Abstract
Selective and targeted delivery of drugs to tumors is a major challenge for an effective cancer therapy and also to overcome the side-effects associated with current treatments. Overexpression of various receptors on tumor cells is a characteristic structural and biochemical aspect of tumors and distinguishes them from physiologically normal cells. This abnormal feature is therefore suitable for selectively directing anticancer molecules to tumors by using ligands that can preferentially recognize such receptors. Several subtypes of integrin receptors that are crucial for cell adhesion, cell signaling, cell viability, and motility have been shown to have an upregulated expression on cancer cells. Thus, ligands that recognize specific integrin subtypes represent excellent candidates to be conjugated to drugs or drug carrier systems and be targeted to tumors. In this regard, integrins recognizing the RGD cell adhesive sequence have been extensively targeted for tumor-specific drug delivery. Here we review key recent examples on the presentation of RGD-based integrin ligands by means of distinct drug-delivery systems, and discuss the prospects of such therapies to specifically target tumor cells.
Keywords: integrins, RGD, tumor, targeted delivery, αvβ3, αvβ5, α5β1 and αvβ6
Introduction
Cancer diagnosis, therapy, and monitoring represent fundamental topics of research in medicine and are of utmost importance in healthcare of today’s society. An efficient cancer therapy should possess exceptional abilities not only to ensure a complete removal of the tumor but also to prevent its spreading and invasion to other tissues by metastasis. Current clinical approaches to treat cancer include, and often combine, surgery, chemotherapy, radiation therapy as well as immunotherapy. However, these methods in general still fail to treat highly aggressive metastatic cancers, and present some serious limitations. For instance, irradiation of tumors may damage adjacent healthy tissues, and chemotherapy, which is based on a non-specific systemic distribution regime, requires high drug dosage and promotes severe adverse side effects. For example, the administration of Paclitaxel (PTX), a drug used for the treatment of lung, ovarian, and breast cancers, has been associated with unwanted effects such as hypersensitivity reactions, myelosuppression, and neurotoxicity (1, 2), among others. Doxorubicin (DOX), another drug used in cancer chemotherapy, has also been described to have cardiotoxic side effects (3, 4). Moreover, chemotherapy might turn inefficient due to acquired chemoresistance as exemplified in the case of Gemcitabine – prime therapeutic used to treat pancreatic cancers (5), for DOX (3) and also for PTX (6, 7).
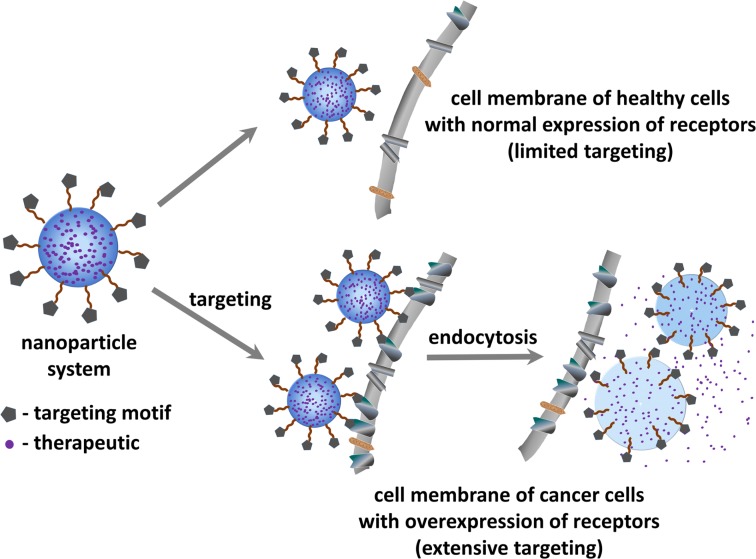
Tumor targeted drug-delivery (Figure 1) represents a promising approach to overcome some of the above mentioned limitations (8). This strategy aims to specifically guide and direct anticancer therapeutics (or imaging agents) to tumor cells without interfering with normal tissues. Such targeted approach relies on the fact that tumor vasculature and tumor cells display a well-differentiated pattern of (over-)expression of specific receptors (i.e., receptors required for tumor angiogenesis), which is consistent with the concept of “Vascular Zip Codes” (9, 10). Targeted drug-delivery methods hence employ small molecules or monoclonal antibodies selective to receptors that are proven to be abnormally expressed on tumors. The conjugation of anticancer drugs to these selective ligands will allow a preferential or selective delivery of the drug to the tumor.
Figure 1.
Schematic representation of the principle of tumor targeted drug delivery for treating cancer.
As a result, this technique benefits from several advantages: (i) non-specific interactions with normal tissues are reduced, and thus the adverse side-effects associated to conventional chemotherapy can be minimized. (ii) Site-directed drug release leads to higher local concentrations at the diseased tissue and thus allows dosage reduction. (iii) Acquired chemoresistance can potentially be reduced by co-delivering other therapeutics capable of regulating cancer multi-drug resistance (MDR). To avail these advantages, well accessible cell surface receptors are preferred over intracellular targets where (complex) drug internalization mechanisms need to be taken into consideration. In this regard, one of the most intensely referred class of proteins for targeted therapy is the integrin family (11).
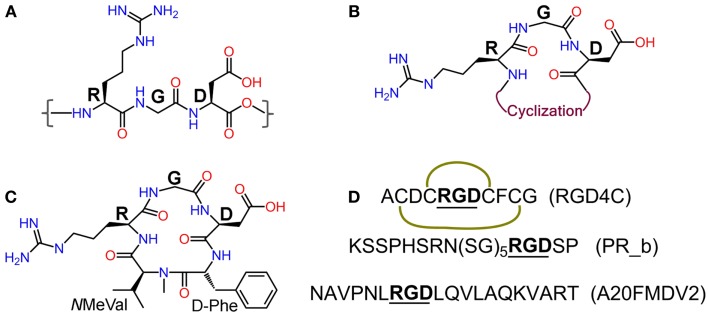
Integrins are heterodimeric transmembrane glycoproteins consisting of an α and a β subunit. In total, 24 different subtypes of integrins that are constituted from 18 α and 8 β subunits have been discovered to date (12). Almost half of them bind to various extra cellular matrix (ECM) proteins such as fibronectin, vitronectin, and collagen through the tripeptide motif Arg-Gly-Asp = RGD [(13), Figure 2], and are vital in the adhesion, signaling, migration, and survival of most cells (14). Integrins have also very important roles in cancer progression and some subtypes have been described to be highly over-expressed on many cancer cells. This is the case of integrins αvβ3, αvβ5, and α5β1, which are crucial mediators of angiogenesis in cancer (8, 15–17). Underlying cause for this is the elevated demand by the enlarging tumor for adequate supply of necessary nutrients and oxygen. In order to meet these demands through blood supply, tumor tissue with a rapidly overgrowing number of cells, signals [via growth factors like vascular endothelial growth factor (VEGF) or basic fibroblast growth factor (bFGF)] for increased angiogenesis, a state known as “angiogenic switch.” Sprouting of new blood vessels and overexpression of integrins in tumor tissues and vasculature are thus key features in the pathophysiology of cancer. Other integrins such as αvβ6 and α6β4 are also observed to be expressed on tumor cells (8). Another pivotal function of integrins is the promotion of cell migration by virtue of their binding to ECM components. This phenomenon is responsible for the process of tumor proliferation, migration, invasion, and metastasis (18). These functional aspects together with the high expression levels found on tumor cells have converted integrins into very interesting proteins for targeted cancer diagnosis and therapy studies.
Figure 2.
(A) Integrin recognition motif RGD; (B) schematic representation of cyclic RGD (cRGD); (C) Cilengitide – c(RGDf-NMeVal); (D) peptide sequences of RGD4C (the green curves indicate disulfide bridges), α5β1 ligand PR_b, and αvβ6 ligand A20FMDV2.
Our review shortly recapitulates recent developments in integrin targeted cancer therapy, with special focus on targeted delivery of chemotherapy or gene therapy via non-viral vectors like nanoparticles (NPs), micelles, vesicles, or other systems grafted with RGD-based integrin ligands. Considering the vastness of the topic, we have only cited a limited amount of recent works. For previous studies and developments in this field other detailed reviews are available (19–22). Applications based on integrin targeting antibodies and therapies involving the blocking of integrin functions with antagonists and other ligands are not subject of this review.
Integrin ligands and integrin targeting
Since the discovery of the integrin recognizing RGD motif by Ruoslahti et al. (13, 23), extensive research has been carried out to develop RGD-based peptide and peptidomimetic integrin ligands (24). Various synthetic strategies have been applied to develop RGD peptide analogs with enhanced biological properties and pharmacokinetics like affinity and selectivity for different integrin subtypes, metabolic stability, and biodistribution. These strategies include the introduction of amino acids flanking the tripeptidic RGD sequence, cyclization, and variation of stereochemical configuration of the constituent amino acids (25), and N-methylation (26, 27) (Figure 2). Cilengitide – c(RGDf-NMeVal) (Figure 2), a very potent antagonist of αvβ3, was developed by using some of these approaches and has been clinically tested by Merck primarily for treatment of glioblastoma multiforme (28, 29). Despite promising preliminary data, its use as anticancer therapeutic has been discontinued due to failure in phase-III clinical trials (Merck press release on Cilengitide studies: http://www.merck.de/de/presse/extNewsDetail.html?newsId=C47977D13865FCB9C1257B1D001EF9CA&newsType=1). Other well-known RGD peptides are cRGDfV (25) – the parent peptide for Cilengitide, cRGDfK (30), and RGD4C (ACDCRGDCFCG) (31). RGD4C is susceptible to be expressed by recombinant methods into proteins and viruses for their targeted delivery. Targeting integrins using cRGDfX, cRGDeV, cRGDyV, and other peptides or peptidomimetics (Figure 2) has also been reported in the literature.
