Summary
Lysine acetylation has emerged as a major posttranslational modification involved in diverse cellular functions. Using a combination of immunoisolation and liquid chromatography coupled to accurate mass spectrometry, we determined the first acetylome of the human malaria parasite Plasmodium falciparum during its active proliferation in erythrocytes with 421 acetylation sites identified in 230 proteins. Lysine-acetylated proteins are distributed in the nucleus, cytoplasm, mitochondrion, and apicoplast. Whereas occurrence of lysine acetylation in a similarly wide range of cellular functions suggests conservation of lysine acetylation through evolution, the Plasmodium acetylome also revealed significant divergence from those of other eukaryotes and even the closely-related parasite Toxoplasma. This divergence is reflected in the acetylation of a large number of Plasmodium-specific proteins and different acetylation sites in evolutionarily conserved acetylated proteins. A prominent example is the abundant acetylation of proteins in the glycolysis pathway but relatively deficient acetylation of enzymes in the citrate cycle. Using specific transgenic lines and inhibitors, we determined that the acetyltransferase PfMYST and lysine deacetylases play important roles in regulating the dynamics of cytoplasmic protein acetylation. The Plasmodium acetylome provides an exciting start point for further exploration of functions of acetylation in the biology of malaria parasites.
Introduction
With ~250 million clinical cases and a death toll of ~0.9 million per year, malaria remains a significant public health problem in many tropical and subtropical countries. Of the four human malaria parasites, Plasmodium falciparum causes the most severe form of disease and the majority of malaria-associated mortality. Whereas recent reduction in global malaria incidence has inspired renewed hopes for malaria elimination and eradication, the malaria control campaign still encounters many challenges. In particular, the parasite is notorious for developing resistance to most currently used antimalarial drugs. Therefore, continued research towards the development of novel diagnostics and therapeutics for malaria is needed, and these efforts require a comprehensive understanding of the parasite’s biology.
Asexual replication of the parasites in red blood cells (RBCs) contributes to malaria-associated morbidity and mortality. Extensive microarray and proteomic analyses have established that the intraerythrocytic developmental cycle (IDC) is governed by highly regulated transcription and translation programs (Le Roch et al., 2003; Luah et al., 2010; Foth et al., 2011). The importance of chromatin-mediated epigenetic regulation of gene expression has been increasingly appreciated, and its roles entail many aspects of parasite biology such as cell cycle regulation, invasion, and virulence (Merrick and Duraisingh, 2010; Miao et al., 2010). Correspondingly, the parasite genome encodes a large suite of chromatin-remodelling and modification enzymes, among which there are at least four lysine acetyltransferases (KATs) and three classes of lysine or histone deacetylases (KDACs or HDACs) (Horrocks et al., 2009; Miao et al., 2010). KATs catalyze the transfer of the acetyl moiety from acetyl-CoA to the ε-position of a lysine residue, whereas KDACs catalyze the removal of the acetyl group from an acetylated lysine. The reversible protein lysine acetylation is a highly regulated post-translational modification found in both prokaryotes and eukaryotes. Since the discovery of protein lysine acetylation almost five decades ago (Allfrey et al., 1964), studies of this modification have focused primarily on histones, the building units of nucleosomes. We and others have identified a multitude of covalent modifications on histones of P. falciparum, including both N-terminal acetylation and lysine acetylation (Miao et al., 2006; Salcedo-Amaya et al., 2009). Histone lysine acetylation, together with other post-translational modifications such as methylation, phosphorylation, ubiquitylation, and sumoylation, profoundly affects chromatin structure and gene expression (Verdone et al., 2005; Shahbazian and Grunstein, 2007).
In addition to histone lysine acetylation, lysine acetylation occurs in cytoplasmic proteins. Recently, advancement in mass spectrometry (MS) allowed characterization of the “acetylomes” in bacteria (Yu et al., 2008; Zhang et al., 2009; Wang et al., 2010), yeast (Henriksen et al., 2012), the protozoan parasite Toxoplasma gondii (Jeffers and Sullivan, 2012), plants (Finkemeier et al., 2011; Wu et al., 2011), Drosophila melanogaster (Weinert et al., 2011), rat (Lundby et al., 2012), and human cells (Kim et al., 2006; Choudhary et al., 2009; Zhao et al., 2010). From these studies, lysine acetylation has emerged as a widespread post-translational modification that may rival protein phosphorylation (Maurer-Stroh et al., 2003). Proteins with acetylated lysines participate in diverse biological functions. Particularly, lysine acetylation is highly prevalent in enzymes catalyzing intermediate metabolism in both bacteria and human cells (Kim et al., 2006; Choudhary et al., 2009; Wang et al., 2010; Zhao et al., 2010). To date, studies on lysine acetylation of non-histone proteins in malaria parasites are very limited. Yet, the fact that some of the Plasmodium KATs such as PfMYST are localized in both nucleus and cytoplasm suggests that regulated protein lysine acetylation occurs in both compartments of the parasite (Miao et al., 2010). In this study, we performed a proteome-wide analysis of the P. falciparum acetylome during the IDC by immunoprecipitation (IP) with specific anti-acetyllysine antibodies and accurate MS. This screen identified 230 lysine-acetylated proteins belonging to considerably diverse functional groups, suggesting that acetylation plays important roles in regulating many cellular processes in Plasmodium. Understanding the mechanism of acetylation and its role in regulating protein functions may open a new venue for development of drugs and vaccines.
Results
Detection of Lysine-Acetylated Proteins
To evaluate overall protein acetylation during the IDC of P. falciparum, Western analysis was performed with protein extracts from parasites at four time points during the IDC with anti-acetyllysine antibodies. Multiple protein bands spanning a wide mass range were detected (Fig. 1A). Several protein bands were strongly reactive to the antibodies. Competition with acetylated BSA confirmed that the protein bands detected were specific. This result also revealed that protein acetylation patterns were similar among different stages of the IDC, and the level of acetylation was the highest in late trophozoite stage, which is correlated with the most elevated hemoglobin digestion and protein synthesis in trophozoites (Fig. 1A).
Fig. 1. Analysis of acetylation in P. falciparum.
