Abstract
A combined kinetic and computational study on our tryptophan-based bifunctional thiourea catalyzed asymmetric Mannich reactions reveals an apparent negative activation enthalpy. The formation of the pre-transition state complex has been unambiguously confirmed and these observations provide an experimental support for the formation of multiple hydrogen bonding network between the substrates and the catalyst. Such interactions allow the creation of a binding cavity, a key factor to install high enantioselectivity.
Hydrogen bonding is an essential interaction in biological systems1. In addition to their role in structural determination, it is crucial in electrophile activation in biocatalysis2,3. For instance, amide hydrolysis catalyzed by serine protease provides a useful starting point for the discussion of electrophile activation by hydrogen bonding interaction in enzymatic catalysis4. The mechanistic study of [3,3]-sigmatropic rearrangement catalyzed by chorismate mutase has also demonstrated hydrogen bonding interaction's importance in binding the substrates in a preferred conformation5,6,7,8. Research in organocatalysis has sought to develop small molecules to utilize hydrogen bonding interactions to achieve enzyme-like activity and selectivity9,10,11,12,13,14. While numerous successful examples have been demonstrated in the past two decades, kinetic understanding and evidence of these systems is still limited.
Thiourea derivatives have been regarded as effective Lewis acid-like catalysts for their ability in electrophilic activation through hydrogen bonding9. The enantioselective hydrocyanation of imines (Strecker reaction) catalyzed by Schiff base derived chiral thiourea catalysts15 and subsequent kinetic and computational studies16 by Jacobsen and co-workers marked one of the early examples of utilizing this form of activation in asymmetric catalysis. The first example of bifunctional thiourea catalyst bearing tertiary amine was reported by Takemoto and co-workers, and they showed that such catalysts led to excellent enantionselectivity in the Michael reaction of malonate esters with nitroalkenes17,18. The activation model proposed indicated that the nitroolefin interacts with the thiourea moiety via double hydrogen bonding, while the tertiary amine holds the malonate via single hydrogen bonding in the activated enol form. In the past decade, novel chiral thiourea derivatives have been developed and proliferated as the most prominent hydrogen bond donor catalysts in a wide variety of asymmetric organic reactions10,16,19,20,21,22,23.
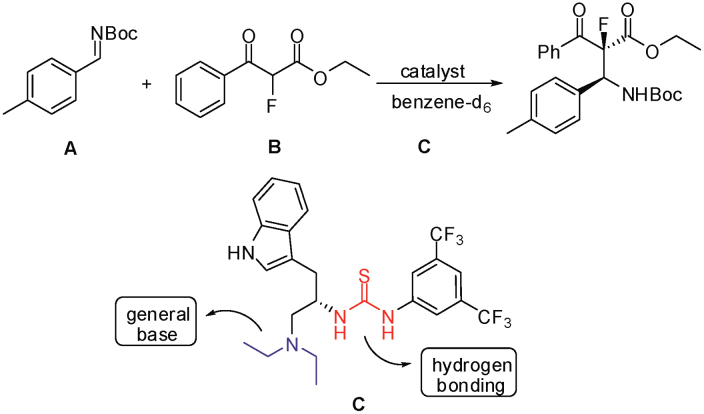
We recently demonstrated that tryptophan-based bifunctional thiourea derivatives are capable of efficiently promoting the asymmetric Mannich reaction of fluorinated ketoesters to afford highly optically enriched fluorine-containing molecules with adjacent quaternary and tertiary stereocenters (Figure 1)24. High enantioselectivity and diastereoselectivity were observed with a wide range of aromatic and heteroaromatic ketoesters with an optimal bifunctional thiourea-based organocatalyst. Preliminary computational studies also suggested the involvement of a “pre-transition-state complex” with multiple hydrogen bonding interactions, and in turn could be responsible for the excellent stereochemical control. In a bid to further deepen our mechanistic understanding of such a catalytic system, we attempted kinetic studies in which experimental data revealed an uncommon apparent negative activation enthalpy. Even though there is precedence of experimentally observed negative activation enthalpy in reactions25,26,27,28,29, the phenomenon in organocatalytic systems is nascent revealing insightful chemical behavior. Concomitant computational studies supported experimental findings and from a theoretical viewpoint we were able to elucidate the origin of the stereochemical outcome and to deduce that the observed apparent negative activation enthalpy arose from a highly extensive and favorable H-bonding effect.