Targeted drug delivery
Targeted delivery can be accomplished by two approaches: the direct conjugation of the targeting motif to the drug or the use of drug vehicular systems grafted with the targeting motif. Of these, the use of carrier systems offers several advantages compared to direct conjugation methods:
Carrier systems have the capacity to present multiple ligands on each particle. This facilitates effective targeting via multiple and simultaneous interactions between the ligands and the receptors, exploiting the concept of multivalency.
Vehicular systems may keep the drug unexposed to physiological systems, thereby protecting it from degradation or alteration, and more importantly, minimizing undesirable non-specific interactions of the drug with normal tissues. Therefore, these systems may remarkably reduce the side effects of the drug.
Targeted carrier systems usually are internalized via receptor-mediated endocytosis and the drug is directly released within cell. This is more effective to attain higher in-cell drug concentrations for amplified therapeutic activity.
Being larger in size (∼>100 nm) than classical drugs, carrier systems are not filtered off by renal pathways (size limit for renal filtration ∼5 nm). This enables a prolonged half-life time of carrier particles in the blood stream and allows for a gradual release of the drug over longer periods of time. Such release kinetics avoid high systemic concentrations of the drug and improves the effectiveness of the administered dose.
The abnormal architecture and permeability of tumor vasculature promotes extravasation of the particles that are in blood circulation. This phenomenon is called enhanced permeability and retention (EPR) effect. Facilitated by this passive transport mechanism, the nano-sized vehicular systems enter into tumor tissues. However, the quick clearance of these NPs from the tissue is prevented by their large size and lead to prolonged retention times in tumor. Hence, the double targeting – passive and active receptor-mediated targeting, enhances therapeutic efficacy.
Among the carrier systems, viral vectors such as retroviruses and adenoviruses have been successfully developed and found to be efficient in targeted gene therapy (32). However, their use is associated with several disadvantages that have precluded their clinical application. In the first place, they can produce unwanted immune responses (33). Also, it is not easy to express viruses composed with targeting moieties that contain unnatural amino acids or chemically modified scaffolds. Moreover, viral vectors can only be used for gene therapy and are not suitable for delivery of chemotherapeutics. Last but not least, they also carry a negative public perception concerning safety (33, 34). Therefore, development of non-viral targeting vectors is a preferred alternative in targeted therapy. In this regard, various kinds of polymer-based nanocarriers have been developed for tumor targeting using integrin ligands including the use of RGD coated virus like particles (VLPs) which use only the capsid of the viruses (35). In the following sections, some representative examples are discussed according to the targeted integrin subtype.
Targeting αvβ3 and αvβ5 Integrins
As previously introduced, the αvβ3 integrin subtype plays a major role in angiogenesis, tumor neovascularization, and tumor metastasis (8). The angiogenic pathways dependent on αvβ3 have been described to be induced by bFGF or tumor necrosis factor α (TNF-α). Its expression is upregulated on angiogenic endothelial cells (36–38) and on various tumor cell lines (39, 40). Antagonistic inhibition of αvβ3 integrin has been shown to suppress angiogenesis (41) and to induce apoptosis (42). The well-established biological roles, high expression on tumor tissues, and the availability of ligands with high affinity, have set αvβ3 the most extensively studied integrin for tumor targeting. The integrin αvβ5 is also involved in angiogenesis but through a distinct pathway stimulated by VEGF or transforming growth factor α (TGF-α) (16). Since most RGD-containing peptidic αvβ3 antagonists also recognize αvβ5, although usually with a lower affinity, these two integrin subtypes are discussed together.
Targeted delivery of chemotherapy using polymeric vehicles
Encapsulation of drugs in polymer-based carrier systems is a practical approach to protect them from degradation in biological system. Furthermore, these systems may reduce the systemic toxicity of the drug and also enhance their safe elimination from the physiological system. In addition, these vehicles often ameliorate the drug’s pharmacokinetic profile and biological distribution within the organism. Phospholipid or polypeptide-based polymers are commonly employed to prepare drug-delivery vehicles as they are akin to biological molecular components and thus display low toxicity and are easily biodegradable. Since the physicochemical properties of these polymers can be easily tuned to produce liposomes, micelles, or NPs, via well-established protocols, these materials are frequently used to construct drug-delivery vehicles. In fact, liposomes have already been used for the formulation and delivery of DOX (4). These vehicles may additionally be PEGylated to improve their aqueous solubility and to reduce non-specific interactions with plasma proteins and membranes. Besides encapsulation, drugs can as well be bound to these systems by chemical methods. This enables drug stability and also secured pH-sensitive release of drugs in situ. These sorts of carrier systems have been equipped with integrin targeting ligands and experimented for their capabilities as targeted drug-delivery systems in cancer treatment. Some illustrative recent works are listed in Table 1.
Table 1.
Outline of representative recent examples of polymer-based targeted delivery studies using αvβ3 and/or αvβ5 integrin ligands.
| Carrier system | Targeting motif | Drug | Cellular system | Results and characteristics (reference) |
|---|---|---|---|---|
| Cholesterol/DOPE/DSPC/DSPE-(PEO)4-cRGDfK/DSPE-mPEG2000 | cRGDfK | DOX | R40P murine pancreatic and SN12C renal carcinoma cells | Fifteen fold increase in drug efficacy relative to animals treated with free drug (95) |
| PLG-PEG micelles | cRGDfC | DOX | U87MG human glioblastoma cells | pH-sensitive drug release, higher cellular uptake, higher accumulation at tumor sites as monitored by positron emission tomography (PET) and ex vivo fluorescence experiments (96) |
| PLGA-4-arm-PEG branched NPs | cRGDfC | – | Pancreatic tumor in mice and U87MG glioma cells | Efficient uptake by U87MG glioma cells over-expressing αvβ3. Highest accumulation at tumor site as monitored by whole body imaging. Low in vivo inherent physiological toxicity for the NPs (97) |
| PGA-PTX-E-[c(RGDfK)]2 conjugate NPs | cRGDfK | PTX | 4T1 murine breast cancer tumors | Augmented antitumor activity and reduced systemic toxicity for PTX, blockade of endothelial cell migration to VEGF and adhesion to fibrinogen. Lysosomal enzyme assisted release of PTX is observed (98) |
| PLGA-PEG NPs | GRGDS and RGD peptidomimetic | PTX and DOX | HUVECs and syngenic TLT cells | High cellular uptake in vitro, improved anticancer efficacy and higher survival rate of mice (99) |
| cRGDyK-PEG-PLA-PTX micelle | cRGDyK | PTX | Intracranial glioblastoma model | 2.5-Fold increase in antiglioblastoma cell cytotoxicity effect over non-targeted system, improved drug accumulation, increase in life time of diseased mice (100) |
| FOR OTHER STUDIES USING PLGA-PLL NPs PLEASE SEE REF. (101, 102) | ||||
| HPMA copolymers | cRGDfK | Geldanamycin | PC-3 and DU145 prostate cancer cell lines | Tumor growth inhibition activity as efficient as free drug, decrease in IC50 values for targeted conjugates. Improvements in biodistribution profile, both in vitro and in vivo antiangiogenic, and antitumor activities for targeted systems (103–105) |
| HPMA copolymers | cRGDfK | Docetaxel | PC-3 and DU145 prostate cancer cell lines | Inhibition of PC3, DU145 cell growth and also of HUVECs in vitro. In vivo tumor regression is also observed (106) |
| PCL-PEEP and Mal-PEG-PCL micelles | Tf and cRGDfK | PTX | BMEC and U87MG glioma cells | Double targeting by Tf and RGD ligand. Uptake of micelles increased 2.4 times for BMEC compared to micelles lacking Tf. High drug accumulation in brain upon IV injection (107) |
| HPAE-co-PLA/DPPE polymer NPs | Tf and cRGDfK | PTX | HUVECs and HeLa cells | In vitro cytotoxicity for NPs coated with cRGD is increased 10 times in αvβ3-expressing HUVECs while Tf targeting to Tf receptor over-expressed HeLa cells lead to twofold increase. pH-sensitive intracellular drug release (108) |
| PFC (perfluorocarbon) NPs | Non-peptidic αvβ3 antagonist | Fumagillin | Vx-2 adenocarcinoma tumor | Diminished development of tumor neovasculature and reduced tumor growth are observed at much lower drug concentrations compared to the previous concentration used in rodent and human clinical trials (109) |
| P(PEGMEMA) based micelles | RGD | Albendazole | OVCAR-3 ovarian cancer cells | Improved cellular uptake of polymeric micelles and 80% cell deaths at a micelle concentration of 10 μg mL−1 (110) |
Targeted delivery of chemotherapy using protein-based NPs
Although polymer-based vehicle systems are a common choice for drug delivery, their long-term biological toxicity might be an issue and needs to be carefully assessed. For this reason, protein-based NPs are considered an attractive alternative for targeted therapy due to their high biocompatibility, biodegradable properties, and water solubility. With regard to this, albumin is one of the proteins that has been most majorly explored for drug delivery. For example, linking c(RGDyK)C to albumin NPs loaded with Gemcitabine showed an increased in vitro and in vivo antitumor efficacy in BxPC-3 pancreatic cancer cell lines compared to NPs without the targeting sequence (43). The conjugation of cyclic RGD to albumin not only lead to successful targeting but also increased the intracellular uptake of NPs and Gemcitabine as monitored by florescence studies. The αvβ3-mediated uptake of the RGD-conjugated components into pancreatic cells was further confirmed by competitive inhibition studies using soluble RGD ligands. In another study (44), Fluorouracil-bearing cRGDfK-albumin nanospheres have shown significant improvement in binding to αvβ3-expressing HUVEC cells in vitro. A considerable improvement in prevention of lung metastasis and angiogenesis, and in tumor regression was observed in vivo in B16F10 tumor-bearing mice as compared with the activity of the free drug. The binding of nanospheres conjugated with RGD to endothelial cells was eightfold higher than that of nanospheres without RGD or conjugated with the RAD sequence (which does not bind to integrins). Similarly, enhanced homing to tumors and endothelial cell binding were reported for cRGDfK-PEG-albumin NPs that were linked to the antimitotic agent monomethyl-auristatin-E (MMAE) (45). These studies were carried out on HUVECs and C26 carcinoma-bearing mice. Two kinds of target systems were prepared with an RGD peptide linked to albumin either by a PEG chain (RGD-PEG-MMAE-HSA) or a short alkyl chain (RGD-MMAE-HSA). After IV administration in mice, fluorescent studies showed colocalization of both carrier systems with the tumor vasculature and tumor cells.