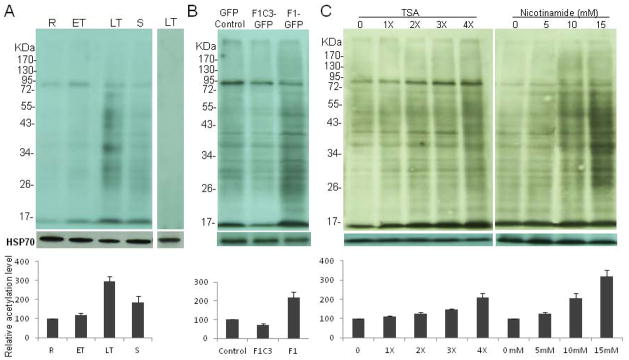
(A) Overall acetylation of P. falciparum in the intraerythrocytic development stages. Proteins were isolated from ring (R), early trophozoite (ET), late trophozoite (LT) and schizont (S). The right panel shows the reaction of anti-acetyllysine antibodies to LT proteins blocked by acetylated BSA. (B) Acetylation of cytoplasmic proteins in trophozoites of GFP-control and parasite lines with overexpression of a truncated inactive KAT PfMYST (F1C3-GFP) and overexpression of the full-length active PfMYST (F1-GFP). (C) Effect of HDAC inhibitors (TSA and nicotinamide) on cytoplasmic protein acetylation. Parasites were treated from ring stage with 1 to 4 times of IC50 of TSA or 5 to 15 mM of nicotinamide for 15 h. Equal amounts of protein lysates were separated by SDS-PAGE, and the acetylated proteins were detected with anti-acetyllysine antibodies. Equal protein loading was evidenced by Western blotting with anti-HSP70 antibodies. The graphs under the respective Western blots show the relative signal intensities determined by densitometry (mean + standard deviation) from three replicates.
KATs and HDACs in Protein Acetylation
Of the four known KATs in P. falciparum, PfMYST was found localized in both cytoplasm and nucleus, suggesting a role in acetylation of cytoplasmic proteins (Miao et al., 2010). To determine the involvement of PfMYST in cytoplasmic protein acetylation, we compared the overall cytoplasmic protein acetylation among trophozoites of three parasite lines, GFP-control, F1-GFP (over-expressing a full-length active version of PfMYST) and F1C3-GFP (over-expressing a truncated inactive version of PfMYST) (Miao et al., 2010). Overexpression of active PfMYST dramatically increased the overall acetylation levels of the cytoplasmic proteins, especially in the molecular mass range of 26–95 kDa (Fig. 1B). In contrast, overexpression of the inactive PfMYST reduced the overall cytoplasmic protein acetylation levels. This result indicated that PfMYST played a major role in the acetylation of cytoplasmic proteins (Miao et al., 2010). To evaluate the effect of HDAC inhibitors on protein acetylation, we treated the parasites with trichostatin A (TSA) and nicotinamide (Fig. 1C). The P. falciparum genome encodes five HADCs (one class I, two class II, and two class III). TSA inhibits class I and II HADCs, whereas nicotinamide inhibits class III HDACs, the sirtuins (Prusty et al., 2008; Patel et al., 2009). 3D7 parasites incubated with TSA at either 1 × or 2 × IC50 (Patel et al., 2009) for 15 h did not cause significant changes in the overall acetylation patterns of cytoplasmic proteins. However, at higher TSA concentrations (3× or 4 × IC50), much higher levels of acetylation were detected (Fig. 1C). Similarly, incubation of parasites with 5, 10 or 15 mM of nicotinamide (Prusty et al., 2008) resulted in increases of the overall acetylation, suggesting that both class I and II HDAC and the Sir2 proteins participate in the deacetylation of cytoplasmic proteins (Fig. 1C).
P. falciparum Acetylome and Distribution in Cellular Compartments
To identify the P. falciparum acetylome during IDC, we used a similar procedure as described before (Kim et al., 2006; Choudhary et al., 2009), which integrates immunoisolation with accurate MS. Proteins from both nuclear and cytoplasmic fractions of late trophozoite stage were digested by endoproteinase Lys-C and trypsin and the resulting peptides were affinity-purified with immobilized anti-acetyllysine antibodies. The enriched peptides were analyzed by 2D-LC/MS/MS and the obtained MS/MS spectra were used to search the P. falciparum databases with the MASCOT search algorithm (Fig. 2A). This search has essentially filtered out peptides from human protein contamination. To ensure the accuracy of the search, positive identifications were verified by manual inspections of the MS/MS spectra (the raw data can be found at http://enot.psu.edu/research/labs/liwang-cui). Since very few proteins except histones were previously reported to be acetylated in P. falciparum (Miao et al., 2006; Salcedo-Amaya et al., 2009; Leiva et al., 2012), this survey greatly expanded the inventory of lysine-acetylated proteins in this parasite with 421 lysine acetylation sites identified in 230 proteins. Although the acetylome is by no means exhaustive, the identification of gametocyte-specific proteins (e.g., pfg27) in the asexual acetylome, which might be due to very minor gametocyte contamination, suggests in-depth coverage of the acetylome. 291 and 183 lysine acetylation sites were identified in 167 and 96 proteins from the cytosolic and nuclear fractions, respectively (Fig. 2B, Tables S1). Whereas the two fractions shared 33 lysine-acetylated proteins, the acetylation sites were not entirely conserved, with 25 of them having different acetylation sites. Further classification of subcellular distribution of the acetylated proteins by their cellular compartment annotations was performed for the cytosol acetylome. Particular attention was paid to the mitochondrion, an organelle derived from an endosymbiotic α-proteobacterium, where widespread protein acetylation has been observed in other eukaryotes (Kim et al., 2006; Choudhary et al., 2009; Zhao et al., 2010; Lundby et al., 2012). In the P. falciparum acetylome, however, only nine acetylated proteins are predicted to localize in the mitochondrion (Fig. 2B). Another organelle derived from endosymbiosis in the apicomplexan parasites is the apicoplast, a nonphotosynthetic plastid (Ralph et al., 2004). Of the ~500 proteins predicted to localize to the apicoplast, 10 acetylated proteins were identified from this study (Fig. 2B). Among them, three belong to translational machinery (two ribosomal proteins and one aminoacyl-tRNA synthetases), one functions in fatty acid synthesis (acyl-CoA binding protein), and four are conserved Plasmodium proteins with unknown functions.
Fig. 2. Identification of protein acetylation in P. falciparum trophozoites.
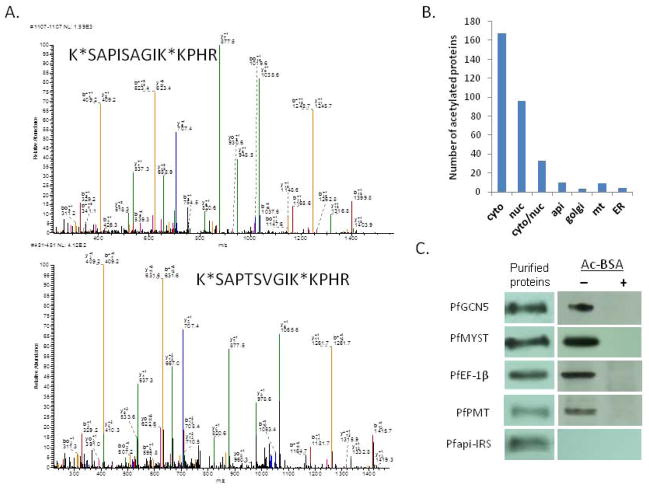
(A) Two representative MS results showing the modification of H3 and H3.3 at K28 and K37. Each peptide was fragmented by MS/MS and the fragments observed were consistent with the sequence of the peptide as shown on top of each MS/MS spectrum. Note that b ions are counting from N-terminus and y ions from C-terminus. Overall the Ms/Ms data unambiguously confirmed that both peptides were diacetylated and they differ by two amino acids. (B) Localization of acetylated proteins in different cellular compartments. (C) Verification of acetylation of five proteins by immuno-precipitation and Western-blot with anti-acetyllysine antibodies.