Figure 1. Mannich reaction of fluorinated ketoester and imine.
Results
Determining the overall rate of reaction
Kinetic studies were conducted to verify the termolecularity of the reaction (Supporting information). When the reaction was carried out with 10 equivalent excess of ketoester B, the -ln([A]/[A]0) plot versus time of imine A gave a straight line (R2 = 0.999), which indicated that the reaction is first-order with respect to A. In the same light, it was determined that the reaction is first-order with respect to B with 10 equivalent excess of imine A. The order in catalyst was also examined by plotting the kinetic rate constant (kobs) against the loading of the catalyst C (Trp-1), which satisfy that the reaction is first-order with respect to C. As the overall reaction rate order is 3, this confirms that this catalyst system is a termolecular process and reaction rate would be dependent on the concentrations of A, B and C. Another general base catalyst, 1,4-diazabicyclo[2.2.2]octane (DABCO) was used for the comparison with C. Employing previous protocol, it was found that the reaction rate still first-order with respect to each substrate and the catalyst.
Apparent negative activation enthalpy
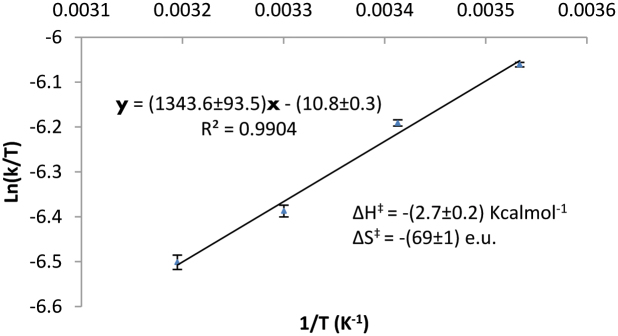
Activation parameters were acquired by the means of Eyring plot analysis with the measurement of reactions rates at 10–40°C30. In sharp contrast to analogous studies done by Takemoto17, it was found that the reaction rate decreased as the reaction temperature was raised within the experimentally accessible temperature range (Table 1) and the activation enthalpy ΔHobs‡ was extrapolated to be negative (Figure 2)! The activation entropy ΔSobs‡ of −74.7 e.u. agrees well with a termolecular reaction. Large activation entropy observed is not without precedence as reported in other pioneering computational and kinetic works in thiourea catalysis26,27. It was noted that the corresponding DABCO studies found the observed activation enthalpy to be positive and the reaction rates increased with elevated temperatures (see supporting information).
Table 1. Activation parameters for Mannich reaction of imine and fluorinated ketoester catalyzed by thiourea C at different temperatures.
| Entry | T (K) | kobs (M−1s−1) | k (M−2s−1) | ee (%) |
|---|---|---|---|---|
| 1 | 283 | (7.9 ± 0.04) × 10−3 | 0.66 ± 0.0033 | 93 |
| 2 | 293 | (7.2 ± 0.05) × 10−3 | 0.60 ± 0.0042 | 93 |
| 3 | 303 | (6.1 ± 0.08) × 10−3 | 0.51 ± 0.0067 | 93 |
| 4 | 313 | (5.6 ± 0.09) × 10−3 | 0.47 ± 0.0075 | 93 |
Figure 2. Eyring plots showing temperature dependence behaviour of the Mannich reaction catalyzed by Trp-1 measured by1H NMR.
DFT investigations on Mannich reactions with Trp-1
This reaction was modeled with DFT calculations to elucidate the observed enantioselectivity and to appraise the energetics. It is well-accepted that the pendent amine on the catalyst can first act as a base to assist the formation of the ketoenolate from the β-ketoester substrate. The resulting protonated amino group together with the thiourea moiety can then bind the ketoenolate and activate the imine electrophile. Two pre-transition-state models of different reactant arrangements derived from theoretical investigations have been proposed in the literature (Figure 3)31,32,33,34,35,36,37,38,39,40. Our initial efforts were thus focused on the identification of the pre-transition-state complex between the substrate and catalyst. Hydrogen bonding is believed to be the keystone interaction for the binding of catalyst and substrates leading to a spatially preferred conformation dictated by the catalyst, a phenomenon that is a reminiscence of enzyme-substrate(s) complexation in biological systems.
Figure 3. Proposed models for catalyst-substrate complex.