Besides the use of albumin as drug-delivery system, spider silk is a protein that holds great promise for application in targeted therapies. Due to its water solubility, excellent biocompatibility, and unique mechanical properties, spider silk has attracted growing interest in a number of biomedical areas. Spider silks are currently under investigation for the encapsulation and controlled release of drugs and growth factors, with so far optimistic outcomes (46). Scheibel’s group has prepared spider silks containing the integrin recognition motifs GRGDSP or cRGDfK by either recombinant expression or chemical methods, respectively (47). These RGD functionalized proteins have been used to generate spider silk films that retain the biophysical properties observed for silks prepared using the native proteins. Significant improvements in the attachment and proliferation of BALB/3T3 mouse fibroblasts were observed on films containing the RGD sequence but not on unmodified or RGE-containing silk. These results encourage further exploration of spider silk protein as a prospective carrier system for targeted drug delivery in cancer.
Targeted delivery of chemotherapy using metallic NPs
Gold and other metallic NPs can be used for the polyvalent display of targeting scaffolds (48). Ease of preparation and functionalization as well as unique physicochemical properties make gold NPs very attractive systems for use in cancer diagnosis and therapy. For instance, PEGylated gold NPs coupled to a cRGD peptidomimetic via thiol chemistry showed good affinity and binding to αvβ3-positive PC-3 prostate cancer cells in vitro (49). In another study, Yang et al. have examined the utility of multifunctional PEGylated superparamagnetic iron oxide (SPIO) NPs in targeted drug delivery and PET/Magnetic Resonance Imaging (MRI) (50). To this end, cRGDfC and a common 64Cu chelator were bound to the distal ends of the PEG chains, whereas the drug, DOX, was conjugated to the SPIO particles via pH-sensitive hydrazone bonds. The cRGD-conjugated SPIO nanocarriers exhibited higher cellular uptake and cytotoxicity in U87MG cells compared to cRGD-free systems. Also, in vivo PET imaging of U87MG tumor-bearing mice revealed increased tumor accumulation of cRGD-SPIO NPs compared to cRGD-free counterparts. Intracellular specific drug release by SPIOs was facilitated by pH-selective cleavage of the SPIO-DOX hydrazone linkage. Such multifunctional systems that are able to simultaneously target a cell or tissue, deliver a drug, and provide a diagnosis are known as theranostics, which constitute an upcoming area of research.
Targeted delivery of gene therapy
Delivery of gene therapy using targeted non-viral vehicles has been widely studied (20). A directed delivery of DNA or RNA fragments is required to prevent from using high doses, which otherwise can lead to off-target gene silencing effects. Using carrier systems for gene therapy is advantageous as it reduces the problems of biodegradability, nucleosomal cleavage, and size and charge limited membrane impermeability associated with the delivery of nucleic acids. As mentioned earlier, non-viral vectors are also helpful to overcome complications and safety issues described for viral vectors. Here, we briefly tabulate some recent targeted gene therapy studies (Table 2).
Table 2.
Outline of recent targeted gene delivery studies using αvβ3 and/or αvβ5 integrin ligands.
| Carrier system | Targeting motif | Gene | Cellular system | Results and Characteristics (reference) |
|---|---|---|---|---|
| PEG-PLys polyplex micelle | cRGDfK | Luc-pDNA | HeLa cells and 293T cells | Enhanced transfection efficiency (TE) and perinuclear accumulation of pDNA within 3 h of incubation (111) |
| PEG-PLys polyplex micelle: cross-linked by thiolation | cRGDfK | Luc-pDNA | HeLa cells and 293T cells | Improvements in TE, selection of endocytotic pathways and regulation of intracellular trafficking by cRGD. Preferential caveolae mediated endocytosis is observed. Thiol cross-linking helped polyplex stabilization and pDNA protection (112) |
| PEG-PLys polyplex micelle: cross-linked by thiolation | cRGDfK | sFlt-1 | BxPC-3 pancreatic adenocarcinoma tumors | Upon IV injection, significant tumor-specific TE and gene expression is observed which lead to a decrease in tumor vasculature. Thiol cross-linking has to be optimized to improve results (113, 114) |
| PEG-PEI polyplex micelles | B6 peptide and RGD bicyclo peptide | pCMVLuc | DU145 and PC3 prostate cancer cells | Significant improvement in TE via targeting. RGD helped in initial association of polyplexes to cells whereas the internalization is observed to be mediated by TfR endocytosis (115) |
| PEG-PEI polyplex micelles | Non-cyclic RGD-peptidomimetic | MeWo and A549 cells | Increased binding, uptake, and luciferase transgene expression in model cells (116) | |
| PEG-PEI polyplex micelles | cRGDyK | pORF-hTRAIL | Intracranial U87 glioblastoma tumor xenografts | Higher gene transfection and increased therapeutic efficiency of TRAIL are observed and is reflected in improved longevities of mice (117) |
| DNA/PEI-Au-RGD nanoclusters | Cap-RGD | pEGFP-Luc | HeLa cells | A 5.4- to 35-fold increase in TE corresponding to a low or high density of αvβ3 on HeLa cells. Observed TEs are far higher than that for targeted or untargeted commercial transfection vector – JetPEI. Higher concentration of gold NPs is found to be toxic (118) |
| PEG–oligo(ethane amino) amide polymers | B6 peptide or cRGDfK | pEGFP-Luc | Mouse N2A neuroblastoma and DU145 human prostate adenocarcinoma cells | Selective binding and transfection efficiency are observed which are mediated by the targeting ligands. The carrier systems however required use of endosomolytic agents for release of polyplexes from endosomes (119) |
| DCP-TEPA polycation liposomes | cRGDfK | siLuc2 | B16F10-luc2 murine melanoma cells | Successful targeting, transfection, and knockdown of luc2 expression in vitro in B16F10-luc2 cells and also in vivo as monitored by imaging in mice with tumor-bearing lungs, is observed (120) |
| PEO-b-PCL micelles | RGD4C | mdr1 siRNA and DOX | MDA435/LCC6 cells resistant to DOX | The system is decorated with cell penetrating peptide (TAT) as well. Dual functional micelles showed improved cellular uptake and mdr1 activity leading to lowered P-gp expression both at the mRNA and protein levels. These effects caused reversal of MDR for DOX, which increased DOX accumulation in cytoplasm and nucleus, and enhanced DOX cytotoxicity (121) |
Phototherapy using targeted systems
Gormley et al. have tested the use of targeted gold nanorods (GNRs) for plasmonic photothermal therapy (PPTT) aiming at reducing the amount of heat required in thermal therapy (51). To this end, PEGylated GNRs were prepared and functionalized with cRGDfK via thiol chemistry. Studies on HUVEC and DU145 prostate cancer cells showed effective in vitro selective targeting of RGD-GNRs to both these cell types but not in vivo in a DU145 mice model. The absence of in vivo effects was attributed to faster clearance of GNRs from physiological system due to the presence of negative charges in cRGDfK-functionalized GNRs. On similar lines, for PPTT, Akhavan et al. have projected reduced single layer graphene oxide nanorods (GONRs) functionalized by amphiphilic PEG polymers containing RGD-based peptides (52). RGD-presenting GONRs showed increased radiation absorption compared to non-functionalized GONRs and also improved destruction of U87MG human glioblastoma cells at reduced doses as low as 1 μg mL−1. Irradiation for 8 min with near-infrared radiation at this concentration resulted in remarkable values of cell destruction (≥97%). On the contrary,<11% of cell destruction and 7% of DNA fragmentation were observed for non-targeted nanorods using the same concentration.
