Abstract
Background:
There are limited data on the use of platelet-rich plasma (PRP) for treating chronic plantar fasciitis.
Questions/Purposes:
The purpose of this study was to document the clinical outcomes of patients who were treated with PRP injections for plantar fasciitis to determine the degree to which injections were able to decrease the visual analogue scale (VAS) pain scores and improve patient reported functional scores.
Methods:
This was a retrospective review of 23 consecutive patients treated with PRP for chronic plantar fasciitis (symptoms lasting over 6 months). Patients returned after 4 weeks for a postinjection follow-up. A second injection was given if significant improvement was not obtained by that time. Postinjection foot and ankle outcome scores (FAOS), 12-item short form health survey (SF-12), and VAS scores were collected at a minimum of 6 months follow-up.
Results:
Thirty injections were given in 23 patients, with one patient lost to follow-up. The mean VAS score improved from 7 to 4. The pain, symptoms, and quality of life subscales of the FAOS and SF-12 significantly improved from preinjection scores. Five patients went on to have endoscopic release of the plantar fascia at an average of 94 days after the last injection (range, 22–314 days). Six patients obtained full resolution of symptoms while the majority of patients were able to forgo surgery due to improvement from the PRP injection.
Conclusion:
These results provide preliminary information on the safety and efficiency of PRP injection as treatment for chronic plantar fasciitis.
Electronic supplementary material
The online version of this article (doi:10.1007/s11420-012-9321-9) contains supplementary material, which is available to authorized users.
Keywords: platelet-rich plasma, plantar fasciitis, ultrasound guided, foot and ankle outcome score
Introduction
Plantar fasciitis is one of the most common causes of plantar heel pain encountered in orthopedic practice. As a clinical entity, it is characterized by gradual onset of sharp pain along the medial heel that is worse with the first step taken in the morning or after prolonged sitting. Symptoms are localized most commonly along the medial tubercle of the calcaneus at the origin of the plantar aponeurosis. Plantar fasciitis in many ways is a misnomer, as the pathological process more closely resembles a degenerative process such as that of lateral epicondylitis. Histological findings show no evidence of inflammation; rather, they show myxoid degeneration, microtears, collagen necrosis, and angiofibroblastic hyperplasia [10]. These findings suggest a chronic degenerative process, not an acute inflammatory one. With this in mind, new treatment regimens that initiate a healing response (PRP) rather than suppress an inflammatory process should be effective treatment options.
In general, plantar fasciitis is a self-limiting disease. Conservative treatments, such as stretching, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, and night splints are regarded as the mainstay of plantar fasciitis treatment and provide substantial relief to 80% of patients [5, 13, 17]. Steroid injection into the plantar fascia is an effective treatment of plantar fasciitis when conservative management is unsuccessful. However, the lack of an inflammatory process histologically in plantar fasciitis questions its mode of action. In adults, steroid injection has been associated with rupture of the plantar fascia in 2.4 to 10% of patients as well as attenuation of the plantar fat pad [1, 6–8, 15, 18].
The idea of using an injection of autologous blood to alleviate pain and incite a healing process is an attractive one. Originally, platelets were thought to only act within the concept of clotting. Now it is known that platelets are active in a variety of biochemical pathways and release many bioactive proteins upon coagulation that are responsible for tissue regeneration and healing. A number of growth factors released by platelets during degranulation including platelet-derived growth factor, transforming growth factor β, type I insulin-type factor (IGF-1), vascular endothelial growth factor, and hepatocyte growth factor have been implicated to have beneficial effects in tissue repair and proliferation [4, 12, 14].
Platelet-rich plasma (PRP) has become a popular treatment option for a variety of orthopedic inflammatory conditions. PRP, the plasma fraction of the autologous blood having a platelet concentration above baseline, contains various growth factors that have been found to be involved in a host of biosynthetic pathways. By injecting an aliquot of concentrated platelet-enriched plasma into a localized area, the various growth factors are thought to jumpstart the regenerative processes in degenerative conditions. In chronic conditions, PRP causes a restart of the inflammatory process that commonly ceases following failed conservative treatment, transforming the chronic injury into a new acute injury with the addition of concentrated growth factors. Earlier results of using PRP to treat plantar fasciitis have been favorable, but the results have come from very small studies and only examined whether patients were relieved of the symptoms in a binary fashion of yes or no [3].