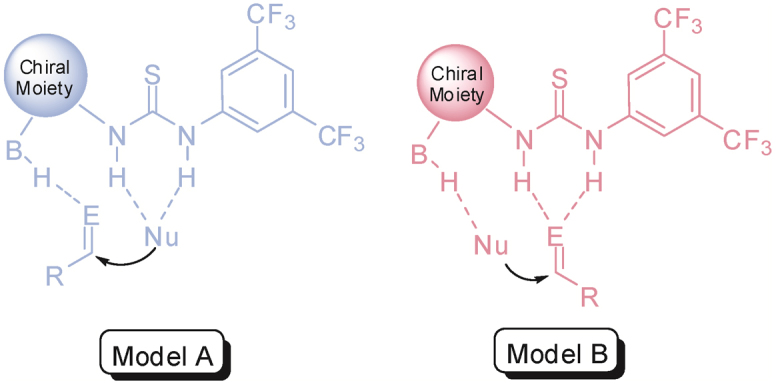
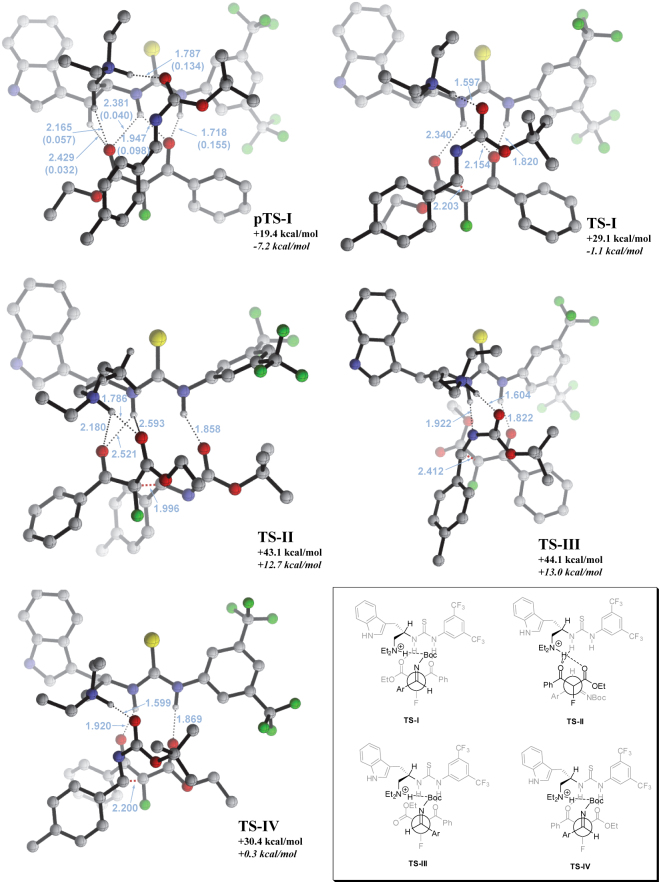
Results from our modeling indicate that the computed pre-transition-state structure pTS-I (model A; +19.4 kcal mol−1 relative to starting materials) is similar in energy to pTS-II (model B; +19.0 kcal mol−1), but the transition state energy barrier of TS-I via pTS-I was found to be significantly lower than that of TS-II via pTS-II by 14.0 kcal mol−1. The vast difference in activation barrier energies for TS-I and TS-II could be rationalized that both electrophile and nucleophile are bound closer and better spatially fitted in the catalyst's ‘assembly pocket’ in pTS-I as compared to pTS-II (C-C bond distance for pTS-I is 3.906 Å and pTS-II is 5.303 Å). In other words, a more transition state-like orientation of the substrates was realized in pTS-I. Moreover, for pTS-I, the α-C-H bonds (to the ammonium group and to the indole ring) are in proximity (2.1 to 2.4 Å) with the ester carbonyl oxygen (Figure 4), alluding to the possibility of the formation of a non-classical C-Hδ+…Oδ− binding pocket. Such secondary interactions have been observed to play an important role in molecular recognition and stereoselective catalytic processes41,42,43. To confirm that such short bond distances are result of hydrogen bonding interactions rather than simple steric arrangement, two-center Mayer bond order analysis44 was carried out. The bond indices were calculated from canonical molecular orbitals in the atomic orbital basis and can be used to analyze the covalent bonding between atoms. The computed bond orders of 0.057 and 0.032 for these α-C-H···O bonding interactions are more significant with reference from our recent DFT studies with different derivatives of thiourea for catalytic organic transformation45,46. The N-H···O hydrogen bonding interactions showed similar to prominently higher bond orders of 0.040, 0.098, and 0.155. Both the C-H and N-H hydrogen bonding interactions contribute to the creation of the binding cavity and subsequently prompt the ketoenolate to be bound such that providing overall stabilization of the charged ketoenolate and locking the nucleophile more rigidly. The imine on the other hand binds via hydrogen bonding to the pendent ammonium group, which firstly enhances the electrophilicity of the sp2 carbon and secondly, fits well above the ketoenolate and is predisposed to nucleophilic addition for the C-C bond formation (Figure 4, top).