Targeting the α5β1 Integrin
In addition to αvβ3 and αvβ5, an upregulated expression of α5β1 in tumor vasculature and other cancer cells has also been described (36, 53–57). α5β1 primarily recognizes fibronectin through the RGD binding motif. Kim et al. have reported that α5β1 inhibition induces cell apoptosis in endothelial cells (58) and also showed that this integrin mediates the migration of endothelial cells. Noteworthy, it has been shown that α5 might substitute the activity of αv during vasculature remodeling (59). For these reasons, targeting of this integrin has also been approached in cancer therapy.
Kokkoli and co-workers have explored α5β1 integrin for targeting cancer cells by using a fibronectin mimetic α5β1-selective RGD-containing peptide, named PR_b (60) (Figure 2). This group produced DPPC-based liposomal NPs covered by PEG and further decorated with PR_b peptide, and studied their targeting capacity in a CT26.WT mouse colon carcinoma experimental model. The quantities of PEG and peptide were fine-tuned in order to optimize the delivery of the nanovector. By increasing the quantity of conjugated peptide, an enhancement in binding of liposomes to cells was observed, whereas the opposite effect was found when the concentration of PEG was augmented. The cytotoxicity of 5-Fluorouracil carried by these PR_b targeted liposomes was found to be comparable to that of the free drug and better than that of the particles containing only the control GRGDSP sequence, confirming the importance of targeting α5β1 on this cancer model. Similar results were obtained in studies using HCT116 and RKO human colon cancer cells (60). This liposomal system has been further investigated for the delivery and cytotoxicity of DOX to MDA-MB 231 breast cancer cells (61). Confocal microscopy experiments showed that these targeted liposomes were internalized in breast cancer cells via an endocytic pathway, and transferred within the first minutes into early endosomes, and after prolonged times into late endosomes and lysosomes. Particularly at high concentrations, the therapeutic effect of encapsulated DOX in MDA-MB 231 cells was comparable to that of the free DOX.
In a recent approach, PR_b targeted polymersomes have also been explored for siRNA delivery (62). T47D breast cancer cells were studied to check the expression of Orai3. The downregulation of Orai3 levels results in cell apoptosis. The delivery of Orai3 by PR_b-conjugated polymersomes decreased the viability of cancer cells but did not affect non-cancerous MCF10A breast cells. When compared to a commercial transfection agent (Lipofectamine RNAiMAX), the observed therapeutic effect of the polymersome formulation is still moderate. However, this method has not shown any systemic toxicity unlike other transfection reagents.
Targeting the αvβ6 Integrin
The integrin subtype αvβ6 is expressed at low or undetectable levels in most adult epithelia, but may be upregulated during inflammation and wound healing (8). αvβ6 preferentially binds to TGF-β1 latency associated peptide (LAP) (63), but can also recognize the ECM proteins tenascin and fibronectin (64). In this regard, αvβ6 is biologically important for the activation of TGF-β1 and has been shown to control TGF-β activity or signaling in fibrosis and to play a crucial role in TGF-β-integrin crosstalk in carcinomas (65). Furthermore, αvβ6 was found to be significantly upregulated in tumor tissues (8) and in certain cancer types including colon (66), ovarian carcinoma (67), and in early stage of non-small cell lung cancer (NSCLC), which is associated with poor patient survival (68, 69). Other studies have shown that αvβ6 expression is correlated with the development of metastasis in gastric cancer and the enhanced survival and invasive potential of carcinoma cells (70, 71). This pathological relevance has turned αvβ6 into a promising target for tumor diagnostics and antitumor therapy.
To date, several linear and cyclic peptides as well as peptidomimetics have been developed to target specifically the αvβ6 integrin subtype (68, 70, 72–74). For instance, the high affinity αvβ6-specific 20-mer peptide H2009.1 (75) was conjugated as a tetramer to a poly-glutamic acid polymer carrying DOX, and was shown to specifically target αvβ6-expressing cells in vitro (76). In another work, the selectivity of this peptide toward αvβ6 was exploited to guide fluorescent quantum dots to lung adenocarcinoma cell line H2009 in vitro (68). Recently, this peptide has also been conjugated to a water soluble PTX conjugate resulting in selective cytotoxicity for the αvβ6-expressing NSCLC cell line (77). The conjugate was able to reduce the rate of tumor growth in vivo, however without an increased benefit over the use of free PTX. Furthermore, the same peptide was used to investigate the multimeric effect on functionalized liposomes (78). In this study, liposomes displaying tetramers of the H2009.1 peptide demonstrated higher drug delivery and toxicity toward αvβ6-expressing cells than liposomes displaying single copies of H2009.1, even if the total number of peptides bound to each liposome was identical. In another approach, H2009.1 was used to functionalize the surface of multifunctional micelles encapsulated with SPIO and DOX for MRI and drug-delivery applications, respectively (79). The functionalized micelles significantly increased cell targeting and uptake in αvβ6-expressing H2009 cells, as verified by MRI and confocal imaging.
A20FMDV2 (80, 81) is another αvβ6-selecitve 20-mer peptide (Figure 2) that can be used for targeted therapies. As an example, this peptide was radiolabeled on solid phase using 4-[18F]fluorobenzoic acid and the conjugate was selectively uptaken by αvβ6-positive tumors but not by αvβ6-negative tumors, as monitored in mice by PET (70). In a similar approach, A20FMDV2 was conjugated to 5-[18F]fluoro-1-pentyne via an azide-based 1,3-dipolar cycloaddition (click chemistry). However, no difference in tumor targeting in vivo was observed for such strategy compared to the previous labeling method (82). 18F-labeled derivatives of the same peptide were described to improve tumor uptake capacity in BxPC-3 (pancreatic cancer) xenograft-bearing mice over [18F]-FDG (83). Recently, A20FMDV2 was conjugated to an 18F-based tracer by copper-free, strain promoted click chemistry. However, the resulting derivative did not show a remarkable in vivo tumor uptake by mouse with mouse model DX3puroβ6-tumor (84). Furthermore, A20FMDV2 was conjugated to DTPA and labeled with 111In for SPECT imaging. In this study, the conjugate showed specific localization in αvβ6-tissues, and displayed increased uptake in an αvβ6-positive tumor and in a mouse xenograft model bearing breast tumors that express αvβ6 endogenously (85). Additionally, A20FMDV2 was incorporated into a recombinant adenovirus type 5 (Ad5) leading to increased cytotoxicity on a panel of αvβ6-positive human carcinoma cell lines in vitro and enhancement in tumor uptake and improved tumor transduction in an αvβ6-positive xenograft model in vivo over the Ad5 wild type (86).
In another approach pursued by the Gambhir research group, cystine knot peptides showing high affinity for αvβ6 but none for the related subtypes αvβ3, αvβ5, and α5β1 were developed and conjugated to 64Cu-DOTA for PET-based tumor imaging (87). Injection of these conjugates into mice bearing either αvβ6-positive BxPC-3 xenografts or αvβ6-negative tumors, and monitoring by PET imaging, showed αvβ6-selective targeting for the tumors expressing αvβ6. In a recent study (88), two cystine knot peptides were labeled with 18F-fluorobenzoate and their capacity to be uptaken by tumor cells assessed in vivo. PET imaging revealed for both peptides specific targeting of αvβ6-positive BxPC-3 xenografted tumors over αvβ6-negative HEK 293 tumors. These results illustrate the potential of the described strategies to be clinically used in PET imaging of αvβ6-over-expressing tumors.
Concluding Remarks
A wide variety of carrier systems have been described to achieve tumor-specific therapeutic effects via integrin targeting. The principal success of this strategy is evidenced by two main observations – the dosage of drug has been usually reduced and an enhanced (and often selective) activity against tumors is achieved. The data obtained from independent studies using different carrier systems are promising and there is therefore hope to bring the targeted delivery methods into practice. However, a number of aspects related to the use of these drug-delivery systems in cancer therapy should be carefully considered.
In the first place, comparative studies between distinct carrier systems are missing. Such studies could provide useful insights on their relative advantages and disadvantages, and help in their further development and optimization. Detailed studies concerning the systemic toxicity and long-term side effects of the drug-delivery vectors in physiological systems are also essential. Another important aspect to optimize the concentration of drugs in cancer therapy would be to evaluate the efficiency of drug uptake with regard to the overall administered dose, but most studies have only rated the efficiency of the targeted systems in comparison to untargeted systems, without mentioning about the concentrations of the drug used. The investigation of the metabolic stability of these systems in gut and liver as well as their bioavailability profile would also be crucial to improve the efficacy of the therapy. Further optimization of such drug formulations could be directed toward new routes of administration, including, though certainly difficult, orally available conjugates.
