Abstract
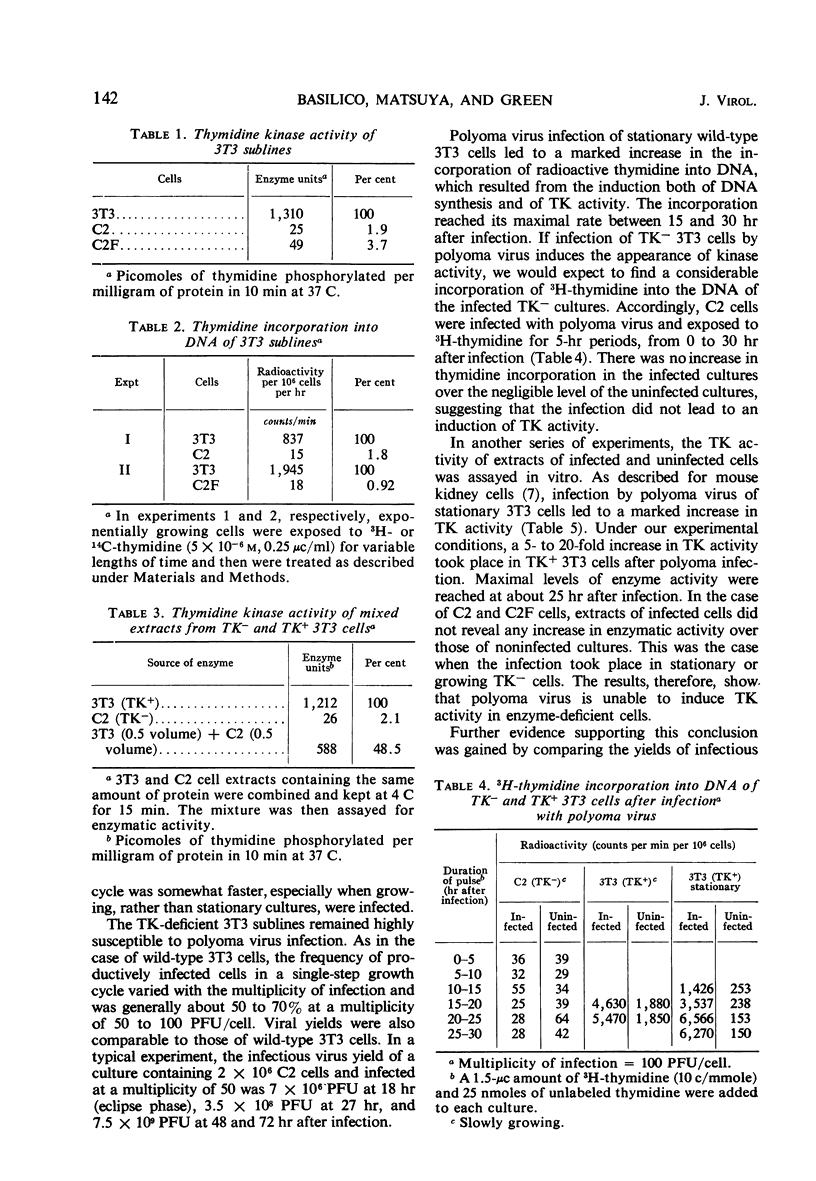
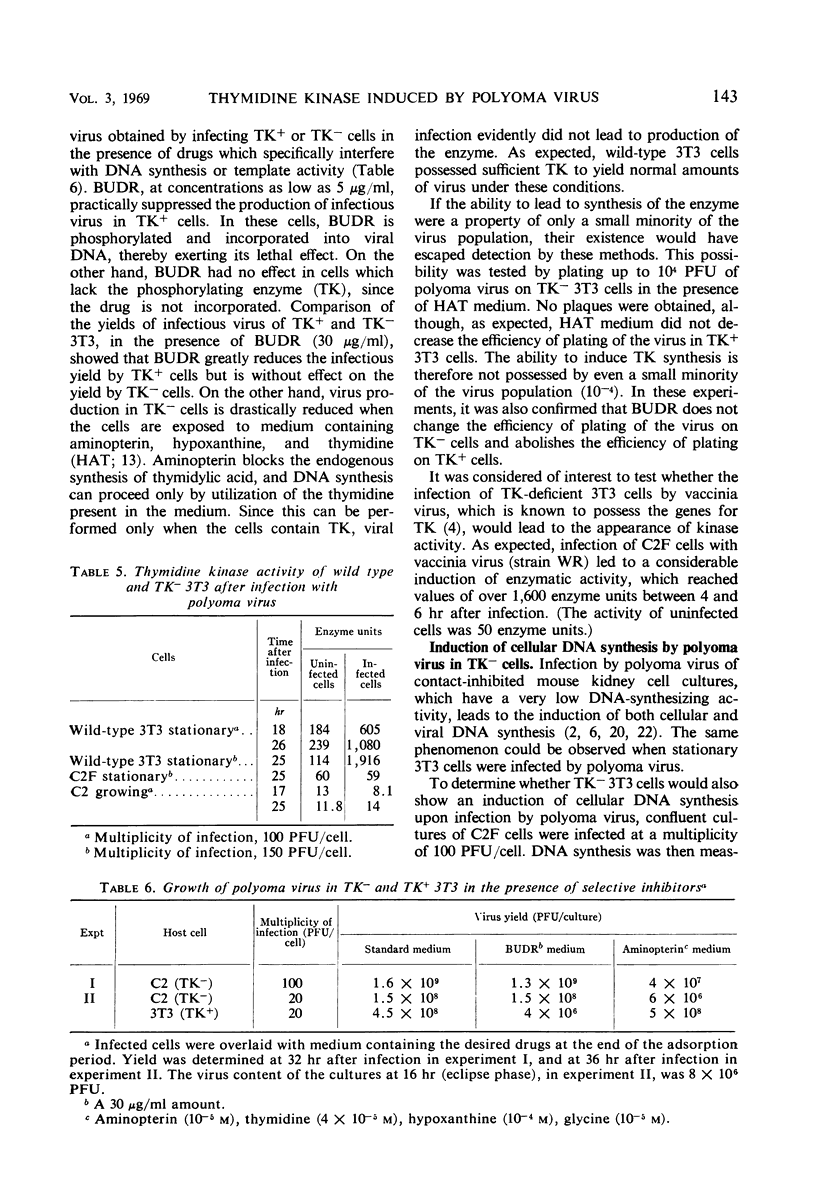
Cells of the 3T3 mouse line efficiently supported the multiplication of polyoma virus, and the infectious process was accompanied by a marked increase in thymidine kinase (TK) activity. Two lines of 5-bromodeoxyuridine-resistant 3T3 cells have been isolated. As expected, these cells incorporated practically no exogenous thymidine into their deoxyribonucleic acid (DNA) and contained negligible TK activity. Like the parental 3T3 cells, TK− lines were susceptible to productive infection by polyoma virus, but infection did not lead to an increase in TK activity. Since kinase activity did appear after infection with another virus (vaccinia) known to contain the gene(s) for that enzyme, it is concluded that TK is not one of the gene products of polyoma virus. As induction of cellular DNA synthesis by polyoma virus occurs normally when the TK− cells are infected in the stationary phase, TK cannot play a role in the determination of this phenomenon.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basilico C., Marin G., di Mayorca G. Requirement for the integrity of the viral genome for the induction of host DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1966 Jul;56(1):208–215. doi: 10.1073/pnas.56.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I. Thymidine kinase from normal, simian virus 40-transformed and simian virus 40-lytically infected cells. J Virol. 1967 Oct;1(5):912–919. doi: 10.1128/jvi.1.5.912-919.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. ISOLATION AND PROPERTIES OF VACCINIA MUTANTS DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Feb;22:214–225. doi: 10.1016/0042-6822(64)90006-6. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. The induction of cancer by viruses. Sci Am. 1967 Apr;216(4):28–37. doi: 10.1038/scientificamerican0467-28. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Vogt M., Dulbecco R. Induction of cellular DNA synthesis by polyoma virus. II. Increase in the rate of enzyme synthesis after infection with polyoma virus in mouse kidney cells. Virology. 1965 Nov;27(3):262–272. doi: 10.1016/0042-6822(65)90105-4. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Dulbecco R. SV 40-specific thymidine kinase. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1888–1894. doi: 10.1073/pnas.58.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. Enzymes of nucleic acid metabolism in cells infected with polyoma virus. Cancer Res. 1966 Apr;26(4):638–646. [PubMed] [Google Scholar]
- Kára J., Weil R. Specific activation of the DNA-synthesizing apparatus in contact-inhibited mouse kidney cells by polyoma virus. Proc Natl Acad Sci U S A. 1967 Jan;57(1):63–70. doi: 10.1073/pnas.57.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littlefield J. W., Basilico C. Infection of thymidine kinase-deficient BHK cells with polyoma virus. Nature. 1966 Jul 16;211(5046):250–252. doi: 10.1038/211250a0. [DOI] [PubMed] [Google Scholar]
- Sheinin R. Studies on the thymidine kinase activity of mouse embryo cells infected with polyoma virus. Virology. 1966 Jan;28(1):47–55. doi: 10.1016/0042-6822(66)90305-9. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]
- Weil R., Michel M. R., Ruschmann G. K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Kaye A. M., Stollar V. Synthesis and transmethylation of DNA in polyoma-infected cultures. Virology. 1965 Oct;27(2):156–169. doi: 10.1016/0042-6822(65)90155-8. [DOI] [PubMed] [Google Scholar]