Confirmation of Lysine-Acetylated Proteins
We selected several newly identified lysine-acetylated proteins including two KATs (PfGCN5 and PfMYST) (Miao et al., 2010), phosphatidylethanolamine N-methyltransferase (PfPMT) (Pessi et al., 2004), and elongation factor 1 β (PfEF1β) (Mamoun and Goldberg, 2001) for verification of acetylation. These proteins were chosen because they are among the most heavily acetylated proteins and there are specific antibodies or tagged parasite lines for these proteins. We also included apicoplast isoleucine tRNA ligase (Pfapi-IRS) (Istvan et al., 2011) as a negative control since no acetyllysine was detected in this protein. These proteins were subjected to IP and Western analysis (Fig. 2C). The purified proteins were first confirmed by their specific antibodies. Anti-acetyllysine antibodies detected the respective proteins, further confirming that they contain acetylated lysine residues, whereas these bands were not detected when acetyl-BSA was included as a competitor (Fig. 2C). In comparison, the negative control protein Pfapi-IRS was detected by specific antibodies, but not the anti-acetyllysine antibodies (Fig. 2C).
Lysine Acetylation in Proteins with Diverse Functions
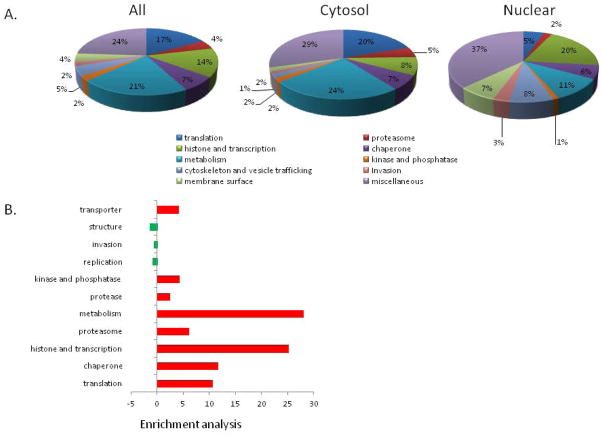
This study identified lysine acetylation in proteins with diverse cellular functions, including histones, regulators of transcription and chromatin structure, splicing, translation, chaperones, cytoskeleton, signaling, metabolic enzymes, and proteasome, suggesting that lysine acetylation regulates diverse cellular processes as reported in the acetylome surveys in other organisms (Kim et al., 2006; Yu et al., 2008; Choudhary et al., 2009; Zhang et al., 2009; Wang et al., 2010; Zhao et al., 2010; Finkemeier et al., 2011; Weinert et al., 2011; Wu et al., 2011; Henriksen et al., 2012; Jeffers and Sullivan, 2012; Lundby et al., 2012). The largest category of lysine-acetylated proteins in both nuclear and cytosol fractions is “hypothetical proteins” that are conserved in all Plasmodium species, which accounts for 24% of the entire identified acetylome. Consistent with the functional distinction between the nucleus and cytoplasm, the second most abundant lysine-acetylated proteins in the nucleus and cytosol belong to the functional categories “histone and transcription” and “metabolism”, respectively (Fig. 3A). Despite evolutionary conservation of pathways targeted for protein lysine acetylation, detailed analysis revealed substantial divergence of the Plasmodium acetylome in both proteins and their acetylation sites. One interesting finding is that the structural protein tubulin is found in other species including Toxoplasma, but was absent in the P. falciparum trophozoite acetylome. Further distinction of the Plasmodium acetylome is indicated in Plasmodium-specific proteins such as those involved in hemoglobin digestion, transporters and membrane or surface proteins (Fig. 3A, Tables S1–S3). To determine which functional categories are specially targeted for lysine acetylation, enrichment analysis was performed. Comparison with the genomic representations of these functional categories showed that proteins involved in translation, transcription, metabolism, and chaperones were significantly enriched in the Plasmodium acetylome, whereas proteins involved in replication, invasion and structure were relatively under-represented (Fig. 3B). Detailed accounts of the acetylated proteins are provided in the Supplemental Materials.
Fig. 3. Acetylation occurs in proteins involving diverse functions in P. falciparum.
(A) Functional classification of lysine-acetylated proteins from all or cytosolic and nuclear fractions. (B) Enrichment analysis. The red bars indicate the proteins at those functional groups are significantly overrepresented, whereas the green bars indicate underrepresented functional groups. P-values were calculated using simulations and were then transformed using the negative natural log for visualization.
Conserved acetylation identified in various metabolic pathways
Lysine-acetylated enzymes were identified in various metabolic pathways including central metabolism, hemoglobin digestion, purine and pyrimidine, phospholipid, polyamine and vitamin B 6 and B9 metabolism (Table S1).
Recent studies highlighted a conserved role of lysine acetylation in the regulation of central carbon metabolism in both prokaryotes and eukaryotes (Kim et al., 2006; Zhang et al., 2009; Wang et al., 2010; Zhao et al., 2010). Metabolic changes dynamically regulate lysine acetylation and activities of key enzymes in the TCA cycle, the urea cycle, and the fatty acid oxidation. Central metabolism in the malaria parasite is different from its host. Living in a glucose-rich environment, the blood-stage Plasmodium relies primarily upon glucose fermentation for energy needs. All enzymes of the complete Embden–Meyerhof–Parnas pathway of glycolysis are encoded in the parasite genome and highly expressed during the IDC (Olszewski and Llinas, 2011). Similar to bacteria (Yu et al., 2008; Zhang et al., 2009; Wang et al., 2010), yeast (Henriksen et al., 2012), Toxoplasma (Jeffers and Sullivan, 2012), D. melanogaster (Weinert et al., 2011), rat (Lundby et al., 2012), and human cells (Kim et al., 2006; Choudhary et al., 2009; Zhao et al., 2010), 10 of 11 the enzymes in this pathway are acetylated with 26 acetylated lysines (Fig. S1, Table S1). Sequence alignment of these enzymes with their orthologs in nine organisms with reported acetylomes showed that 14 of 26 acetyllysines in P. falciparum are conserved in at least one of their respective orthologs (Fig. S2). In addition, although the rest of acetyllysine sites are not conserved, they are located in domains that are rich in acetyllysines in the flanking regions of their orthologs, suggesting that acetyllysines might be important for the functions of these enzymes (Fig. S2). Within the TCA cycle, only isocitrate dehydrogenase was found acetylated in this study.