It should be mentioned that most studies in this field rank the antitumor potency of the targeted systems based on the reduction in tumor volume and size, parameters that will however not entirely assure the success of the therapy. More satisfactory would be to carry out longer experiments to ensure the complete removal of tumors and arrest of resurrections. In this regard, recent findings have suggested that antiangiogenic therapeutics that aim at treating cancer primarily through reduction and control of tumor growth, may, in some cases, indirectly promote cancer invasiveness and metastasis (89, 90). This ultimately alarms development of targeted therapies which can inhibit multiple cellular functions and affecting not only cell survival in situ but also mechanisms involved in the promotion and progression of metastasis. Further investigations on this matter should include the study of targeted therapy on early stage and late stage tumors, and the effect (if any) of these strategies in the development of drug resistance mechanisms by some tumors. Additionally, treatment of cancer often necessitates a combination therapy (combination of different therapeutics or therapies). In this respect, it is demanding to study the usage of targeted approaches for delivering multiple drugs or therapies either by a single carrier system or multiple carrier systems. These studies are further pending in literature. Most of the studies on targeted gene delivery have used luciferase model system. Though it is a good analogous system for understanding gene delivery, proper experimental gene therapy studies aimed to treat cancers are to be extensively studied.
The choice of an optimal integrin ligand is another aspect of paramount importance in the design of integrin-based targeted therapies in cancer. This will depend on the differential pattern of integrin expression in cancer cell types and the biological activity and selectivity profiles of the targeting ligands. Many applications have used linear or cyclic RGD peptides to deliver drugs or nucleotides to tumors. Most of these peptides are active for αvβ3; however, it is often ignored that these ligands may target other integrin subtypes as well. This might not be relevant as long as simplified cellular or experimental animal models are investigated. However, it may raise safety concerns if clinical applications in humans are to be envisaged. E.g., the habitually used peptide – c(RGDfX), developed in our group long ago (25, 30), has about 1 nM affinity for αvβ3 and is certainly selective against αIIbβ3 (low affinity for the platelet receptor). Nonetheless, the compound also has affinity in the low nanomolar range for αvβ5 (7.6 nM) and α5β1 (15 nM) (73). Thus, the use of c(RGDfX) might not always provide enough selectivity to distinguish between distinct cell types. In this regard, our group has recently developed (91, 92) and functionalized (93, 94) peptidomimetics which can clearly discriminate between αvβ3 and α5β1. Application of such single integrin subtype selective ligands will enable a selective and controlled delivery of drugs to tumors, taking advantage of the distinct patterns of integrin expression found for each cancer type.
It is on the basis of these considerations that targeted therapy with integrin ligands be translated into clinical studies, and be demonstrated whether such strategy will result in a clear benefit for cancer patients.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
A549, human non-small cell lung carcinoma; ATCC, CCL-185 cells; ATCC, HTB-65 cells; bFGF, basic fibroblast growth factor; BMEC, brain microvascular endothelial cells; Cap-RGD, Ac-CCVVVTGRGDSPSSK-COOH; DCP-TEPA, dicetylphosphate-tetraethylenepentamine; DOPE, dioleoylphosphatidylethanolamine; DPPC, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine; DSPC, distearoylphosphatidylcholine; DSPE, distearoylphosphatidylethanolamine; DTPA, diethylenetriamenepentaacetate; FDG, fluoro-2-deoxy-D-glucose; HEK, human embryonic kidney; HeLa, human cervical carcinoma cells; HPAE-co-PLA/DPPE, poly[(amine-ester)-co-(D,L-lactide)]/1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine copolymer; HPMA, N-(2-hydroxypropyl)methacrylamide; HAS, human serum albumin; HUVEC, human umbilical vein endothelial cells; IV, intravenous; Luc-pDNA, luciferase pDNA; Mal-PEG-PCL, maleimide-poly(ethylene glycol)-block-poly(ɛ-caprolactone); MDR, multi-drug resistance; MeWo, human malignant skin melanoma; MMAE, monomethyl-auristatin-E; MRI, magnetic resonance imaging; NSCLC, non-small cell lung cancer; PCL-PEEP, poly(ɛ-caprolactone)-block-poly-(ethyl ethylene phosphate); pCMVLuc, Photinus pyralis luciferase under control of the CMV enhancer/promoter; PEG, polyethylene glycol; PEI, polyethylenimine; PEO-b-PCL, poly(ethylene oxide)-block-poly(ɛ-caprolactone); PET, positron emission tomography; PGA, poly-glutamic acid; PLA, poly(lactic acid); PLG, poly-L-glutamic acid; PLGA, poly (D,L-lactide-co-glycolide); PLL, poly (L-lysine); PLys, polylysine; pORF-hTRAIL, plasmid expressing the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL); P(PEGMEMA), poly[poly(ethylene glycol) methyl ether methacrylate]; sFlt-1, soluble fms-like tyrosinekinase-1 (pDNA encoding the soluble form of VEGF receptor-1); SPECT, single-photon emission computed tomography; TAT peptide, CGRKKRRQRRR; Tf, transferrin; TfR, transferrin receptor; TLT, transplantable liver tumors; VEGF, vascular endothelial growth factors.
References
- 1.Kumar S, Mahdi H, Bryant C, Shah JP, Garg G, Munkarah A. Clinical trials and progress with paclitaxel in ovarian cancer. Int J Womens Health (2010) 2:411–27 10.2147/IJWH.S7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol (1990) 8:1263–8 [DOI] [PubMed] [Google Scholar]
- 3.Prados J, Melguizo C, Ortiz R, Vélez C, Alvarez PJ, Arias JL, et al. Doxorubicin-loaded nanoparticles: new advances in breast cancer therapy. Anticancer Agents Med Chem (2012) 12:1058–70 10.2174/187152012803529646 [DOI] [PubMed] [Google Scholar]
- 4.Lao J, Madani J, Puértolas T, Álvarez M, Hernández A, Pazo-Cid R, et al. Liposomal doxorubicin in the treatment of breast cancer patients: a review. J Drug Deliv (2013) 456409:1–12 10.1155/2013/456409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergman AM, Pinedo HM, Peters GJ. Determinants of resistance to 2’,2’-difluorodeoxycytidine (gemcitabine). Drug Resist Updat (2002) 5:19–33 10.1016/S1368-7646(02)00002-X [DOI] [PubMed] [Google Scholar]
- 6.Yusuf RZ, Duan Z, Lamendola DE, Penson RT, Seiden MV. Paclitaxel resistance: molecular mechanisms and pharmacologic manipulation. Curr Cancer Drug Targets (2003) 3:1–19 10.2174/1568009033333754 [DOI] [PubMed] [Google Scholar]
- 7.Galletti E, Magnani M, Renzulli ML, Botta M. Paclitaxel and docetaxel resistance: molecular mechanisms and development of new generation taxanes. ChemMedChem (2007) 2:920–42 10.1002/cmdc.200600308 [DOI] [PubMed] [Google Scholar]
- 8.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer (2010) 10:9–22 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruoslahti E. Specialization of tumour vasculature. Nat Rev Cancer (2002) 2:83–90 10.1038/nrc724 [DOI] [PubMed] [Google Scholar]
- 10.Teesalu T, Sugahara KN, Ruoslahti E. Mapping of vascular ZIP codes by phage display. Methods Enzymol (2012) 503:35–56 10.1016/B978-0-12-396962-0.00002-1 [DOI] [PubMed] [Google Scholar]
- 11.Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci (2012) 33:405–12 10.1016/j.tips.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 12.Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res (2010) 339:269–80 10.1007/s00441-009-0834-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature (1984) 309:30–3 10.1038/309030a0 [DOI] [PubMed] [Google Scholar]
- 14.Cabodi S, Di Stefano P, Leal Mdel P, Tinnirello A, Bisaro B, Morello V, et al. Integrins and signal transduction. Adv Exp Med Biol (2010) 674:43–54 10.1007/978-1-4419-6066-5_5 [DOI] [PubMed] [Google Scholar]
- 15.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov (2010) 9:804–20 10.1038/nrd3266 [DOI] [PubMed] [Google Scholar]
- 16.Weis SM, Cheresh DA. αV integrins in angiogenesis and cancer. Cold Spring Harb Perspect Med (2011) 1:a006478. 10.1101/cshperspect.a006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev (2010) 29:223–37 10.1007/s10555-010-9211-x [DOI] [PubMed] [Google Scholar]
