arterial wall in vivo. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999;19:870–6. doi: 10.1161/01.atv.19.4.870. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Magnusson B, Svensson PA, Wiklund O, Boren J, Carlsson LM, et al. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26:1871–6. doi: 10.1161/01.ATV.0000229665.78997.0b. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. Journal of Clinical Investigation. 2001;107:1255–62. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakouane-Giudicelli M, Alfaidy N, Bayle P, Tassin de Nonneville A, Studer V, Rozenberg P, et al. Hypoxia-inducible factor 1 controls the expression of the uncoordinated-5-B receptor, but not of Netrin-1, in first trimester human placenta. International Journal of Developmental Biology. 2011;55:981–7. doi: 10.1387/ijdb.103276md. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nature Cell Biology. 2003;5:781–92. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Hueper WC. Artereosclerosis: the anoxemia theory. Archives of Pathology. 1944;38:162–81. [Google Scholar]
- Hulten LM, Levin M. The role of hypoxia in atherosclerosis. Current Opinion in Lipidology. 2009;20:409–14. doi: 10.1097/MOL.0b013e3283307be8. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. Journal of Clinical Investigation. 1993;92:883–93. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Karakurum M, Shreeniwas R, Chen J, Pinsky D, Yan SD, Anderson M, et al. Hypoxic induction of interleukin-8 gene expression in human endothelial cells. Journal of Clinical Investigation. 1994;93:1564–70. doi: 10.1172/JCI117135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar G, Seppanen M, Eskola O, Lindholm P, Gronroos TJ, Forsback S, et al. 18F-EF5: a new PET tracer for imaging hypoxia in head and neck cancer. Journal of Nuclear Medicine. 2008;49:1944–51. doi: 10.2967/jnumed.108.053785. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, et al. A microRNA signature of hypoxia. Molecular and Cellular Biology. 2007;27:1859–67. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahdesmaki K, Oorni K, Alanne-Kinnunen M, Jauhiainen M, Hurt-Camejo E, Kovanen PT. Acidity and lipolysis by group V secreted phospholipase A(2) strongly increase the binding of apoB-100-containing lipoproteins to human aortic proteoglycans. Biochimica et Biophysica Acta. 2012;1821:257–67. doi: 10.1016/j.bbalip.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Liu ML, Reilly MP, Casasanto P, McKenzie SE, Williams KJ. Cholesterol enrichment of human monocyte/macrophages induces surface exposure of phosphatidylserine and the release of biologically-active tissue factor-positive microvesicles. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:430–5. doi: 10.1161/01.ATV.0000254674.47693.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaul KL, Hutchinson NI, Parsons JN, Bayne EK, Tocci MJ. Analysis of IL-1 and TNF-alpha gene expression in human rheumatoid synoviocytes and normal monocytes by in situ hybridization. Journal of Immunology. 1990;145:4154–66. [PubMed] [Google Scholar]
- Mahy P, De Bast M, Gillart J, Labar D, Gregoire V. Detection of tumour hypoxia: comparison between EF5 adducts and [18F]EF3 uptake on an individual mouse tumour basis. European Journal of Nuclear Medicine and Molecular Imaging. 2006;33:553–6. doi: 10.1007/s00259-005-0049-3. [DOI] [PubMed] [Google Scholar]
- Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO Journal. 2001;20:5197–206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C, Muthana M, Lewis CE. Hypoxia regulates macrophage functions in inflammation. Journal of Immunology. 2005;175:6257–63. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- Na TY, Lee HJ, Oh HJ, Huh S, Lee IK, Lee MO. Positive cross-talk between hypoxia inducible factor-1alpha and liver X receptor alpha induces formation of triglyceride-loaded foam cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:2949–56. doi: 10.1161/ATVBAHA.111.235788. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Fujii H, Sumiyoshi S, Wight TN, Sueishi K. Early human atherosclerosis: accumulation of lipid and proteoglycans in intimal thickenings followed by macrophage infiltration. Arteriosclerosis, Thrombosis, and Vascular Biology. 2007;27:1159–65. doi: 10.1161/ATVBAHA.106.134080. [DOI] [PubMed] [Google Scholar]
- Pallottini V, Guantario B, Martini C, Totta P, Filippi I, Carraro F, et al. Regulation of HMG-CoA reductase expression by hypoxia. Journal of Cellular Biochemistry. 2008;104:701–9. doi: 10.1002/jcb.21757. [DOI] [PubMed] [Google Scholar]
- Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, et al. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circulation Research. 2011;109:1141–52. doi: 10.1161/CIRCRESAHA.111.246363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plosch T, Gellhaus A, van Straten EM, Wolf N, Huijkman NC, Schmidt M, et al. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: a reason for alterations in preeclampsia? Placenta. 2010;31:910–8. doi: 10.1016/j.placenta.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–53. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13531–6. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nature Immunology. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- Rowe AH, Argmann CA, Edwards JY, Sawyez CG, Morand OH, Hegele RA, et al. Enhanced synthesis of the oxysterol 24(S), 25-epoxycholesterol in macrophages by inhibitors of 2,3-oxidosqualene:lanosterol cyclase: a novel mechanism for the attenuation of foam cell formation. Circulation Research. 2003;93:717–25. doi: 10.1161/01.RES.0000097606.43659.F4. [DOI] [PubMed] [Google Scholar]
- Rydberg EK, Krettek A, Ullstrom C, Ekstrom K, Svensson PA, Carlsson LM, et al. Hypoxia increases LDL oxidation and expression of 15-lipoxygenase-2 in human macrophages. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24:2040–5. doi: 10.1161/01.ATV.0000144951.08072.0b. [DOI] [PubMed] [Google Scholar]
- Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circulation Research. 2008;103:1173–80. doi: 10.1161/CIRCRESAHA.108.178533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K, Lawn RM, Wade DP. ABC1 gene expression and ApoA-I-mediated cholesterol efflux are regulated by LXR. Biochemical and Biophysical Research Communications. 2000;274:794–802. doi: 10.1006/bbrc.2000.3243. [DOI] [PubMed] [Google Scholar]
- Silvola JM, Saraste A, Forsback S, Laine VJ, Saukko P, Heinonen SE, et al. Detection of hypoxia by [18F]EF5 in atherosclerotic plaques in mice. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:1011–5. doi: 10.1161/ATVBAHA.110.221440. [DOI] [PubMed] [Google Scholar]
- Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. Journal of Pathology. 2009;218:7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- Tabas I. Free cholesterol-induced cytotoxicity. A possible contributing factor to macrophage foam cell necrosis in advanced atherosclerotic lesions. Trends in Cardiovascular Medicine. 1997;7:256–63. doi: 10.1016/S1050-1738(97)00086-8. [DOI] [PubMed] [Google Scholar]
- Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3781–6. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugocsai P, Hohenstatt A, Paragh G, Liebisch G, Langmann T, Wolf Z, et al. HIF-1beta determines ABCA1 expression under hypoxia in human macrophages. International Journal of Biochemistry and Cell Biology. 2010;42:241–52. doi: 10.1016/j.biocel.2009.10.002. [DOI] [PubMed] [Google Scholar]
- van Gils JM, Derby MC, Fernandes LR, Ramkhelawon B, Ray TD, Rayner KJ, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nature Immunology. 2012;13:136–43. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–71. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]