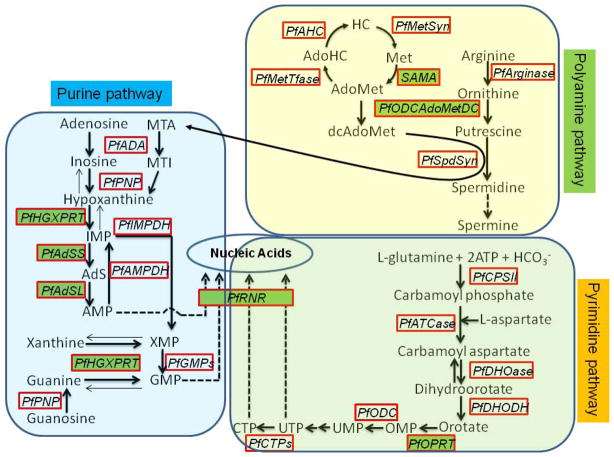
Plasmodium purine and pyrimidine metabolic pathways are distinct from those of the human host and they are extensively pursued for novel drug development (Cassera et al., 2011). P. falciparum is a purine auxotroph, salvaging host cell purines for synthesis of cofactors and nucleic acids. Unlike purines, pyrimidines exist at only low concentrations in human erythrocytes and P. falciparum primarily synthesizes pyrimidines de novo (Cassera et al., 2011). Eventually, ribonucleotide diphosphate reductase converts purine and pyrimidine nucleotides to deoxyribonucleotides (Wilson et al., 1952; Bracchi-Ricard et al., 2005). In the purine salvage and pyrimidine synthesis pathways (Cassera et al., 2011), six enzymes were lysine-acetylated (Fig. 4). In addition, two enzymes involved in the biosynthesis of polyamines were acetylated.
Fig. 4. Acetylation of enzymes in the purine, pyrimidine and polyamine metabolic pathways in P. falciparum. Purine pathway.
AMP, adenosine 5′-monophosphate; IMP, inosine 5′-monophosphate; XMP, xanthosine 5′-monophosphate; GMP, guanosine 5′-monophosphate; MTA, methylthioadenosine; MTI, methylthioinosine; AdS, adenoylsuccinate; PfADA, P. falciparum adenosine deaminase; PfPNP, P. falciparum purine nucleoside phosphorylase; PfHGXPRT, P. falciparum hypoxanthine-guanine-xanthine phosphoribosyl transferase; PfAMPDA, P. falciparum adenosine 5′-monophosphate deaminase; PfIMPDH, P. falciparum inosine 5′-monophosphate dehydrogenase; PfGMPs, P. falciparum guanosine 5′-monophosphate synthase; PfAdSS, adenylosuccinate synthetase; PfAdSL, adenylosuccinate lyase. Pyrimidine pathway: OMP, orotidine 5′-monophosphate; UMP, uridine 5′-monophosphate; UTP, uridine 5′-triphosphate; CTP, cytidine 5′-triphosphate; PfCPSII, P. falciparum carbamoyl phosphate synthetase II; PfATCase, P. falciparum aspartate carbamoyltransferase; PfDHOase, P. falciparum dihydroorotase; PfDHODH, P. falciparum dihydroorotate dehydrogenase; PfOPRT, P. falciparum orotate phosphoribosyltransferase; PfODC, P. falciparum orotidine 5′-monophosphate decarboxylase; PfCTPs, P. falciparum cytidine 5′-triphosphate synthase. Polyamine Pathway: AdoMet, S-adenosylmethionine; AdoHC, S-adenosylhomocysteine; HC, homocysteine; Met, methionine; dcAdoMet, decarboxylated S-adenosylmethionine; PfSpdSyn, P. falciparum spermidine synthase; PfODCAdoMetDC, P. falciparum ornithine decarboxylase/S-adenosylmethionine decarboxylase; PfMetTfase, P. falciparum methyltransferase(s); PfAHC, P. falciparum S-adenosyl homocysteinase; PfMetSyn, P. falciparum methionine synthase; SAMS, P. falciparum S-adenosylmethionine synthase. The arrows indicate the direction of net flux. The metabolically un-favored direction is depicted with light arrows on reversible steps. Multiple arrows indicate pathways not shown entirely. The enzymes are boxed and acetylated enzymes are shown in green blocks.
RNA splicing and translation factors
This study identified 37 lysine-acetylated proteins in this functional group, accounting for 17% of total lysine-acetylated proteins (Fig. 2B). These proteins include ribosome proteins, splicing factors, translation initiation factors, elongation factors and aminoacyl-tRNA synthetases, suggesting that acetylation might play a profound role in regulating RNA splicing and translation in Plasmodium. Among them, elongation factor 1-α was heavily acetylated at 8 lysine residues, similar to the observations made in its orthologs in other eukaryotes (human, rat, Drosophila, plant and Toxoplasma) (Choudhary et al., 2009; Finkemeier et al., 2011; Weinert et al., 2011; Wu et al., 2011; Jeffers and Sullivan, 2012; Lundby et al., 2012). In addition, K304 is a conserved site among plant (Finkemeier et al., 2011; Wu et al., 2011), human (Choudhary et al., 2009) and Plasmodium (Fig. S3). Of the five cytosolic aminoacyl-tRNA synthetases (glutaminyl-tRNA synthetase, valine-tRNA ligase, glutamate-tRNA ligase, seryl-tRNA synthetase and isoleucine-tRNA ligase), isoleucine-tRNA synthetase (Istvan et al., 2011) is heavily acetylated at 7 lysine residues, which is in stark contrast with the monoacetylation detected in the homologs of human and Toxoplasma. This difference may suggest a regulatory mechanism of acetylation for the cytosolic isoleucine-tRNA synthetase in Plasmodium. Aminoacyl-tRNA synthetases are potential targets for intervention, and inhibitors of isoleucine-tRNA synthetase and seryl-tRNA synthetase are found to inhibit P. falciparum growth (Istvan et al., 2011; Hoepfner et al., 2012).
Histones, chromatin-associated proteins, and transcriptional factors
Among the covalent modifications, histone lysine acetylation is highly abundant and acetylation sites are evolutionarily conserved. This study confirmed 19 of the 29 known acetylation sites, including almost all known acetylation sites in H4, H2A.Z and H2B.X (Miao et al., 2006; Salcedo-Amaya et al., 2009). However, this study failed to detect known acetylation sites in H2A and K18, K23, K56 in H3, and K9, K14, K18 and K23 in H3.3, which could be due to intrinsic low abundance of acetylation at these sites in trophozoites or low affinity of antibodies to these sites. In addition, five new acetylation sites in histones were detected: H3K36, H2A.ZK36, H3.3K36, and two sites (K23, K26) in CenH3. Unlike acetylation in core histones, the functional significance of lysine acetylation in minor or variant histones is less understood.
