Abstract
Peptide nucleic acids containing 2-pyrimidinone (P) and 3-oxo-2,3-dihydropyridazine (E) heterocycles recognized C-G and U-A inversions in a polypurine tract of double helical RNA with high affinity and sequence selectivity at pH 6.25. E-modified PNA bound strongly to bacterial A-site RNA, while no binding was observed to the human A-site RNA.
Recent discoveries of important roles that non-coding RNAs play in regulating gene expression make them attractive targets for molecular recognition.1 However, most non-coding RNAs are in a double helical conformation and molecular recognition of such structures is a formidable problem. Designing small molecules that selectively recognize RNA using hydrophobic or electrostatic interactions has been a challenging and involved process frustrated by the conformational flexibility of non-helical RNA, which is the most common target for small molecules.2 Herein, we show that RNA can be recognized with high affinity and sequence selectivity using nucleobase-modified PNA that forms stable triple helix in the major groove of double helical RNA.
Hydrogen bonding to nucleobases is an inherently effective means of selective recognition of helical nucleic acids. Dervan and co-workers pioneered this concept in DNA recognition using minor groove-binding polyamides3 and major groove-binding oligonucleotides (triple helix).4 Compared to DNA, the minor groove of RNA is wide and shallow and less suited for molecular recognition. The major groove of RNA is deep and narrow, which complicates triple helical binding. RNA triple helices are little studied. Relatively stable RNA triple helices are formed via parallel binding of a pyrimidine oligoribonucleotide to a purine tract of the double helical RNA.5 The sequence selectivity is achieved through Hoogsteen hydrogen bonding, which allows formation of U*A-U and C*G-C triplets (Figure 1).
Fig 1.
Hoogsteen triplets recognition of purines and pyrimidines.
Practical applications of triple helical recognition of nucleic acids are limited by (1) low affinity of the third strand oligonucleotide for the double helix caused, at least in part, by electrostatic repulsion between the negatively charged phosphate backbones and (2) the requirement for long homopurine tracts, as only U*A-U and C*G-C triplets are used in the common triple helical recognition. We recently reported6 that the problem of low affinity could be overcome by using peptide nucleic acid (PNA), a neutral oligonucleotide analogue that does not suffer from the electrostatic repulsion. Although binding of PNA to double helical DNA has been extensively explored,7 triple helix formation between PNA and double helical RNA was unknown before our study.6 This discovery inspired a hypothesis that triple helix forming PNA may serve as a general ligand for sequence selective recognition of biologically relevant RNA helices.
Despite significant research acitivity,8 the requirement for long purine tracts remains a major limitation of triple helical recognition. Biologically relevant double-helical RNAs typically do not contain long polypurine stretches. However, it is common to find seven or more contiguous purines interrupted by one or two pyrimidines in ribosomal RNAs9 and microRNAs.10 Thus, if the sequence range of triple helical recognition could be expanded to recognize isolated pyrimidines, the approach could be rendered useful for fundamental studies in RNA biology as well as practical biomedical applications.
We recently found that 5-methylisocytidine when installed in PNA recognized the C-G inversion in polypurine tract of RNA with slightly higher affinity than the natural nucleobases, though the sequence selectivity of recognition was low.11 Herein we show that 2-pyrimidinone (P) and 3-oxo-2,3-dihydropyridazine (E) recognized C-G and U-A inversions (Figure 1) with high affinity and sequence selectivity at pH 6.25. While E-modified PNA showed good binding affinity for the complementary bacterial A-site RNA, no binding was observed to the human A-site RNA, which has a different sequence. The use of P base in PNA had no precedent before our study, although derivatives of pyrimidinone have been used as oligonucleotide modifications for recognition of C-G inversions in polypurine tracts of DNA.12 The E base was originally designed as a PNA modification by Nielsen and co-workers,13 who used it to recognize T-A inversions in polypurine tracts of DNA. Apart from our preliminary study using 5-methylisocytidine,11 recognition of pyrimidine inversions in polypurine tracts of double helical RNA had no previous precedents.