Figure 4. Geometry of key intermediate and transition states showing bond distances (Å) of non-covalent interactions (blue) (mPW1PW91/6-31 + G(d,p)).
Mayer bond order for pTS-I are in parenthesis and blue. Reported energies are relative to starting materials: free energy in black non-italic bold and enthalpy in black italic bold. Dotted lines show hydrogen bonding (black) and C-C bond formation (orange). Non-interacting hydrogen atoms have been omitted for visual clarity. Atoms are color coded for each element: carbon (grey); hydrogen (white); oxygen (red); nitrogen (blue); fluorine (green); sulfur (yellow). Insert showing the Newman projection of the transition states from the perspective of the newly formed C-C bonds.
The imine molecule could bind in two different faces relative to the ketoenolate: Re or Si (as viewed by the approach of the nucleophile) and the ketoenolate could also bind to the protonated catalyst in two different orientations, thus determining the overall stereoselective outcome. Computationally, it was found that the transition state leading to the Re-attack, TS-I, is 15.0 kcal mol−1 more favorable than the Si-attack through TS-III. The preference for TS-I is obvious as the s-trans-imine can be bound more closely to the ketoenolate. In order for the Si-face attack on the imine to materialize, the imine has to be the s-cis form which is energetically penalized due to the steric repulsion between the Boc and the 4-methylphenyl groups (TS-III, Figure 4). Modeling for the second orientation of the ketoenolate binding to the catalyst was carried out and the corresponding transition state TS-IV is slightly endergonic by 1.3 kcal mol−1 compared to TS-I, accounting for the 9:1 diastereoselectivity for the reaction. These observations provide the rationale to the constant excellent enantioselectivity obtained for all ketoester substrates yet moderate diastereoselectivity when the ketoester has substituents of similar size (e.g. methyl and ethyl groups)24. It is also noted that the calculated differences in activation free energies of these transition states were similar to those in activation enthalpies, suggesting that the observed selectivities are not due to the entropic control in Sohtome and Nagasawa's systems42,47.
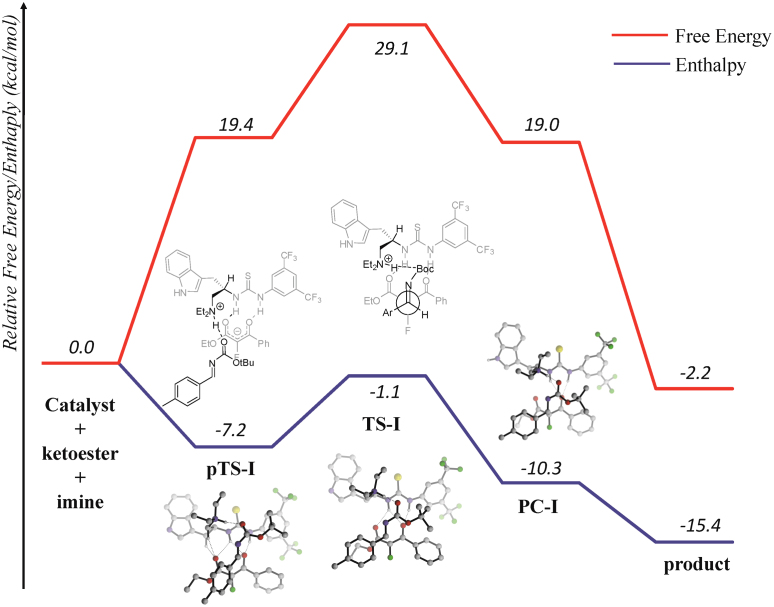
When measuring the reaction rate, one is looking at the overall free energy barrier, i.e. TS-I, and it occurs that the overall enthalpy change of transition state TS-I is −1.1 kcal mol−1 relative to starting substrates. Comparing the enthalpy change for intermediate pTS-I to starting materials (−7.2 kcal mol−1) suggests a distinct feature of an exothermic elementary step due to formation of hydrogen bonds with C and substrates. As our experiments revealed inverse dependence of reaction rate with temperature, it is clear from the reaction profile how negative activation enthalpy originated (Figure 5).