The purpose of this study was to retrospectively review a larger series of patients who have been treated with PRP injections for chronic plantar fasciitis with symptoms lasting over 6 months to determine if PRP injections (1) provided relief of symptoms, (2) alleviated the need to undergo surgery, and (3) decreased the visual analogue scale (VAS) pain score and improved patient reported functional scores.
Materials and Methods
Study approval was obtained from the institutional review board. We retrospectively reviewed with follow-up 24 consecutive patients who were treated with PRP injection for chronic plantar fasciitis. Chronic plantar fasciitis was defined as characteristic symptoms lasting longer than 6 months. The diagnosis was made clinically by the appropriate history as well as pain localized along the plantar fascia at the plantar medial heel. All patients had failed various conservative treatments such as rest, ice, stretching, anti-inflammatory medications, and physical therapy. Patients with previous steroid injections in the inferior heel, diabetes mellitus, and lack of preinjection questionnaire information were excluded. The study subjects were composed of 5 males and 19 females, with an average age of 47 (range, 25–63 years). The average duration of symptoms before injection was 9 months (range, 6 to 12 months).
All patients were instructed to stop taking NSAIDs 2 weeks prior to injection. PRP injections were done using sterile technique under ultrasound guidance by a single surgeon. Blood was drawn into a 60 cm3 syringe preloaded with 8 cm3 anticoagulant citrate dextrose-A (ACDA) giving a ratio of 1 cm3 ACDA to 7 cm3 of blood. The syringe was then put into a centrifuge for 12 to 16 min with the time depending on the volume of blood drawn. The centrifuge first did a preliminary spin at a lower rate per minute for about 7 min, after which the machine extracted red blood cells. The machine then went into higher rate of spin for about 7 min for the final separation of platelets from the rest of the plasma. White blood cells were present, but the amount varied from case to case. The injection site was prepped with povidone-iodine and anesthetized locally with 1 mL of lidocaine. Between 2 and 3 mL of PRP was delivered into the plantar fascia under ultrasound guidance, using a technique described as peppering whereby a needle was repeatedly inserted and reinserted into the affected area.
After injection, patients were placed in a controlled ankle motion (CAM) walker for the first 2 weeks and were instructed to weight bear as tolerated. Starting in the third week after injection, patients were instructed to conduct self-stretching of the plantar fascia. NSAIDs continued to be disallowed for the 4 weeks postinjection. All patients were asked to return to the office 4 weeks after the injection for follow-up. Patients were given the VAS, foot and ankle outcome survey (FAOS), and 12-item short form health survey (SF-12) prior to the injection and at the postinjection office visit. The VAS is a validated standard of care assessment evaluating pain numerically on a scale from 0 (no pain) to 10 (worst pain) [16]. The FAOS is a self-administered survey that contains five subscales: pain, symptoms, function in activities of daily living, function in sports and recreation, and overall foot-and-ankle-related quality of life. The survey is designed to rate the patient’s subjective assessment of his or her function during the week leading up to taking the survey. A normalized score is calculated for each subscale, ranging from 0 (extreme symptoms) to 100 (no symptoms) [9, 11].
Patients who were responding favorably to the first injection were encouraged to continue the stretching regimen. If patients had little or no response to the injection at 4 weeks, they were offered another injection for up to 3 months. Since we know of no data that discuss the timing of repeat PRP injections, we chose 4 weeks as a reasonable time period to see some clinical results. In this study, the same aforementioned protocol was used to give the second injection and the same postinjection protocol was instructed. The same pre- and postinjection questionnaires were collected. If symptoms did not improve after the second injection, a surgical option was recommended. For patients who eventually elected surgical treatment, the postinjection scores were collected prior to the operation. Patients were contacted at a minimum of 6 months after injection with an average follow-up of 6.7 months (range, 6 to 10 months).
Paired samples t test (two tailed) was used to assess statistical difference in pre- and postinjection VAS, all five subscales of the FAOS, and the SF-12.
Results
A total of 29 injections were given to 24 patients. One patient was lost to follow-up. Eighteen patients were treated with one injection and five patients received two injections in the same foot. For the five patients who had two injections, the average length between injections was 7 weeks (range, 4–14 weeks) (Table 1). No complications were reported. Eight patients failed to experience adequate relief of symptoms whereby the VAS scores of six of these patients did not change postoperatively and the VAS scores of two patients increased. The postinjection FAOS and SF-12 scores were reported to be worse in only one patient. All patients were placed in a CAM walker following the injection and all, but two patients who required crutches for less than 24 h were able to ambulate immediately following the injection.
Table 1.