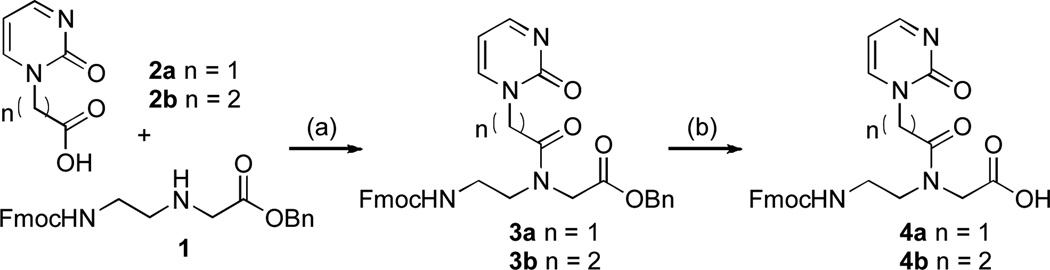
Our study started with synthesis of Fmoc-protected P, Pex and E PNA monomers 4a, 4b and 7, respectively (Schemes 1 and 2). N-(3-dimethylaminopropyl)-N´-ethylcarbodiimide (EDC) and 3-hydroxy-1,2,3-benzotriazine-4(3H)-one (DhbtOH) mediated coupling of the commercially available (2-oxo-1(2H)-pyrimidinyl)acetic acid 2a and Fmoc-protected PNA backbone 114 followed by hydrogenation gave P PNA monomer 4a.
Scheme 1.
Synthesis of P and Pex PNA monomers. Steps: (a) EDC, DhbtOH, DMF, 40 °C, 12 h, 3a 67%, 3b 65%; (b) Pd/C, H2, MeOH:CH2Cl2, rt, 3h, 4a 91%, 4b 84%.
Scheme 2.
Synthesis of E PNA monomer. Steps: (a) DhbtOH, DCC, DMF, 0 °C, 1h then rt, overnight, 89%; (b) Pd/C, H2, ethanol, rt, 3h, 73%.
Analogous N,N’-dicyclohexylcarbodiimide (DCC) mediated coupling of 1 with the known carboxylic acid 513 followed by hydrogenation gave E PNA monomer 7 (Scheme 2). Inspired by the design of the E base, which features longer linker between the backbone of PNA and the heterocycle, we also prepared an extended variant of P, Pex PNA monomer 4b (Scheme 1). Our hypothesis was that the one carbon longer linker in 4b would relax the conformation of PNA’s backbone allowing better positioning of the P base for recognition of the C-G inversion.
Monomers 4a, 4b and 7 were used in a standard PNA synthesis protocol on an Expedite 8909 DNA synthesizer to prepare modified PNA nonamers PNA3, PNA4 and PNA5, respectively (Figure 2). The target RNA hairpins HRP1-HRP4 (containing a variable base pair) were chosen from our previous studies.6,11 We expected that the modified PNAs would form the following matched triplexes: the P-modified PNA3 and PNA4 with HRP3 and the E-modified PNA5 with HRP4. The binding affinity and sequence selectivity were studied using isothermal titration calorimetry (ITC), as previously described by us.6
Fig 2.
Sequences of RNA hairpins and PNA ligands.
ITC results (Table 1, Figures S1–19) showed that at pH 5.5 the unmodified PNA1 and PNA2 had excellent binding affinity (Ka > 8 × 108) and good selectivity for the matched hairpins HRP1 and HRP2, respectively. Replacement of the variable base in PNA with the P base (monomer 4a) led to an overall decrease in binding affinity for PNA3, while good binding selectivity was maintained (Table 1, entry 3). In support of our hypothesis that an extended linker would improve binding affinity, PNA4 featuring the Pex base (monomer 4b) had significantly higher affinity than PNA3 for the matched target HRP3 (cf., Table 1, entries 3 and 4). However, the higher affinity was accompanied by somewhat lower sequence selectivity of PNA4. Surprisingly, PNA5 featuring the E base had high binding affinity to all hairpins tested without any sequence selectivity. This result was in contrast to the original report13 that E base demonstrated high selectivity for T and U over other nucleobases in DNA at pH 7. The discrepancy prompted us to investigate the binding of modified PNAs at higher and more physiologically relevant pH.
Table 1.
Binding of PNA to RNA hairpins at pH 5.5 a
| Entry | PNA (variable base) |
HRP1 (G-C) |
HRP2 (A-U) |
HRP3 (C-G) |
HRP4 (U-A) |
|---|---|---|---|---|---|
| 1 | PNA1 (C) | 890 | 96 | 74 | 3 |
| 2 | PNA2 (T) | 72 | 820 | 62 | 8 |
| 3 | PNA3 (P) | 9 | 3 | 46 | 3 |
| 4 | PNA4 (Pex) | 47 | 172 | 293 | 6 |
| 5 | PNA5 (E) | 270 | 210 | ND b | 38 |
Association constants Ka × 106 M−1 in sodium acetate buffer, pH 5.5
Not determined.