Besides histones, quite a number of proteins associated with chromatin biology were found to be lysine-acetylated. The two KATs PfGCN5 and PfMYST contain 7 and 11 acetyllysines, respectively (Fig. 5, Table S1). In addition, the Elp3-like KAT and another putative histone acetyltransferase were also acetylated. In PfMYST, acetylated lysine 428 and its corresponding lysine residues in yEsa1 K262, SAS2 K168, hMOF K274 and TgMYST-A K288 were also found acetylated, and this lysine is located in the active domain of these enzymes and its acetylation is essential for activity (Choudhary et al., 2009; Jeffers and Sullivan, 2012; Yuan et al., 2012). Similar to the observation in metazoan acetylomes, acetylation in Plasmodium also potentially targets protein complexes. A member of the PfGCN5 KAT complex, PfADA2, contains 9 acetyllysines (Fan et al., 2004a). Two PHD domain proteins potentially associated with the PfGCN5 complex (PF10_0079 and PF14_0315) were also abundantly acetylated with 18 and 14 acetyllysines, respectively (Fig. 5). Interestingly, two PHD domain containing proteins (TGME49_024260 and TGME49_034700) were also found acetylated at 6 and 4 lysines in T. gondii, respectively (Jeffers and Sullivan, 2012). In addition to these KATs and related proteins, other chromatin-associated proteins were found acetylated including two high mobility group proteins (PfHMGB1 and PfHMGB2), PCNA, a SWI/SNF factor, two bromodomain-containing proteins, and a putative histone lysine methyltransferase (Table S1).
Fig. 5. Acetylation sites in P. falciparum KATs and their associated proteins.
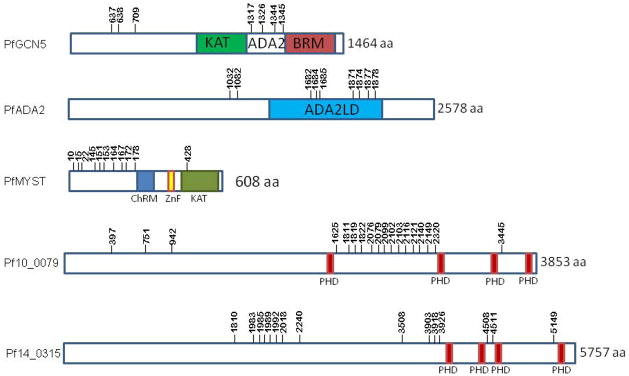
The structures of two key KATs, PfGCN5 and PfMYST, and their associated proteins, PfADA2, Pf10_0079, Pf14_0315 are depicted. The acetylated sites are shown with short bars with numbers indicating the positions of lysine residues. KAT: lysine acetyltransferase activity domain, ADA2: ADA2 binding domain, BRM: bromodomain, ADA2LD: ADA2 like-domain, ChRM: chromodomain, Znf: zinc finger. PHD: PHD domain.
Other protein groups
Chaperones are a group of proteins that are enriched in lysine acetylation with 16 lysine-acetylated proteins identified, which are similar to the profiles of acetylated chaperones documented in human, rat and Drosophila (Kim et al., 2006; Choudhary et al., 2009; Zhao et al., 2010; Weinert et al., 2011; Lundby et al., 2012), suggesting a high level of conservation in the acetylation network of chaperones. It is worth to mention that PfHsp90 contains five acetyllysine sites and two proteins (Hsp101 and PTEX150) of the recently identified novel protein export complex PTEX (Plasmodium Translocon of EXported proteins) (de Koning-Ward et al., 2009) were also acetylated.
This study revealed 12 lysine-acetylated proteins in protein ubiquitylation and sumoylation pathways, including 6 subunits of the 26S proteasome complex, a deubiquinating/deneddylating enzyme (Artavanis-Tsakonas et al., 2006), a ubiquitin-ribosomal fusion protein, SUMO, a bacteria-like proteasomal predecessor PfhslV (Ramasamy et al., 2007), a putative ubiquitin ligase, and a Dsk2-like proteasomal regulator. It contrast, none of the proteins in the human proteasome complex were found acetylated (Choudhary et al., 2009), highlighting a major difference in the ubiquitylation system in Plasmodium compared to its host.
As seen in the Toxoplasma tachyzoite acetylome, hypothetical proteins comprise the largest category of lysine-acetylated proteins in Plasmodium (Fig. 3A, Table S1). Furthermore, more than twenty acetylated proteins lack homologs in metazoan species. Among them are membrane surface proteins important for cytoadherence and invasion.
Acetylation of KATs and their activities
In order to understand the effect of lysine acetylation on the enzymatic activities of the two KATs PfGCN5 and PfMYST, GFP-tagged PfGCN5 and PfMYST were purified from trophozoite stage parasites of transgenic parasite lines treated with or without HDAC inhibitor (TSA and nicotinomide) treatment. Anti-GFP antibodies showed that similar amounts of purified PfGCN5 or PfMYST were used for in vitro HAT assays (Fig. 6). Anti-acetyllysine antibodies revealed that HDAC inhibitor treatment resulted in higher levels of acetylation in PfGCN5 and PfMYST (Fig. 6). When core and nucleosomal histones were used as the substrates, increased acetylation of PfGCN5 did not significantly change its HAT activities. However, higher acetylation of PfMYST was associated with a significant decrease of its HAT activity on H4 in both core and nucleosomal histones (Fig. 6). It is noteworthy that autoacetylation of PfGCN5 and PfMYST in the in vitro reactions was not obvious, although autoacetylation of recombinant PfGCN5 HAT domain was previously detected (Fan et al., 2004b).
Fig. 6. Effect of lysine acetylation on HAT activities of PfGCN5 and PfMYST.