Patients with single or double injections
| Number of patients | Number of injections used | Time between injections | |
|---|---|---|---|
| Single injection | 18 | 18 | |
| Double injection | 5 | 10 | 7 weeks |
| Lost to follow-up | 1 | 1 | |
| Total | 24 | 29 |
Six of the eight patients who did not experience adequate relief of symptoms went on to receive surgical treatment. Of these six patients, two reported relief from the PRP injection: one reported a decrease in the VAS score and one reported improved FAOS and SF-12 scores while the remaining four reported no change. Five of the six surgical patients elected for surgical treatment before a second injection was performed. The average length of time between the last injection and surgery was 3 months (range, 22 to 314 days).
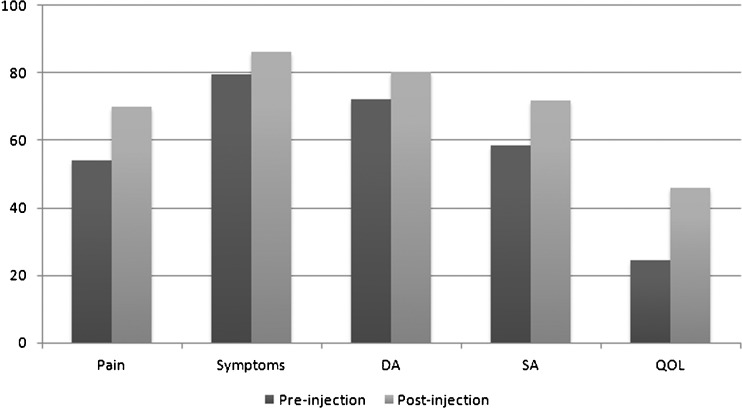
The mean postinjection VAS score of 4.5 (95% CI, 3.4–5.6) significantly improved from the mean preinjection score of 6.91 (95% CI, 6.3–7.5) with a p < 0.001 (Fig. 1). Four patients reported a VAS score of 0.0 at follow-up and an additional four patients had VAS scores of 2.0 or below. The postinjection SF-12 score of 76.7 (95% CI, 69.2–84.1) improved significantly (p = 0.004) from the preinjection score of 59.1 (95% CI, 51–67.2) (Fig. 2) Three of the five FAOS subgroups postinjection scores including pain, symptoms, and quality of life showed significant improvement from the preinjection scores, from 54.1 to 70.1 (p = 0.001), from 79.7 to 86.1 (p = 0.005), and from 24.6 to 45.9 (p = 0.003), respectively (Fig. 3). The subgroup that showed the most improvement was pain, indicating that patients did better clinically postinjection. Daily activity and sports activity did improve after the injection but the difference was not statistically significant (Table 2). Throughout the course of the study, no complications due to the PRP injection were reported.
Fig. 1.
The pre- and post-injection VAS scores are shown demonstrating overall improvement.
Fig. 2.
Pre- and post-injection SF-12 scores are projected here demonstrating overall improvement following the PRP injection.
Fig. 3.
The pre- and post-injection FAOS scores are displayed. Daily activities (DA), sports activities (SA), and overall quality of life (QOL) subscales are displayed individually.
Table 2.
Paired samples t tests for all patients
| Test characteristic | Pre-injection (95% CI) | Post-injection (95% CI) | Mean difference | p value |
|---|---|---|---|---|
| Pain | 54.1 (48.3–59.8) | 70.1 (62.5–77.7) | 16 | 0.001a |
| Symptoms | 79.7 (75.2–84.1) | 86.1 (81.3–91.0) | 6.4 | 0.005a |
| DA | 72.3 (65.5–79.2) | 80.3 (74.2–86.4) | 8 | 0.094 |
| SA | 58.5 (49.6–67.4) | 71.8 (64.2–79.5) | 13.3 | 0.075 |
| QOL | 24.6 (20.9–28.2) | 45.9 (32–59.9) | 21.3 | 0.003a |
| SF-12 | 59.1 (51–67.2) | 76.7 (69.2–84.1) | 17.6 | 0.004a |
| VAS | 6.9 (6.3–7.5) | 4.5 (3.3–5.6) | 2.4 | <0.001a |
aSignificant at α = 0.05
DA daily activities, SA sports activities, QOL overall quality of life, SF-12 12-item short form health survey, VAS visual analogue scale
Discussion
The purpose of our study was to determine the success of PRP in the relief of plantar fasciitis symptoms. We found that SF-12 scores, VAS scores, and three of five FAOS subgroup scores significantly improved. Eight patients did not feel that they received adequate relief of symptoms, and six of these patients subsequently received surgical treatment.