- 18.Moschos SJ, Drogowski LM, Reppert SL, Kirkwood JM. Integrins and cancer. Oncology (Williston Park, N Y) (2007) 21:13–20 [PubMed] [Google Scholar]
- 19.Temming K, Schiffelers RM, Molema G, Kok RJ. RGD-based strategies for selective delivery of therapeutics and imaging agents to the tumour vasculature. Drug Resist Updat (2005) 8:381–402 10.1016/j.drup.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 20.Park J, Singha K, Son S, Kim J, Namgung R, Yun CO, et al. A review of RGD-functionalized nonviral gene delivery vectors for cancer therapy. Cancer Gene Ther (2012) 19:741–8 10.1038/cgt.2012.64 [DOI] [PubMed] [Google Scholar]
- 21.Danhier F, Le Breton A, Préat V. RGD-based strategies to target αvβ3 integrin in cancer therapy and diagnosis. Mol Pharm (2012) 9:2961–73 10.1021/mp3002733 [DOI] [PubMed] [Google Scholar]
- 22.Chen K, Chen X. Integrin targeted delivery of chemotherapeutics. Theranostics (2011) 1:189–200 10.7150/thno/v01p0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruoslahti E. The RGD story: a personal account. Matrix Biol (2003) 22:459–65 10.1016/S0945-053X(03)00083-0 [DOI] [PubMed] [Google Scholar]
- 24.Meyer A, Auernheimer J, Modlinger A, Kessler H. Targeting RGD recognizing integrins: drug development, biomaterial research, tumor imaging and targeting. Curr Pharm Des (2006) 12:2723–47 10.2174/138161206777947740 [DOI] [PubMed] [Google Scholar]
- 25.Aumailley M, Gurrath M, Müller G, Calvete J, Timpl R, Kessler H. Arg-Gly-Asp constrained within cyclic pentapeptides. Strong and selective inhibitors of cell adhesion to vitronectin and laminin fragment P1. FEBS Lett (1991) 291:50–4 10.1016/0014-5793(91)81101-D [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee J, Gilon C, Hoffman A, Kessler H. N-methylation of peptides: a new perspective in medicinal chemistry. Acc Chem Res (2008) 41:1331–42 10.1021/ar8000603 [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee J, Rechenmacher F, Kessler H. N-methylation of peptides and proteins: an important element for modulating biological functions. Angew Chem Int Ed Engl (2013) 52:254–69 10.1002/anie.201205674 [DOI] [PubMed] [Google Scholar]
- 28.Dechantsreiter MA, Planker E, Mathä B, Lohof E, Hölzemann G, Jonczyk A, et al. N-methylated cyclic RGD peptides as highly active and selective αvβ3 integrin antagonists. J Med Chem (1999) 42:3033–40 10.1021/jm970832g [DOI] [PubMed] [Google Scholar]
- 29.Mas-Moruno C, Rechenmacher F, Kessler H. Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem (2010) 10:753–68 10.2174/187152010794728639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haubner R, Gratias R, Diefenbach B, Goodman SL, Jonczyk A, Kessler H. Structural and functional aspects of RGD-containing cyclic pentapeptides as highly potent and selective integrin αvβ3 antagonists. J Am Chem Soc (1996) 118:7461–72 10.1021/ja9603721 [DOI] [Google Scholar]
- 31.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Nat Biotechnol (1995) 13:265–70 10.1038/nbt0395-265 [DOI] [PubMed] [Google Scholar]
- 32.Hajitou A. Targeted systemic gene therapy and molecular imaging of cancer contribution of the vascular-targeted AAVP vector. Adv Genet (2010) 69:65–82 10.1016/S0065-2660(10)69008-6 [DOI] [PubMed] [Google Scholar]
- 33.Smaglik P. Merck blocks “safer” gene therapy trials. Nature (2000) 403:817. 10.1038/35002743 [DOI] [PubMed] [Google Scholar]
- 34.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007 – an update. J Gene Med (2007) 9:833–42 10.1002/jgm.1100 [DOI] [PubMed] [Google Scholar]
- 35.Hovlid ML, Steinmetz NF, Laufer B, Lau JL, Kuzelka J, Wang Q, et al. Guiding plant virus particles to integrin-displaying cells. Nanoscale (2012) 4:3698–705 10.1039/c2nr30571b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer (2008) 8:604–17 10.1038/nrc2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science (1994) 264:569–71 10.1126/science.7512751 [DOI] [PubMed] [Google Scholar]
- 38.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by αvβ3-targeted magnetic resonance imaging. Nat Med (1998) 4:623–6 10.1038/nm0598-623 [DOI] [PubMed] [Google Scholar]
- 39.Beck V, Herold H, Benge A, Luber B, Hutzler P, Tschesche H, et al. ADAM15 decreases integrin αvβ3/vitronectin-mediated ovarian cancer cell adhesion and motility in an RGD-dependent fashion. Int J Biochem Cell Biol (2005) 37:590–603 10.1016/j.biocel.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer αv-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem (2004) 15:41–9 10.1021/bc0300403 [DOI] [PubMed] [Google Scholar]
- 41.Brooks PC, Strömblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest (1995) 96:1815–22 10.1172/JCI118227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meerovitch K, Bergeron F, Leblond L, Grouix B, Poirier C, Bubenik M, et al. A novel RGD antagonist that targets both αvβ3 and α5β1 induces apoptosis of angiogenic endothelial cells on type I collagen. Vascul Pharmacol (2003) 40:77–89 10.1016/S1537-1891(02)00339-7 [DOI] [PubMed] [Google Scholar]
- 43.Ji S, Xu J, Zhang B, Yao W, Xu W, Wu W, et al. RGD-conjugated albumin nanoparticles as a novel delivery vehicle in pancreatic cancer therapy. Cancer Biol Ther (2012) 13:206–15 10.4161/cbt.13.4.18692 [DOI] [PubMed] [Google Scholar]
- 44.Dubey PK, Singodia D, Verma RK, Vyas SP. RGD modified albumin nanospheres for tumour vasculature targeting. J Pharm Pharmacol (2011) 63:33–40 10.1111/j.2042-7158.2010.01180.x [DOI] [PubMed] [Google Scholar]
- 45.Temming K, Meyer DL, Zabinski R, Dijkers EC, Poelstra K, Molema G, et al. Evaluation of RGD-targeted albumin carriers for specific delivery of auristatin E to tumor blood vessels. Bioconjug Chem (2006) 17:1385–94 10.1021/bc060087z [DOI] [PubMed] [Google Scholar]
- 46.Wenk E, Wandrey AJ, Merkle HP, Meinel L. Silk fibroin spheres as a platform for controlled drug delivery. J Control Release (2008) 132:26–34 10.1016/j.jconrel.2008.08.005 [DOI] [PubMed] [Google Scholar]
- 47.Wohlrab S, Müller S, Schmidt A, Neubauer S, Kessler H, Leal-Egaña A, et al. Cell adhesion and proliferation on RGD-modified recombinant spider silk proteins. Biomaterials (2012) 33:6650–9 10.1016/j.biomaterials.2012.05.069 [DOI] [PubMed] [Google Scholar]
- 48.Arnold M, Cavalcanti-Adam EA, Glass R, Blümmel J, Eck W, Kantlehner M, et al. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem (2004) 5:383–8 10.1002/cphc.200301014 [DOI] [PubMed] [Google Scholar]
- 49.Arosio D, Manzoni L, Araldi EM, Scolastico C. Cyclic RGD functionalized gold nanoparticles for tumor targeting. Bioconjug Chem (2011) 22:664–72 10.1021/bc100448r [DOI] [PubMed] [Google Scholar]
- 50.Yang X, Hong H, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, et al. cRGD-functionalized, DOX-conjugated, and 64Cu-labeled superparamagnetic iron oxide nanoparticles for targeted anticancer drug delivery and PET/MR imaging. Biomaterials (2011) 32:4151–60 10.1016/j.biomaterials.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gormley AJ, Malugin A, Ray A, Robinson R, Ghandehari H. Biological evaluation of RGDfK-gold nanorod conjugates for prostate cancer treatment. J Drug Target (2011) 19:915–24 10.3109/1061186X.2011.623701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akhavan O, Ghaderi E, Emamy H. Nontoxic concentrations of PEGylated graphene nanoribbons for selective cancer cell imaging and photothermal therapy. J Mater Chem (2012) 22:20626–33 10.1039/c2jm34330d [DOI] [Google Scholar]
- 53.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin α5β1 with the central cell-binding domain of fibronectin. Am J Pathol (2000) 156:1345–62 10.1016/S0002-9440(10)65005-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S, Harris M, Varner JA. Regulation of integrin αvβ3-mediated endothelial cell migration and angiogenesis by integrin α5β1 and protein kinase A. J Biol Chem (2000) 275:33920–8 10.1074/jbc.M003668200 [DOI] [PubMed] [Google Scholar]
- 55.Jayne DG, Heath RM, Dewhurst O, Scott N, Guillou PJ. Extracellular matrix proteins and chemoradiotherapy: α5β1 integrin as a predictive marker in rectal cancer. Eur J Surg Oncol (2002) 28:30–6 10.1053/ejso.2001.1182 [DOI] [PubMed] [Google Scholar]
- 56.Jia Y, Zeng ZZ, Markwart SM, Rockwood KF, Ignatoski KM, Ethier SP, et al. Integrin fibronectin receptors in matrix metalloproteinase-1-dependent invasion by breast cancer and mammary epithelial cells. Cancer Res (2004) 64:8674–81 10.1158/0008-5472.CAN-04-0069 [DOI] [PubMed] [Google Scholar]