Figure 5. Reaction profile diagram for the asymmetric Mannich reaction (mPW1PW91/6-31 + G(d,p)).
Discussion
From our combined theoretical and kinetic mechanistic study for the enantioselective bifunctional thiourea catalyzed Mannich reaction, first experimental evidence was revealed for an apparent negative activation enthalpy. Since the apparent enthalpy of activation is the sum of enthalpies of the complexation in pre-transition state and of the bond formation step, this important observation suggests that the multitude of non-covalent bonding interactions between the catalyst and substrates certainly translate to a more exothermic enthalpic change for the complexation step (pTS-1, Figure 5) with ΔH = −7.2 kcal mol−1. As a result of the highly exothermic adduct-forming step and only slightly endothermic (+6.1 kcal.mol) corresponding activation enthalpy for the elementary C-C bond formation step, the observed enthalpy was analyzed to be −3.1 kcal mol−1 (ΔHobs‡). The empirical treatment of ΔGobs‡ = ΔHobs‡ − TΔSobs‡ for our kinetic measurements, for which ΔHobs‡ and ΔSobs‡ are assumed to be constant for a small change in temperature, allowed us to observe an apparent negative activation enthalpy due to the exothermic change from starting intermediates to the transition state as being clarified computationally at the same time. These observations unambiguously confirm the multistep nature of the mechanism in the chiral bifunctional thiourea catalyzed reactions, i.e. a pre-transition-state complex is formed. Consistent with the kinetic indications, our calculation results further suggest the formation of multiple hydrogen bonding interactions between the substrates and the catalyst after the pendent amine group deprotonates the pro-nucleophile (ketoester), supporting the analogous model proposed by Pápai and co-workers48. These interactions allow the creation of a binding cavity to direct the incoming imine substrate to install high enantioselectivities.
Methods
Kinetics experiments were recorded on a 500 MHz spectrometer. Chemical shifts were reported in parts per million (ppm), and the residual proton peak was used as an internal reference: proton (in Benzene-d6: δ 7.16). Benzene-d6 was dried from sodium/benzophenone. Stock solutions in deuterated benzene were used in kinetics experiments and 1,4-dimethoxybenzene was used as internal standard. Computations were carried out with density functional theory (DFT) employing Gaussian 09 suite of program49. The modified Perdew-Wang exchange functional mPW1PW91 theory50,51,52 and the all electron split-valence Pople basis set 6−31 + G(d,p)53,54,55 containing polarization functions on heavy atoms and hydrogen and diffuse functions for heavy atoms was used as it was shown to be accurate for modeling Mannich reactions56. Frequency analyses were carried out to verify minimum structures having only positive eigenvalues of the Hessian matrix or transition state structures showing a single negative eigenvalue.
Author Contributions
Y.L. and K.-W.H. conceived and designed the experiments. X.H., T.C. and J.L. carried out synthesis and kinetic experiments. R.L. performed DFT analysis. All authors participated in the mechanistic discussions and contributed to the writing and revising of the manuscript.
Supplementary Material
SUPPLEMENTARY INFO
Acknowledgments
This work was supported by National University of Singapore (Y.L. and K.-W.H.) and King Abdullah University of Science and Technology (K.-W.H.).
References
- Perrin C. L. & Nielson J. B. “Strong” Hydrogen Bonds in Chemistry and Biology. Annu. Rev. Phys. Chem. 48, 511–544 (1997). [DOI] [PubMed] [Google Scholar]
- Pauling L. Molecular Architecture and Biological Reactions. Chem. Eng. News 24, 1375–1377 (1946). [Google Scholar]
- Zhang X. & Houk K. N. Why Enzymes Are Proficient Catalysts: Beyond the Pauling Paradigm. Acc. Chem. Res. 38, 379–385 (2005). [DOI] [PubMed] [Google Scholar]
- Wharton C. W. in Comprehensive Biological Catalysis. Vol. 1 (ed Michael Sinnott) 345–379 (Academic Press, 1998).