Limitations of our study include this study’s retrospective nature and the limited number of patients with the lack of a control group. As PRP preparation devices are different, the concentration of PRP prepared from each device will be different. The concentrations will also be dependent on the original sample of blood, which can differ tremendously between patients as well. Postinjection regimen should also be an important area of study; however, the use of CAM walkers in this study was not likely to contribute to any additional improvement as all patients have failed more conservative therapy. This study reports our experience with PRP injection to treat plantar fasciitis, but without a control over many of the aforementioned variables, it is hard to conclude the true clinical implications. Further randomized controlled trails should be conducted to more closely examine each variable to determine the efficacy of ultrasound-guided injection of PRP to treat plantar fasciitis.
Kalaci et al. conducted a randomized multicenter prospective trial with 100 patients divided into four equal treatment groups that included autologous blood alone, local anesthetic combined with peppering, corticosteroid alone, and corticosteroid combined with peppering. At a 3-week and 6-month follow-up, patients with corticosteroid injection combined with peppering showed the most significant improvement. Interestingly, the group that received corticosteroid injection combined with peppering did better than the group that just received a corticosteroid injection [6]. Given that there was improvement of the corticosteroid injection combined with peppering compared with just injection, it is plausible to think the repeated introduction of the needle into the plantar fascia is in and of itself efficacious as a mechanical stimulation mechanism to aid the healing process of plantar aponeurosis. Conversely, the increased improvement can also be due to the creation of channels by the needle into the aponeurosis that allow for corticosteroids or PRP to travel deeper into the tissue. The present study used a peppering technique in conjunction with PRP injection under ultrasound guidance.
Barrett et al. enrolled nine patients in an office study to evaluate PRP injection treated plantar fasciitis. Patients were injected with 3 mL of PRP while anesthetized with a posterior tibial and sural nerve block. Six of the nine patients achieved complete symptomatic relief after 2 months. Two of the three initially unsuccessful patients eventually found complete relief following an additional PRP injection [3]. The results of our study demonstrated similar results from a larger series of patients. Scores from all subscales of the FAOS as well as SF-12 showed improvement over preinjection scores. Due to the self-limiting nature of plantar fasciitis, the most improvement was expected in the pain, symptoms, and quality-of-life subscales of the FAOS and not as greatly expected in the daily activity or sports activity subscales. Fifteen out of 23 patients injected with PRP had a significantly decreased VAS score before going on to achieve full symptomatic relief. The remaining eight patients experienced increase in functional scores and decrease of VAS score but did not find complete symptomatic relief.
Lee et al. looked at 61 patients with plantar fasciitis for greater than 6 weeks and randomly assigned the patients to either a steroid injection or an injection of autologous blood [9]. Patients were assessed at 6 weeks, 3 months, and 6 months. At 6 weeks and 3 months, patients in the steroid group had significantly lower VAS scores than those receiving autologous blood, but these effects evened out by 6 months, and both sets of patients improved significantly by 6 months. These patients only had symptoms for a period of at least 6 weeks; the stage and nature of tissue repair is most likely different than that of patients with chronic symptoms lasting more than 6 months. In a situation in which the steroid injection was thought to decrease the acute inflammatory process, PRP injection was able to match the effect of steroid injection. With the equal efficacy, the decrease in risk of plantar fascia rupture and fat pad attenuation was significant enough to utilize PRP injections before steroid injections.
A study performed by Aksahin et al. compared the effects of corticosteroid injections and PRP injections to treat plantar fasciitis. Their study consisted of 60 patients who did not respond to conservative treatment for at least 3 months prior to either injection. The patients were placed into two groups in which 30 patients were treated with a corticosteroid injection and 30 patients were treated with a PRP injection. They found no significant difference in pain or patient satisfaction, thus demonstrating that PRP injections are as effective as corticosteroid injections without the risks of fat pad attenuation, plantar fascia rupture, and calcaneal osteomyelitis that corticosteroid injections pose [2].
Six of our patients eventually elected for surgical treatment, but five out of the six did not wait for the recommended period of time or receive a second injection before scheduling for surgery. Many of these patients may have obtained complete relief of symptoms if a second injection had been given. Surgical treatments were performed as per patients’ requests mainly due to lack of satisfactory improvement. Many of these patients did, in fact, show improvement in the postinjection scores but were frustrated by the time course of the treatment and no longer wished to receive another injection. The majority of these patients obtained surgery within 20 days, with an outlier at 314 days. For the patients that experienced symptomatic improvement after the PRP injections required no further medical treatment. This approach in these patients avoided the risk of the known complications of steroid injections and surgery.