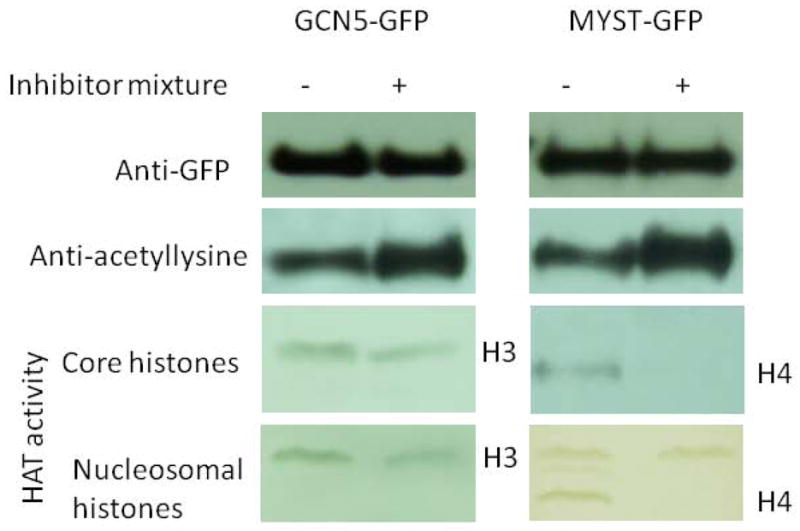
GFP-tagged PfGCN5 and PfMYST were immunoprecipitated from P. falciparum trophozoites cultured without or with the inhibitor mixture (TSA and nicotinamide). Blots with anti-GFP antibodies indicate approximately equal amounts of purified PfGCN5 or PfMYST used in the HAT assays. The overall acetylation levels of the purified proteins were estimated by Western blots with anti-acetyllysine antibodies. HAT activities of purified PfGCN5 and PfMYST were determined using core histones and nucleosomal histones.
Acetylation motifs and preference
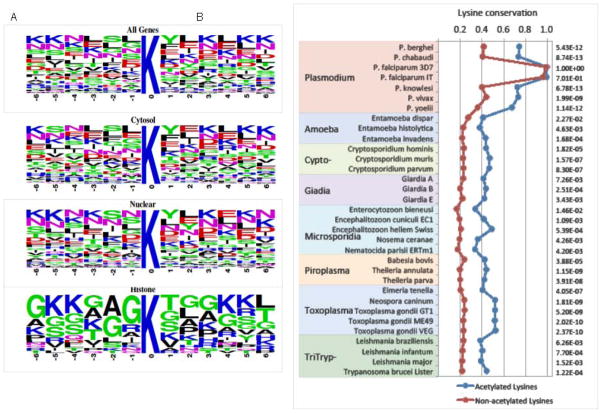
To elucidate the acetylation motifs in Plasmodium, we analyzed the composition of amino acids surrounding the acetylated lysines (Fig. 7A). Similar to other studies, we could not obtain definitive motifs but only identified a set of patterns. In metazoans, acetylated lysines of nuclear and cytosolic proteins tend to be flanked by F at the −2 position, Y at the +1 position and additional stretches of Ks on both sides (Choudhary et al., 2009; Weinert et al., 2011). However, the sequence motifs differ by subcellular compartments. In the rat acetylomes, a strong preference for glycine in position −1 and proline in position +1 is observed on nuclear proteins, whereas cytoplasmic proteins are enriched with glutamate in the vicinity of the acetylation site (Lundby et al., 2012). In P. falciparum, the acetylation motifs in histones are better defined and a GK motif is highly conserved (Fig. 7A). However, at +1 position T is the most commonly found, compared to the most common residues of A and S in human (Choudhary et al., 2009) and Toxoplasma (Jeffers and Sullivan, 2012) histones, respectively. Consistently in Plasmodium there is a relative enrichment of lysines in the flanking sequences, but other motifs are less clearly defined. Whereas Y at +1 position is conserved in cytosolic and nuclear proteins, S instead of F is the most common at −2 position.
Fig. 7. Acetylation motifs and conservation of acetylation sites.
(A) Relative abundance of each amino acid residues surrounding sites of acetylated lysine in all acetylated proteins, cytosolic proteins, nuclear proteins and histones. (B) Lysine conservation of P. falciparum acetylated lysines in other pathogen species. Acetylated lysines are significantly more conserved than non-acetylated lysines.
Evolutionarily conserved acetylation sites may indicate functional significance of acetylation on protein functions. To determine whether acetylation sites are evolutionarily conserved, we compared the degree of conservation in acetylated versus non-acetylated lysine residues across 32 protozoan species and microsporidia. Orthologs of acetylated Plasmodium proteins were retrieved from genome sequences of EupathDB using BLASTP, and the conservation of acetylated lysines was determined by sequence alignment. The results showed that acetylated lysines in Plasmodium are significantly more conserved comparing to non-acetylated lysines across a wide range of protozoan parasites and microsporidia (Fig. 7B).
Conservation of acetylation in the Apicomplexa
Due to the close phylogenetic relationship between Plasmodium and Toxoplasma, we performed a qualitative comparison of the acetylome data obtained to date for each parasite. In each parasite, the functional breakdown of acetylated proteins was similar, with 71 proteins detected as acetylated in both species (Table 1). Among some of the commonalities, histone acetylation, along with the acetylation of KATs and components of KAT complexes, are shared features between the parasite acetylomes. Moreover, the acetylation marks largely appear to cluster within the same regions of both P. falciparum and T. gondii KAT homologues (Fig. S4), suggesting that lysine acetylation may regulate the formation and function of these acetylation complexes. Consistent with this idea, acetylation marks are enriched in the ADA2-interaction domain of both species’ GCN5 KATs. PfMYST and the two MYSTs in T. gondii are heavily acetylated within the N-terminal extension upstream from the KAT domain, a region in which the function has yet to be defined. Acetylation of K428 within the KAT domain of PfMYST is an example of an acetyl mark that appears conserved across eukaryotes. The equivalent lysine is acetylated in TgMYST-A as well as in yeast and human MYST proteins; this particular PTM has been demonstrated to regulate nucleosome interaction and acetylation (Sun et al., 2011; Yuan et al., 2012). Also noteworthy is that acetylated proteins exist within all cellular compartments beyond the nucleus, including the mitochondrion and apicoplast, although the types of proteins detected as acetylated within these compartments were not absolutely conserved. Phosphoglycerate kinase was found to be acetylated in each parasite’s apicoplast, and four mitochondrial proteins were acetylated in both species (fumarase, isocitrate dehydrogenase, Hsp60, and Hsp70). AP2 factors were found to be acetylated in both species, but it is not possible at present to judge if the AP2s in question are orthologous. Other common features can be identified with respect to metabolic proteins, including components of translation. For example, three amino-acyl tRNA transferases are acetylated in both apicomplexan parasites, but the lysines targeted are not the same. Acetylation of ribosomal proteins is common and includes three acetyl-sites on homologous residues. Several orthologous heat shock proteins are acetylated in both parasites, with two of these acetyl marks being absolutely conserved. A large number of metabolic enzymes were detected as acetylated in both Plasmodium and Toxoplasma, with thirteen proteins common to both acetylomes and six of the acetylated lysines being homologous. Finally, there are a number of hypothetical proteins with no known function conserved in both parasites that are commonly subject to acetylation (Table 1).
Table 1.