- Chook Y. M., Gray J. V., Ke H. & Lipscomb W. N. The Monofunctional Chorismate Mutase from Bacillus subtilis: Structure Determination of Chorismate Mutase and Its Complexes with a Transition State Analog and Prephenate, and Implications for the Mechanism of the Enzymatic Reaction. J. Mol. Bio. 240, 476–500 (1994). [DOI] [PubMed] [Google Scholar]
- Lee A., Stewart J. D., Clardy J. & Ganem B. New insight into the catalytic mechanism of chorismate mutases from structural studies. Chemistry & biology 2, 195–203 (1995). [DOI] [PubMed] [Google Scholar]
- Lee A. Y., Karplus P. A., Ganem B. & Clardy J. Atomic structure of the buried catalytic pocket of Escherichia coli chorismate mutase. J. Am. Chem. Soc. 117, 3627–3628 (1995). [Google Scholar]
- Ganem B. The Mechanism of the Claisen Rearrangement: Déjà Vu All Over Again. Angew. Chem. Int. Ed. 35, 936–945 (1996). [Google Scholar]
- Schreiner P. R. Metal-free organocatalysis through explicit hydrogen bonding interactions. Chem. Soc. Rev. 32, 289–296 (2003). [DOI] [PubMed] [Google Scholar]
- Yamamoto H. & Futatsugi K. “Designer Acids”: Combined Acid Catalysis for Asymmetric Synthesis. Angew. Chem. Int. Ed. 44, 1924–1942 (2005). [DOI] [PubMed] [Google Scholar]
- Akiyama T., Itoh J. & Fuchibe K. Recent Progress in Chiral Brønsted Acid Catalysis. Adv. Synth. Catal. 348, 999–1010 (2006). [Google Scholar]
- Doyle A. G. & Jacobsen E. N. Small-Molecule H-Bond Donors in Asymmetric Catalysis. Chem. Rev. 107, 5713–5743 (2007). [DOI] [PubMed] [Google Scholar]
- Connon S. J. Asymmetric catalysis with bifunctional cinchona alkaloid-based urea and thiourea organocatalysts. Chem. Commun. 2499–2510 (2008). [DOI] [PubMed] [Google Scholar]
- Yu X. & Wang W. Hydrogen-Bond-Mediated Asymmetric Catalysis. Chem. Asian J. 3, 516–532 (2008). [DOI] [PubMed] [Google Scholar]
- Sigman M. S. & Jacobsen E. N. Schiff Base Catalysts for the Asymmetric Strecker Reaction Identified and Optimized from Parallel Synthetic Libraries. J. Am. Chem. Soc. 120, 4901–4902 (1998). [Google Scholar]
- Vachal P. & Jacobsen E. N. Structure-Based Analysis and Optimization of a Highly Enantioselective Catalyst for the Strecker Reaction. J. Am. Chem. Soc. 124, 10012–10014 (2002). [DOI] [PubMed] [Google Scholar]
- Okino T., Hoashi Y., Furukawa T., Xu X. & Takemoto Y. Enantio- and Diastereoselective Michael Reaction of 1,3-Dicarbonyl Compounds to Nitroolefins Catalyzed by a Bifunctional Thiourea. J. Am. Chem. Soc. 127, 119–125 (2005). [DOI] [PubMed] [Google Scholar]
- Okino T., Hoashi Y. & Takemoto Y. Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts. J. Am. Chem. Soc. 125, 12672–12673 (2003). [DOI] [PubMed] [Google Scholar]
- Steinhagen H. & Helmchen G. Asymmetric Two-Center Catalysis—Learning from Nature. Angew. Chem. Int. Ed. 35, 2339–2342 (1996). [Google Scholar]
- Wuest J. D. Multiple Coordination and Activation of Lewis Bases by Multidentate Lewis Acids. Acc. Chem. Res. 32, 81–89 (1998). [Google Scholar]
- Shibasaki M. & Yoshikawa N. Lanthanide Complexes in Multifunctional Asymmetric Catalysis. Chem. Rev. 102, 2187–2210 (2002). [DOI] [PubMed] [Google Scholar]
- Ma J.-A. & Cahard D. Towards Perfect Catalytic Asymmetric Synthesis: Dual Activation of the Electrophile and the Nucleophile. Angew. Chem. Int. Ed. 43, 4566–4583 (2004). [DOI] [PubMed] [Google Scholar]