- 57.Chen J, De S, Brainard J, Byzova TV. Metastatic properties of prostate cancer cells are controlled by VEGF. Cell Commun Adhes (2004) 11:1–11 10.1080/15419060490471739 [DOI] [PubMed] [Google Scholar]
- 58.Kim S, Bakre M, Yin H, Varner JA. Inhibition of endothelial cell survival and angiogenesis by protein kinase A. J Clin Invest (2002) 110:933–41 10.1172/JCI0214268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, et al. Endothelial α5 and αv integrins cooperate in remodeling of the vasculature during development. Development (2010) 137:2439–49 10.1242/dev.049551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg A, Tisdale AW, Haidari E, Kokkoli E. Targeting colon cancer cells using PEGylated liposomes modified with a fibronectin-mimetic peptide. Int J Pharm (2009) 366:201–10 10.1016/j.ijpharm.2008.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shroff K, Kokkoli E. PEGylated liposomal doxorubicin targeted to α5β1-expressing MDA-MB-231 breast cancer cells. Langmuir (2012) 28:4729–36 10.1021/la204466g [DOI] [PubMed] [Google Scholar]
- 62.Pangburn TO, Georgiou K, Bates FS, Kokkoli E. Targeted polymersome delivery of siRNA induces cell death of breast cancer cells dependent upon Orai3 protein expression. Langmuir (2012) 28:12816–30 10.1021/la300874z [DOI] [PubMed] [Google Scholar]
- 63.Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin αvβ6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell (1999) 96:319–28 10.1016/S0092-8674(00)80545-0 [DOI] [PubMed] [Google Scholar]
- 64.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem (2000) 275:21785–8 10.1074/jbc.R000003200 [DOI] [PubMed] [Google Scholar]
- 65.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep (2010) 11:97–105 10.1038/embor.2009.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bates RC, Bellovin DI, Brown C, Maynard E, Wu B, Kawakatsu H, et al. Transcriptional activation of integrin β6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J Clin Invest (2005) 115:339–47 10.1172/JCI23183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmed N, Pansino F, Baker M, Rice G, Quinn M. Association between αvβ6 integrin expression, elevated p42/44 kDa MAPK, and plasminogen-dependent matrix degradation in ovarian cancer. J Cell Biochem (2002) 84:675–86 10.1002/jcb.10080 [DOI] [PubMed] [Google Scholar]
- 68.Elayadi AN, Samli KN, Prudkin L, Liu YH, Bian A, Xie XJ, et al. A peptide selected by biopanning identifies the integrin αvβ6 as a prognostic biomarker for nonsmall cell lung cancer. Cancer Res (2007) 67:5889–95 10.1158/0008-5472.CAN-07-0245 [DOI] [PubMed] [Google Scholar]
- 69.Prudkin L, Liu DD, Ozburn NC, Sun MH, Behrens C, Tang X, et al. Epithelial-to-mesenchymal transition in the development and progression of adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol (2009) 22:668–78 10.1038/modpathol.2009.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hausner SH, DiCara D, Marik J, Marshall JF, Sutcliffe JL. Use of a peptide derived from foot-and-mouth disease virus for the noninvasive imaging of human cancer: generation and evaluation of 4-[18F]fluorobenzoyl A20FMDV2 for in vivo imaging of integrin αvβ6 expression with positron emission tomography. Cancer Res (2007) 67:7833–40 10.1158/0008-5472.CAN-07-1026 [DOI] [PubMed] [Google Scholar]
- 71.Kawashima A, Tsugawa S, Boku A, Kobayashi M, Minamoto T, Nakanishi I, et al. Expression of αv integrin family in gastric carcinomas: increased αvβ6 is associated with lymph node metastasis. Pathol Res Pract (2003) 199:57–64 10.1078/0344-0338-00355 [DOI] [PubMed] [Google Scholar]
- 72.Goodman SL, Hölzemann G, Sulyok GAG, Kessler H. Nanomolar small molecule inhibitors for αvβ6, αvβ5, and αvβ3 integrins. J Med Chem (2002) 45:1045–51 10.1021/jm0102598 [DOI] [PubMed] [Google Scholar]
- 73.Bochen A, Marelli UK, Otto E, Pallarola D, Mas-Moruno C, Di Leva FS, et al. Biselectivity of isoDGR peptides for fibronectin binding integrin subtypes α5β1 and αvβ6: conformational control through flanking amino acids. J Med Chem (2013) 56:1509–19 10.1021/jm301221x [DOI] [PubMed] [Google Scholar]
- 74.Hsiao JR, Chang Y, Chen YL, Hsieh SH, Hsu KF, Wang CF, et al. Cyclic αvβ6-targeting peptide selected from biopanning with clinical potential for head and neck squamous cell carcinoma. Head Neck (2010) 32:160–72 10.1002/hed.21166 [DOI] [PubMed] [Google Scholar]
- 75.Oyama T, Sykes KF, Samli KN, Minna JD, Johnston SA, Brown KC. Isolation of lung tumor specific peptides from a random peptide library: generation of diagnostic and cell-targeting reagents. Cancer Lett (2003) 202:219–30 10.1016/j.canlet.2003.08.011 [DOI] [PubMed] [Google Scholar]
- 76.Guan H, McGuire MJ, Li S, Brown KC. Peptide-targeted polyglutamic acid doxorubicin conjugates for the treatment of αvβ6-positive cancers. Bioconjug Chem (2008) 19:1813–21 10.1021/bc800154f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S, Gray BP, McGuire MJ, Brown KC. Synthesis and biological evaluation of a peptide-paclitaxel conjugate which targets the integrin αvβ6. Bioorg Med Chem (2011) 19:5480–9 10.1016/j.bmc.2011.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray BP, Li SZ, Brown KC. From phage display to nanoparticle delivery: functionalizing liposomes with multivalent peptides improves targeting to a cancer biomarker. Bioconjug Chem (2013) 24:85–96 10.1021/bc300498d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guthi JS, Yang SG, Huang G, Li SZ, Khemtong C, Kessinger CW, et al. MRI-visible micellar nanomedicine for targeted drug delivery to lung cancer cells. Mol Pharm (2007) 7:32–40 10.1021/mp9001393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DiCara D, Rapisarda C, Sutcliffe JL, Violette SM, Weinreb PH, Hart IR, et al. Structure-function analysis of Arg-Gly-Asp helix motifs in αvβ6 integrin ligands. J Biol Chem (2007) 282:9657–65 10.1074/jbc.M610461200 [DOI] [PubMed] [Google Scholar]
- 81.Logan D, Abughazaleh R, Blakemore W, Curry S, Jackson T, King A, et al. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature (1993) 362:566–8 10.1038/362566a0 [DOI] [PubMed] [Google Scholar]
- 82.Hausner SH, Marik J, Gagnon MK, Sutcliffe JL. In vivo positron emission tomography (PET) imaging with an αvβ6 specific peptide radiolabeled using 18F-“click” chemistry: evaluation and comparison with the corresponding 4-[18F]fluorobenzoyl- and 2-[18F]fluoropropionyl-peptides. J Med Chem (2008) 51:5901–4 10.1021/jm800608s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hausner SH, Abbey CK, Bold RJ, Gagnon MK, Marik J, Marshall JF, et al. Targeted in vivo imaging of integrin αvβ6 with an improved radiotracer and its relevance in a pancreatic tumor model. Cancer Res (2009) 69:5843–50 10.1158/0008-5472.CAN-08-4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hausner SH, Carpenter RD, Bauer N, Sutcliffe JL. Evaluation of an integrin αvβ6-specific peptide labeled with [18F]fluorine by copper-free, strain-promoted click chemistry. Nucl Med Biol (2013) 40:233–9 10.1016/j.nucmedbio.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 85.Saha A, Ellison D, Thomas GJ, Vallath S, Mather SJ, Hart IR, et al. High-resolution in vivo imaging of breast cancer by targeting the pro-invasive integrin αvβ6. J Pathol (2010) 222:52–63 10.1002/path.2745 [DOI] [PubMed] [Google Scholar]
- 86.Coughlan L, Vallath S, Saha A, Flak M, McNeish IA, Vassaux G, et al. In vivo retargeting of adenovirus type 5 to αvβ6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J Virol (2009) 83:6416–28 10.1128/JVI.00445-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kimura RH, Teed R, Hackel BJ, Pysz MA, Chuang CZ, Sathirachinda A, et al. Pharmacokinetically stabilized cystine knot peptides that bind αvβ6 integrin with single-digit nanomolar affinities for detection of pancreatic cancer. Clin Cancer Res (2012) 18:839–49 10.1158/1078-0432.CCR-11-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hackel BJ, Kimura RH, Miao Z, Liu H, Sathirachinda A, Cheng Z, et al. 18F-fluorobenzoate-labeled cystine knot peptides for PET imaging of integrin αvβ6. J Nucl Med (2013) 54(7):1101–5 10.2967/jnumed.112.110759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell (2009) 15:167–70 10.1016/j.ccr.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 90.Shojaei F. Anti-angiogenesis therapy in cancer: current challenges and future perspectives. Cancer Lett (2012) 320:130–7 10.1016/j.canlet.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 91.Heckmann D, Meyer A, Marinelli L, Zahn G, Stragies R, Kessler H. Probing integrin selectivity: rational design of highly active and selective ligands for the α5β1 and αvβ3 integrin receptor. Angew Chem Int Ed Engl (2007) 46:3571–4 10.1002/anie.200700008 [DOI] [PubMed] [Google Scholar]