Acetylated proteins shared between Plasmodium falciparum (Pf3D7) and Toxoplasma gondii.
| Pf3D7 ID | Protein Name | T. gondii ID | Protein Name |
|---|---|---|---|
| MAL8P1.69 | 14-3-3 protein, putative | TGME49_063090 | 14-3-3 protein, putative |
| MAL8P1.142 | 20S proteasome beta subunit | TGME49_080710 | proteasome A-type and B-type domain-containing protein |
| MAL7P1.300 | 40S ribosomal protein S29, putative | TGME49_042340 | 40S ribosomal protein S29, putative |
| PFC1020c | 40S ribosomal protein S3A, putative | TGME49_032710 | 40S ribosomal protein S3a, putative |
| PF14_0141 | 60S ribosomal protein L10, putative | TGME49_088720 | 60S ribosomal protein L10, putative |
| PFF0885w | 60S ribosomal protein L27a, putative | TGME49_110490 | ribosomal protein L22, putative |
| PF10_0272 | 60S ribosomal protein L3, putative | TGME49_027360 | 60S ribosomal protein L3, putative |
| PF13_0346 | 60S ribosomal protein L40/UBI, putative | TGME49_089750 | ubiquitin / ribosomal protein CEP52 fusion protein, putative |
| PFI0755c | 6-phosphofructokinase (PFK9) | TGME49_026960 | phosphofructokinase, putative |
| PFF1350c | acetyl-CoA synthetase, putative | TGME49_066640 | acetyl-coenzyme A synthetase, putative |
| PF13_0131 | acetyltransferase, GNAT family, putative | TGME49_030060 | acetyltransferase domain-containing protein |
| PFL2215w | actin I (ACT1) | TGME49_009030 | actin |
| PF11_0197 | acyl-CoA-binding protein, putative | TGME49_034510 | acyl-CoA-binding protein, putative |
| PF14_0241 | basic transcription factor 3b, putative | TGME49_057090 | NAC domain containing protein |
| PFL0635c | bromodomain protein, putative | TGME49_064640 | bromodomain domain-containing protein |
| PF14_0510 | co-chaperone p23 (P23) | TGME49_121520 | P23 co-chaperone, putative |
| PFF0835w | conserved Plasmodium protein | TGME49_032440 | hypothetical protein |
| MAL8P1.95 | conserved Plasmodium protein | TGME49_060440 | 46 kDa FK506-binding nuclear protein, putative |
| PFL0640w | conserved Plasmodium protein | TGME49_008800 | hypothetical protein, conserved |
| PFF1295w | conserved protein, unknown function | TGME49_115250 | melanocyte proliferating gene 1, putative |
| PFC0170c | dihydrolipoamide acyltransferase, putative | TGME49_119920 | dihydrolipoamide branched chain transacylase, E2 subunit, putative |
| PF13_0305 | elongation factor 1-alpha | TGME49_086420; TGME49_094800 | elongation factor 1-alpha, putative; elongation factor 1-alpha, putative |
| PFI0645w | elongation factor 1-beta (EF-1beta) | TGME49_026410 | elongation factor 1-beta, putative |
| PF14_0486 | elongation factor 2 | TGME49_005470 | elongation factor 2, putative |
| PF10_0155 | enolase (ENO) | TGME49_068850 | enolase 2 |
| PF14_0655 | eukaryotic initiation factor 4A (eIF4A) | TGME49_050770 | eukaryotic translation initiation factor 4A |
| PF08_0071 | Fe-superoxide dismutase (FeSOD) | TGME49_116310 | superoxide dismutase |
| PF14_0425 | fructose-bisphosphate aldolase | TGME49_036040 | fructose-1,6-bisphosphate aldolase |
| PFI1340w | fumarate hydratase, putative | TGME49_067330 | fumarase, putative (Mitochondrial localization) |
| PF14_0511 | glucose-6-phosphate dehydrogenase-6- phosphogluconolactonase (G6PDH) | TGME49_078830 | glucose-6-phosphate dehydrogenase, putative |
| PF08_0132 | glutamate dehydrogenase, putative (GDHc) | TGME49_049390 | NAD-specific glutamate dehydrogenase, putative |
| PF13_0257 | glutamate--tRNA ligase, putative | TGME49_063870 | glutamyl-tRNA synthetase, putative |
| PF14_0598 | glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | TGME49_089690; TGME49_069190 | glyceraldehyde-3-phosphate dehydrogenase |
| PF11_0183 | GTP-binding nuclear protein ran/tc4 (RAN) | TGME49_048340 | GTP-binding nuclear protein RAN/TC4 |
| PF10_0153 | heat shock protein 60 (HSP60) | TGME49_047550 | heat shock protein 60 (Mitochondrial localization) |
| PF08_0054 | heat shock protein 70 (Hsp70) | TGME49_073760 | heat shock protein 70, putative (Mitochondrial localization) |
| PFI0875w | heat shock protein 70 (Hsp70-2) | TGME49_111720 | heat shock protein 70, putative |
| PF07_0029 | heat shock protein 90 (HSP90) | TGME49_088380 | heat shock protein 90 |
| PFF1155w | hexokinase (HK) | TGME49_065450 | hexokinase |
| PFL0145c | high mobility group protein (HMGB1) | TGME49_010410 | high mobility group protein |
| MAL8P1.72 | high mobility group protein (HMGB2) | TGME49_010410 | high mobility group protein |
| PF08_0034 | histone acetyltransferase GCN5 (GCN5) | TGME49_043440 | histone acetyltransferase GCN5, putative |
| PF11_0192 | histone acetyltransferase, putative (MYST) | TGME49_118330; TGME49_007080 | MYST-family histone acetyltransferase-A; histone acetyltransferase |
| PFC0920w | histone H2A variant, putative (H2A.Z) | TGME49_100200 | histone H2A |
| PF07_0054 | histone H2B variant, putative (H2Bv) | TGME49_009910; TGME49_105160; TGME49_051870 | histone H2B variant 1; histone H2B, putative; histone H2B, putative |
| PFF0510w | histone H3 (H3) | TGME49_061240 | histone H3 |
| PFF0865w | histone H3 variant, putative (H3.3) | TGME49_018260 | histone H3.3 variant |
| PF11_0061 | histone H4 (H4) | TGME49_039260 | histone H4, putative |
| PFE1370w | hsp70 interacting protein, putative | TGME49_032660 | 58 kDa phosphoprotein, putative |
| PF14_0324 | Hsp70/Hsp90 organizing protein (HOP) | TGME49_052220 | Hsc70/Hsp90-organizing protein, putative |
| PF11_0189 | insulinase, putative | TGME49_014490 | M16 family peptidase, putative |
| PF13_0242 | isocitrate dehydrogenase (NADP), mitochondrial precursor (IDH) | TGME49_113140 | isocitrate dehydrogenase, putative (Mitochondrial localization) |
| PF13_0179 | isoleucine-tRNA ligase, putative | TGME49_007640 | isoleucine-tRNA synthetase, putative |
| PF13_0141 | L-lactate dehydrogenase (LDH) | TGME49_032350 | lactate dehydrogenase |
| PF14_0439 | M17 leucyl aminopeptidase (LAP) | TGME49_090670 | cytosol aminopeptidase |
| PF10_0036 | N-acetyltransferase, putative | TGME49_019760 | N-terminal acetyltransferase complex subunit ARD1, putative |
| PFE0630c | orotate phosphoribosyltransferase (OPRT) | TGME49_059660 | orotate phosphoribosyltransferase, putative |
| PF13_0234 | phosphoenolpyruvate carboxykinase (PEPCK) | TGME49_089650 | phosphoenolpyruvate carboxykinase |
| PFI1105w | phosphoglycerate kinase (PGK) | TGME49_022020; TGME49_118230 | phosphoglycerate kinase, putative; phosphoglycerate kinase, putative (Apicoplast localization) |