- Muñiz K. Bifunctional Metal–Ligand Catalysis: Hydrogenations and New Reactions within the Metal–(Di)amine Scaffold. Angew. Chem. Int. Ed. 44, 6622–6627 (2005). [DOI] [PubMed] [Google Scholar]
- Han X., Kwiatkowski J., Xue F., Huang K.-W. & Lu Y. Asymmetric Mannich Reaction of Fluorinated Ketoesters with a Tryptophan-Derived Bifunctional Thiourea Catalyst. Angew. Chem. Int. Ed. 48, 7604–7607 (2009). [DOI] [PubMed] [Google Scholar]
- Shimomura T., Tölle K. J., Smid J. & Szwarc M. Energy and Entropy of Activation of Propagation by the Free Polystyryl Anions and their Ion Pairs. The Phenomenon of "Negative" Activation Energy. J. Am. Chem. Soc. 89, 796–803 (1967). [Google Scholar]
- Liu M. T. H. & Bonneau R. Laser Flash Photolysis Study of Substituent Effects on the Rate of 1,2-H Migration in a Series of Benzylchlorocarbenes. J. Am. Chem. Soc. 114, 3604–3607 (1992). [Google Scholar]
- Boyd A. A. & Lesclaux R. The Temperature Dependence of the Rate Coefficients for β-Hydroxyperoxy Radical Self Reactions. Int. J. Chem. Kinetics 29, 323–331 (1997). [Google Scholar]
- Olson L. P., Kuwata K. T., Bartberger M. D. & Houk K. N. Conformation-Dependent State Selectivity in O-O Cleavage of ONOONO: An “Inorganic Cope Rearrangement” Helps Explain the Observed Negative Activation Energy in the Oxidation of Nitric Oxide by Dioxygen. J. Am. Chem. Soc. 124, 9469–9475 (2002). [DOI] [PubMed] [Google Scholar]
- Feria L., Gonalez C. & Castro M. Ab Initio Study of the CH3O2 Self-Reaction in Gas Phase: Elucidation of the CH3O2 + CH3O2 --> 2CH3O + O2 Pathway. Int. J. Quantum Chem. 99, 605–615 (2004). [Google Scholar]
- Eyring H. The Activated Complex and the Absolute Rate of Chemical Reactions. Chem. Rev. 17, 65–77 (1935). [Google Scholar]
- Schreiner P. R. & Wittkopp A. H-Bonding Additives Act Like Lewis Acid Catalysts. Org. Lett. 4, 217–220 (2002). [DOI] [PubMed] [Google Scholar]
- Kirsten M., Rehbein J., Hiersemann M. & Strassner T. Organocatalytic Claisen Rearrangement: Theory and Experiment. J. Org. Chem. 72, 4001–4011 (2007). [DOI] [PubMed] [Google Scholar]
- Kotke M. & Schreiner P. R. Generally Applicable Organocatalytic Tetrahydropyranylation of Hydroxy Functionalities with Very Low Catalyst Loading. Synthesis 5, 779–790 (2007). [Google Scholar]
- Schmidtchen F. P. & Berger M. Artificial Organic Host Molecules for Anions. Chem. Rev. 97, 1609–1646 (1997). [DOI] [PubMed] [Google Scholar]
- Beer P. D. & Gale P. A. Anion Recognition and Sensing: The State of the Art and Future Perspectives. Angew. Chem. Int. Ed. 40, 486–516 (2001). [PubMed] [Google Scholar]
- 35 Years of Synthetic Anion Receptor Chemistry 1968–2003. (ed Gale, P. A.) Coord. Chem. Rev. 240, 1–226 (2003). [Google Scholar]
- Gomez D. E., Fabbrizzi L., Licchelli M. & Monzani E. Urea vs. thiourea in anion recognition. Org. Biomol. Chem. 3, 1495–1500 (2005). [DOI] [PubMed] [Google Scholar]
- Hammar P., Marcelli T., Hiemstra H. & Himo F. Density Functional Theory Study of the Cinchona Thiourea- Catalyzed Henry Reaction: Mechanism and Enantioselectivity. Adv. Synth. Catal. 349, 2537–2548 (2007). [Google Scholar]
- Miyabe H. & Takemoto Y. Discovery and Application of Asymmetric Reaction by Multi-Functional Thioureas. Bull. Chem. Soc. Jpn. 81, 785–795 (2008). [Google Scholar]