We conclude that these results support the use of PRP injection and argue that it should be considered as a safe alternative treatment modality for chronic plantar fasciitis.
Electronic supplementary material
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)
Disclosures
Informed Consent: Martin J. O’Malley, MD, J. Turner Vosseller, MD, Yang Gu, BS declare they have no conflict of interest.
Human/Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration on 1975, as revised in 2008 (5).
Informed Consent: Informed consent was obtained from all patients for being included in the study.
Required Author Forms Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Therapeutic Study Level IV. See levels of evidence for a complete description.
References
- 1.Acevedo JI, Beskin JL. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int. 1998;19:91–97. doi: 10.1177/107110079801900207. [DOI] [PubMed] [Google Scholar]
- 2.Aksahin E, Dogruyol D, Yuksel HY, Hapa O, Dogan O, Celebi L, Bicimoglu A. The comparison of the effect of corticosteroids and platelet-rich plasma (PRP) for the treatment of plantar fasciitis. Arch Orthop Trauma Surg. 2012;132:781–785. doi: 10.1007/s00402-012-1488-5. [DOI] [PubMed] [Google Scholar]
- 3.Barrett S, Erredge S. Growth factors for chronic plantar fasciitis. Podiatry Today. 2004;17:37–42. [Google Scholar]
- 4.Gerritsen ME, Tomlinson JE, Zlot C, et al. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br J Pharmacol. 2003;140(4):595–610. doi: 10.1038/sj.bjp.0705494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill LH. Plantar Fasciitis. Diagnosis and Conservative Management. J Am Acad Orthop Surg. 1997;5(2):109–117. doi: 10.5435/00124635-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Kalaci A, Cakici H, Hapa O, et al. Treatment of plantar fasciitis using four different local injection modalities: a randomized prospective clinical trial. J Am Podiatr Med Assoc. 2009;99(2):108–113. doi: 10.7547/0980108. [DOI] [PubMed] [Google Scholar]
- 7.Kim C, Cashdollar MR, Mendicino RW, et al. Incidence of plantar fascia ruptures following corticosteroid injection. Foot Ankle Spec. 2010;3(6):335–337. doi: 10.1177/1938640010378530. [DOI] [PubMed] [Google Scholar]
- 8.Leach R, Jones R, Silva T. Rupture of the plantar fascia in athletes. J Bone Joint Surg Am. 1978;60(4):537–539. [PubMed] [Google Scholar]
- 9.Lee TG, Ahmad TS. Intralesional autologous blood injection compared to corticosteroid injection for treatment of chronic plantar fasciitis. A prospective, randomized, controlled trial. Foot Ankle Int. 2007;28(9):984–990. doi: 10.3113/FAI.2007.0984. [DOI] [PubMed] [Google Scholar]
- 10.Lemont H, Ammirati KM, Usen N. Plantar fasciitis: a degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93(3):234–237. doi: 10.7547/87507315-93-3-234. [DOI] [PubMed] [Google Scholar]
- 11.Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788–794. doi: 10.1177/107110070102201004. [DOI] [PubMed] [Google Scholar]
- 12.Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3–4):165–174. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schepsis AA, Leach RE, Gorzyca J. Plantar fasciitis. Etiology, treatment, surgical results, and review of the literature. Clin Orthop Relat Res. 1991;266:185–196. [PubMed] [Google Scholar]
- 14.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- 15.Tatli YZ, Kapasi S. The real risks of steroid injection for plantar fasciitis, with a review of conservative therapies. Curr Rev Musculoskelet Med. 2009;2(1):3–9. doi: 10.1007/s12178-008-9036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005;14(7):798–804. doi: 10.1111/j.1365-2702.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 17.Wolgin M, Cook C, Graham C, et al. Conservative treatment of plantar heel pain: long-term follow-up. Foot Ankle Int. 1994;15(3):97–102. doi: 10.1177/107110079401500303. [DOI] [PubMed] [Google Scholar]
- 18.Wong MW, Tang YY, Lee SK, et al. Glucocorticoids suppress proteoglycan production by human tenocytes. Acta Orthop. 2005;76(6):927–931. doi: 10.1080/17453670610046118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)
(PDF 510 kb)