- 92.Heckmann D, Meyer A, Laufer B, Zahn G, Stragies R, Kessler H. Rational design of highly active and selective ligands for the α5β1 integrin receptor. Chembiochem (2008) 9:1397–407 10.1002/cbic.200890032 [DOI] [PubMed] [Google Scholar]
- 93.Rechenmacher F, Neubauer S, Polleux J, Mas-Moruno C, De Simone M, Cavalcanti-Adam EA, et al. Functionalizing αvβ3- or α5β1-selective integrin antagonists for surface coating: a method to discriminate integrin subtypes in vitro. Angew Chem Int Ed Engl (2013) 52:1572–5 10.1002/anie.201206370 [DOI] [PubMed] [Google Scholar]
- 94.Rechenmacher F, Neubauer S, Mas-Moruno C, Dorfner PM, Polleux J, Guasch J, et al. A Molecular toolkit for the functionalization of titanium-based biomaterials that selectively control integrin-mediated cell adhesion. Chem Eur J (2013) 19(28):9218–23 10.1002/chem.201301478 [DOI] [PubMed] [Google Scholar]
- 95.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, et al. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A (2008) 105:9343–8 10.1073/pnas.0803728105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiao Y, Hong H, Javadi A, Engle JW, Xu W, Yang Y, et al. Multifunctional unimolecular micelles for cancer-targeted drug delivery and positron emission tomography imaging. Biomaterials (2012) 33:3071–82 10.1016/j.biomaterials.2011.12.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ding H, Yong KT, Roy I, Hu R, Wu F, Zhao L, et al. Bioconjugated PLGA-4-arm-PEG branched polymeric nanoparticles as novel tumor targeting carriers. Nanotechnology (2011) 22:165101. 10.1088/0957-4484/22/16/165101 [DOI] [PubMed] [Google Scholar]
- 98.Eldar-Boock A, Miller K, Sanchis J, Lupu R, Vicent MJ, Satchi-Fainaro R. Integrin-assisted drug delivery of nano-scaled polymer therapeutics bearing paclitaxel. Biomaterials (2011) 32:3862–74 10.1016/j.biomaterials.2011.01.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, et al. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release (2009) 140:166–73 10.1016/j.jconrel.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 100.Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release (2010) 143:136–42 10.1016/j.jconrel.2009.12.020 [DOI] [PubMed] [Google Scholar]
- 101.Liu P, Qin L, Wang Q, Sun Y, Zhu M, Shen M, et al. cRGD-functionalized mPEG-PLGA-PLL nanoparticles for imaging and therapy of breast cancer. Biomaterials (2012) 33:6739–47 10.1016/j.biomaterials.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 102.Yin P, Wang Y, Qiu Y, Hou L, Liu X, Qin J, et al. Bufalin-loaded mPEG-PLGA-PLL-cRGD nanoparticles: preparation, cellular uptake, tissue distribution, and anticancer activity. Int J Nanomedicine (2012) 7:3961–9 10.2147/IJN.S32063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borgman MP, Ray A, Kolhatkar RB, Sausville EA, Burger AM, Ghandehari H. Targetable HPMA copolymer-aminohexylgeldanamycin conjugates for prostate cancer therapy. Pharm Res (2009) 26:1407–18 10.1007/s11095-009-9851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Borgman MP, Aras O, Geyser-Stoops S, Sausville EA, Ghandehari H. Biodistribution of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer drug delivery. Mol Pharm (2009) 6:1836–47 10.1021/mp900134c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greish K, Ray A, Bauer H, Larson N, Malugin A, Pike D, et al. Anticancer and antiangiogenic activity of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer therapy. J Control Release (2011) 151:263–70 10.1016/j.jconrel.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ray A, Larson N, Pike DB, Grüner M, Naik S, Bauer H, et al. Comparison of active and passive targeting of docetaxel for prostate cancer therapy by HPMA copolymer-RGDfK conjugates. Mol Pharm (2011) 8:1090–9 10.1021/mp100402n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang P, Hu L, Yin Q, Feng L, Li Y. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Mol Pharm (2012) 9:1590–8 10.1021/mp200600t [DOI] [PubMed] [Google Scholar]
- 108.Xu Q, Liu Y, Su S, Li W, Chen C, Wu Y. Anti-tumor activity of paclitaxel through dual-targeting carrier of cyclic RGD and transferrin conjugated hyperbranched copolymer nanoparticles. Biomaterials (2012) 33:1627–39 10.1016/j.biomaterials.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 109.Winter PM, Schmieder AH, Caruthers SD, Keene JL, Zhang H, Wickline SA, et al. Minute dosages of αvβ3-targeted fumagillin nanoparticles impair Vx-2 tumor angiogenesis and development in rabbits. FASEB J (2008) 22:2758–67 10.1096/fj.07-103929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim Y, Pourgholami MH, Morris DL, Stenzel MH. An optimized RGD-decorated micellar drug delivery system for albendazole for the treatment of ovarian cancer: from RAFT polymer synthesis to cellular uptake. Macromol Biosci (2011) 11:219–33 10.1002/mabi.201000293 [DOI] [PubMed] [Google Scholar]
- 111.Oba M, Fukushima S, Kanayama N, Aoyagi K, Nishiyama N, Koyama H, et al. Cyclic RGD peptide-conjugated polyplex micelles as a targetable gene delivery system directed to cells possessing αvβ3 and αvβ5 integrins. Bioconjug Chem (2007) 18:1415–1123 10.1021/bc0700133 [DOI] [PubMed] [Google Scholar]
- 112.Oba M, Aoyagi K, Miyata K, Matsumoto Y, Itaka K, Nishiyama N, et al. Polyplex micelles with cyclic RGD peptide ligands and disulfide cross-links directing to the enhanced transfection via controlled intracellular trafficking. Mol Pharm (2008) 5:1080–92 10.1021/mp800070s [DOI] [PubMed] [Google Scholar]
- 113.Oba M, Vachutinsky Y, Miyata K, Kano MR, Ikeda S, Nishiyama N, et al. Antiangiogenic gene therapy of solid tumor by systemic injection of polyplex micelles loading plasmid DNA encoding soluble flt-1. Mol Pharm (2010) 7:501–9 10.1021/mp9002317 [DOI] [PubMed] [Google Scholar]
- 114.Vachutinsky Y, Oba M, Miyata K, Hiki S, Kano MR, Nishiyama N, et al. Antiangiogenic gene therapy of experimental pancreatic tumor by sFlt-1 plasmid DNA carried by RGD-modified crosslinked polyplex micelles. J Control Release (2011) 149:51–7 10.1016/j.jconrel.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 115.Nie Y, Schaffert D, Rödl W, Ogris M, Wagner E, Günther M. Dual-targeted polyplexes: one step towards a synthetic virus for cancer gene therapy. J Control Release (2011) 152:127–34 10.1016/j.jconrel.2011.02.028 [DOI] [PubMed] [Google Scholar]
- 116.Merkel OM, Germershaus O, Wada CK, Tarcha PJ, Merdan T, Kissel T. Integrin αvβ3 targeted gene delivery using RGD peptidomimetic conjugates with copolymers of PEGylated poly(ethylene imine). Bioconjug Chem (2009) 20:1270–80 10.1021/bc9001695 [DOI] [PubMed] [Google Scholar]
- 117.Zhan C, Meng Q, Li Q, Feng L, Zhu J, Lu W. Cyclic RGD-polyethylene glycol-polyethylenimine for intracranial glioblastoma-targeted gene delivery. Chem Asian J (2012) 7:91–6 10.1002/asia.201100570 [DOI] [PubMed] [Google Scholar]
- 118.Ng QK, Sutton MK, Soonsawad P, Xing L, Cheng H, Segura T. Engineering clustered ligand binding into nonviral vectors: αvβ3 targeting as an example. Mol Ther (2009) 17:828–36 10.1038/mt.2009.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martin I, Dohmen C, Mas-Moruno C, Troiber C, Kos P, Schaffert D, et al. Solid-phase-assisted synthesis of targeting peptide-PEG-oligo(ethane amino)amides for receptor-mediated gene delivery. Org Biomol Chem (2012) 10:3258–68 10.1039/c2ob06907e [DOI] [PubMed] [Google Scholar]
- 120.Yonenaga N, Kenjo E, Asai T, Tsuruta A, Shimizu K, Dewa T, et al. RGD-based active targeting of novel polycation liposomes bearing siRNA for cancer treatment. J Control Release (2012) 160:177–81 10.1016/j.jconrel.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 121.Xiong XB, Uludag H, Lavasanifar A. Virus-mimetic polymeric micelles for targeted siRNA delivery. Biomaterials (2010) 31:5886–93 10.1016/j.biomaterials.2010.03.075 [DOI] [PubMed] [Google Scholar]