Initial ITC experiments did not detect binding of the unmodified PNA2 to HRP2 at pH 7 (Ka < 103). However, good affinity was observed at pH 6.25 (Table 2, Figures S20–35), which has biological relevance being in the range of cytoplasmic pH of acidophilic bacteria.15 Changing the pH to 6.25 reduced the affinity of unmodified PNAs and PNA4, while the sequence selectivity was significantly improved (cf., Tables 1 and 2).
Table 2.
Binding of PNA to RNA hairpins at pH 6.25 a
| Entry | PNA (variable base) |
HRP1 (G-C) |
HRP2 (A-U) |
HRP3 (C-G) |
HRP4 (U-A) |
|---|---|---|---|---|---|
| 1 | PNA1 (C) | 8.1 | LB b | LB b | LB b |
| 2 | PNA2 (T) | 7.5 | 20.0 | LB b | 0.9 |
| 3 | PNA4 (Pex) | LB b | LB b | 4.4 | LB b |
| 4 | PNA5 (E) | 3.0 | 0.5 | LB b | 28.0 |
Association constants Ka × 106 M−1 in sodium acetate buffer, pH 6.25
Ka estimated <0.01; the low binding prevented more accurate curve fit..
Interestingly, the increase of pH restored the expected binding profile for PNA5, which now had the highest affinity for the matched HRP4 (bold in entry 4, Table 2). It is conceivable that lower pH may have favored protonation or alternative tautomeric forms of E, which may explain the high affinity and low selectivity of PNA5 at pH 5.5. Most importantly, at pH 6.25 the affinity of PNAs targeting single isolated pyrimidine interruption was similar to the affinity of unmodified PNAs targeting all-purine strands (see bold numbers in Table 2). As observed in our previous study,6 the binding stoichiometry (Table S1) was consistent with a 1:1 PNA-RNA triple helix. The P and E bases showed excellent sequence selectivity for the target C-G and U-A base pairs, respectively.
The binding of PNAs to RNA hairpins was further confirmed by UV thermal melting experiments, which gave the expected biphasic melting curves typical for triple helices (Figure 3, for full set of data, see Figures S37–40). The low temperature transition was assigned to the dissociation of the PNA from the triple helical structure. The high temperature transition was assigned to the melting of the hairpin to single strand. The triple helix melting for the matched PNA4-HRP3 (solid line in Figure 3) was shifted 14 °C higher than the transitions of the mismatched complexes. Overall, the melting data were consistent with triple helix formation and confirmed our ITC results.
Fig 3.
UV thermal melting curves of Pex-modified PNA4 bound to HRP1-HRP4. Solid line is the matched PNA4-HRP3 complex.
The encouraging results obtained in our model system (Figure 2) prompted us to check if nucleobase-modified PNAs could recognize purine-rich strands in biologically important RNAs. Intriguingly, the sequence of ribosomal A-site conserved among several pathogenic bacteria, such as E. coli, P. aeruginosa and S. aureus, features a stretch of eight purines (Figure 4, bold in HRP5) interrupted by single uridine.9 ITC experiments (Figure S36) showed that the E-modified PNA6 recognized the purine-rich strand of bacterial A-site with affinity similar to that observed in our model hairpins (Ka = 5.2 × 106). The binding stoichiometry was close to 1:1, as expected for the triple helix. In contrast, we observed no binding of PNA6 to HRP6, which features the sequence of the human ribosomal A-site.
Fig 4.
inding of E-modified PNA6 to models of bacterial (HRP5) and human (HRP6) A-site RNAs.
Remarkably, the non-canonical A*G and A*A base pairs and the looped out adenosine did not significantly lower the stability of the PNA-RNA complex. We expected that of PNA6 would show excellent selectivity for HRP5 over HRP6 because the purine-rich strands of human and bacterial A-site sequences had only four out of nine nucleosides common (bold in HRP6). This is in contrast to the A-rich loop, the target of aminoglycoside antibiotics, which is remarkably similar for different organisms.
In summary, PNA nonamers modified with P and E nucleobases recognized single isolated pyrimidine interruptions in polypurine tracts of double helical RNA with similar affinity and sequence selectivity than unmodified PNAs binding to all-purine strands of RNA at pH 6.25. The binding stoichiometry (1:1 complex) and the sequence selectivity (Table 2) were consistent with the triple helix formations, as demonstrated in our previous study.6 Preliminary results suggested that the approach could be further developed to recognize complex biologically relevant RNAs featuring bulges and non-canonical base pairs, such as pre-microRNAs and ribosomal RNAs.9,10 Although recognition of pre-microRNA is a relatively new area of research, promising results have already been reported that binding of helix-threading peptides inhibit maturation of pre-microRNA.16
Supplementary Material
Acknowledgments
We thank Binghamton University and NIH (R01 GM071461) for financial support of this research.