- Almaşi D., Alonso D. A., Gómez-Bengoa E. & Nájera C. Chiral 2-Aminobenzimidazoles as Recoverable Organocatalysts for the Addition of 1,3-Dicarbonyl Compounds to Nitroalkenes. J. Org. Chem. 74, 6163–6168 (2009). [DOI] [PubMed] [Google Scholar]
- Yang H. & Wong M. W. Oxyanion Hole Stabilization by C–H⋯O Interaction in a Transition State - A Three-Point Interaction Model for Cinchona Alkaloid-Catalyzed Asymmetric Methanolysis of meso-Cyclic Anhydrides. J. Am. Chem. Soc. 135, 5808–5818 (2013). [DOI] [PubMed] [Google Scholar]
- Sohtome Y. et al. Entropy-Controlled Catalytic Asymmetric 1,4-Type Friedel–Crafts Reaction of Phenols Using Conformationally Flexible Guanidine/Bisthiourea Organocatalyst. Angew. Chem. Int. Ed. 49, 7299–7303 (2010). [DOI] [PubMed] [Google Scholar]
- Cannizzaro C. E. & Houk K. N. Magnitudes and Chemical Consequences of R3N + − C−H···O = C Hydrogen Bonding. J. Am. Chem. Soc. 124, 7163–7169 (2002). [DOI] [PubMed] [Google Scholar]
- Mayer I. Bond order and valence: Relations to Mulliken's population analysis. Int. J. Quantum Chem. 26, 151–154 (1984). [Google Scholar]
- Zhu B. et al. Direct Asymmetric Vinylogous Aldol Reaction of Allyl Ketones with Isatins: Divergent Synthesis of 3-Hydroxy-2-Oxindole Derivatives. Angew. Chem. Int. Ed. 52, 6666–6670 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Highly Enantio- and Diastereoselective Reactions of γ-Substituted Butenolides Through Direct Vinylogous Conjugate Additions. Angew. Chem. Int. Ed. 51, 10069–10073 (2012). [DOI] [PubMed] [Google Scholar]
- Sohtome Y., Tanaka S., Takada K., Yamaguchi T. & Nagasawa K. Solvent-Dependent Enantiodivergent Mannich-Type Reaction: Utilizing a Conformationally Flexible Guanidine/Bisthiourea Organocatalyst. Angew. Chem. Int. Ed. 49, 9254–9257 (2010). [DOI] [PubMed] [Google Scholar]
- Hamza A., Schubert G., Soós T. & Pápai I. Theoretical Studies on the Bifunctionality of Chiral Thiourea-Based Organocatalysts: Competing Routes to C−C Bond Formation. J. Am. Chem. Soc. 128, 13151–13160 (2006). [DOI] [PubMed] [Google Scholar]
- Gaussian 09 v. revision A.02 (Gaussian, Inc., Wallingford CT, 2009).
- Perdew J. P. in Electronic Structure of Solids '91. Vol. 11 (eds Ziesche, P. & Eschrig, H.) (Akademie Verlag, 1991).
- Adamo C. & Barone V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 108, 664–675 (1998). [Google Scholar]
- Lynch B. J., Zhao Y. & Truhlar D. G. Effectiveness of Diffuse Basis Functions for Calculating Relative Energies by Density Functional Theory. J. Phys. Chem. A 107, 1384–1388 (2003). [Google Scholar]
- Ditchfield R., Hehre W. J. & Pople J. A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 54, 724–728 (1971). [Google Scholar]
- Hariharan P. C. & Pople J. A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chem. Acc. 28, 213–222 (1973). [Google Scholar]
- Hehre W. J., Ditchfield R. & Pople J. A. Self Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 56, 2257–2261 (1972). [Google Scholar]
- Wheeler S. E., Moran A., Pieniazek S. N. & Houk K. N. Accurate Reaction Enthalpies and Sources of Error in DFT Thermochemistry for Aldol, Mannich, and α-Aminoxylation Reactions. J. Phys. Chem. A 113, 10376–10384 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY INFO