Footnotes
Electronic Supplementary Information (ESI) available: Details of PNA synthesis, ITC experiments and data, UV melting data and copies of NMR spectra. See DOI: 10.1039/b000000x/
Footnotes should appear here. These might include comments relevant to but not central to the matter under discussion, limited experimental and spectral data, and crystallographic data.
Notes and references
- 1.Meister G, Tuschl T. Nature. 2004;431:343. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]; Plasterk RHA. Cell. 2006;124:877. doi: 10.1016/j.cell.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Thomas JR, Hergenrother PJ. Chem. Rev. 2008;108:1171. doi: 10.1021/cr0681546. [DOI] [PubMed] [Google Scholar]; Sucheck SJ, Wong CH. Curr. Opin. Chem. Biol. 2000;4:678. doi: 10.1016/s1367-5931(00)00142-3. [DOI] [PubMed] [Google Scholar]; Chow CS, Bogdan FM. Chem. Rev. 1997;97:1489. doi: 10.1021/cr960415w. [DOI] [PubMed] [Google Scholar]
- 3.Dervan PB, Edelson BS. Curr. Opin. Struct. Biol. 2003;13:284. doi: 10.1016/s0959-440x(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 4.Moser HE, Dervan PB. Science. 1987;238:645. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- 5.Roberts RW, Crothers DM. Science. 1992;258:1463. doi: 10.1126/science.1279808. [DOI] [PubMed] [Google Scholar]; Han H, Dervan PB. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3806. doi: 10.1073/pnas.90.9.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]; Escude C, Francois JC, Sun JS, Ott G, Sprinzl M, Garestier T, Helene C. Nucleic Acids Res. 1993;21:5547. doi: 10.1093/nar/21.24.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Zengeya T, Rozners E. J. Am. Chem. Soc. 2010;132:8676. doi: 10.1021/ja101384k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen ME, Bentin T, Nielsen PE. Nucleic Acids Res. 2009;37:4498. doi: 10.1093/nar/gkp437. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nielsen PE. Curr. Opin. Mol. Ther. 2010;12:184. [PubMed] [Google Scholar]
- 8.Fox KR, Brown T. Q. Rev. Biophys. 2005;38:311. doi: 10.1017/S0033583506004197. [DOI] [PubMed] [Google Scholar]
- 9. http://www.rna.ccbb.utexas.edu/ [Google Scholar]
- 10.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. Nucleic Acids Res. 2008;36:D154. doi: 10.1093/nar/gkm952. http://www.mirbase.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zengeya T, Li M, Rozners E. Bioorg. Med. Chem. Lett. 2011;21:2121. doi: 10.1016/j.bmcl.2011.01.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gianolio DA, McLaughlin LW. J. Am. Chem. Soc. 1999;121:6334. [Google Scholar]; Prevot-Halter I, Leumann CJ. Bioorg. Med. Chem. Lett. 1999;9:2657. doi: 10.1016/s0960-894x(99)00451-5. [DOI] [PubMed] [Google Scholar]; Buchini S, Leumann CJ. Angew. Chem., Int. Ed. 2004;43:3925. doi: 10.1002/anie.200460159. [DOI] [PubMed] [Google Scholar]; Ranasinghe RT, Rusling DA, Powers VEC, Fox KR, Brown T. Chem. Commun. 2005:2555. doi: 10.1039/b502325d. [DOI] [PubMed] [Google Scholar]; Rusling DA, Powers VEC, Ranasinghe RT, Wang Y, Osborne SD, Brown T, Fox KR. Nucleic Acids Res. 2005;33:3025–3032. doi: 10.1093/nar/gki625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eldrup AB, Dahl O, Nielsen PE. J. Am. Chem. Soc. 1997;119:11116. [Google Scholar]
- 14.Wojciechowski F, Hudson RHE. J. Org. Chem. 2008;73:3807. doi: 10.1021/jo800195j. [DOI] [PubMed] [Google Scholar]
- 15.Slonczewki JL, Fujisawa M, Dopson M, Krulwich TA. Adv. Microb. Physiol. 2009;55:1. doi: 10.1016/S0065-2911(09)05501-5. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurthy M, Simon K, Orendt AM, Beal PA. Angew. Chem., Int. Ed. 2007;46:7044. doi: 10.1002/anie.200702247. [DOI] [PubMed] [Google Scholar]; Gooch BD, Beal PA. J. Am. Chem. Soc. 2004;126:10603. doi: 10.1021/ja047818v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.