| PF11_0208 | phosphoglycerate mutase, putative (PGM1) | TGME49_097060 | phosphoglycerate mutase 1, putative |
| PFL1170w | polyadenylate-binding protein, putative (PABP) | TGME49_024850 | polyadenylate-binding protein, putative |
| PFF0320c | polypyrimidine tract binding protein, putative | TGME49_090660 | polypyrimidine track-binding protein, putative |
| PFF1025c | pyridoxine biosynthesis protein PDX1 (PDX1) | TGME49_037140 | ethylene inducible protein, putative |
| PFF1300w | pyruvate kinase (PyrK) | TGME49_056760 | pyruvate kinase, putative |
| PF08_0019 | receptor for activated c kinase (RACK) | TGME49_016880 | receptor for activated C kinase, RACK protein, putative |
| PFL1720w | serine hydroxymethyltransferase (SHMT) | TGME49_034190 | glycine hydroxymethyltransferase, putative |
| PF07_0073 | seryl-tRNA synthetase, putative | TGME49_051690 | seryl-tRNA synthetase, putative |
| PF10_0266 | small subunit rRNA processing stabilizing factor, putative | TGME49_111410 | U3 small nucleolar ribonucleoprotein protein MPP10, putative |
| PFF1185w | SNF2 helicase, putative (ISWI) | TGME49_036970 | SNF2 family N-terminal domain containing protein |
| PF11_0331 | TCP-1/cpn60 chaperonin family, putative | TGME49_029990 | TCP-1/cpn60 family chaperonin, putative |
| PF10_0143 | transcriptional coactivator ADA2 (ADA2) | TGME49_017050 | transcriptional co-activator ADA2-A |
Discussion
We have screened acetylated proteins during Plasmodium IDC and identified 230 lysine-acetylated proteins with 421 acetylation sites. These acetylated proteins belong to diverse functional groups, including those that perform evolutionarily conserved functions as well as Plasmodium specific. Compared with acetylome from bacteria, yeast, Drosophila, rat and human cells, the number of protein lysine acetylation detected in Plasmodium, as well as in another apicomplexan parasite, T. gondii (with 753 lysine acetylation sites in 486 proteins in tachyzoites), is more similar to those in prokaryotes than in metazoans (Jeffers and Sullivan, 2012). A more detailed comparison revealed some interesting parallels as well as dramatic differences between the Plasmodium and Toxoplasma acetylomes. The unconserved features of each parasite acetylome may be due to differences in technique or current coverage, but may also reflect adaptations of the parasites to selective pressures from different living environments, suggesting adaptive regulation of parasite-specific pathways by acetylation. The similarity in the types of proteins that are acetylated, or in some cases even the lysine residue targeted for acetylation, likely underscores a vital biological role for this modification across Apicomplexa.
Overall, the acetylation motifs in most organisms studied so far are not well defined. This, in turn, limits the power of algorithms for proteome-wide prediction of acetylation substrates (Basu et al., 2009). Furthermore, the acetylation motifs appear to differ between proteins of different cellular compartments (Lundby et al., 2012). This is likely due to differential distributions of various KATs, which may have different sequence preferences. Indeed, prediction algorithms based on the preferred sequence features of different KAT families improve the power of prediction (Li et al., 2012). In Plasmodium, the cytosolic and nuclear acetylomes also show tremendous differences in the acetylation motifs, and this distinction is likely attributed to the different subcellular localizations of the KATs. In particular, PfMYST is abundantly present in both cytosol and nucleus, whereas PfGCN5 is restricted to the nucleus (Miao et al., 2010). Similar localization patterns have been observed for these KATs in T. gondii. Except histones, the substrate targets and sequence preferences of these KATs are not known. Therefore, future studies aimed at identifying the substrates of individual KATs are needed to elucidate the functional division of KATs.
Lysine acetylation, which uses acetyl-CoA, is tightly linked to the cellular metabolic status and energy flux. It is thus not surprising that lysine acetylation of key enzymes in the central carbon metabolic pathways is evolutionarily conserved from bacteria to human (Kim et al., 2006; Zhang et al., 2009; Wang et al., 2010; Zhao et al., 2010). Accordingly, concentrations of metabolic fuels directly regulate the acetylation status and activities of metabolic enzymes (Wang et al., 2010; Zhao et al., 2010). Consistent with the essential role of mitochondrion in energy metabolism, a large number of mitochondrial proteins are found acetylated in other species (Kim et al., 2006; Choudhary et al., 2009; Zhao et al., 2010; Lundby et al., 2012). Similarly, enzymes of various metabolic pathways make up the most abundant group of acetylated proteins in the Plasmodium cytoplasmic acetylome. However, the central carbon metabolism pathways in Plasmodium are very divergent from the canonical pathways. The blood stage malaria parasites, living in a glucose-rich environment, rely primarily on glucose fermentation with the formation of lactate rather than oxidative phosphorylation for its energy need. Likewise, protein lysine acetylation is highly abundant in the glycolysis pathway but not in the TCA cycle, and there is a relative dearth of acetylated proteins in Plasmodium mitochondrion. It is also noteworthy that proteins of many pathways unique to the malaria parasite, such as purine salvage and phospholipid biosynthesis, are also abundantly acetylated. Currently, many of these pathways are actively pursued for the development of novel antimalarial drugs.
Histone acetylation is an important epigenetic mark for euchromatin in gene regulation in malaria parasites (Salcedo-Amaya et al., 2009). In addition to histone acetylation, this study identified a large number of proteins potentially involved in chromatin biology and transcription. The two KATs PfGCN5 and PfMYST play essential roles in gene expression, cell cycle progression, and DNA repair (Cui et al., 2007; Miao et al., 2010). These two KATs are among the most heavily acetylated proteins in the nuclear acetylome. In addition, manipulation of the acetylation levels of PfMYST resulted in reduced HAT activity of this enzyme on histone H4. Moreover, other members of the PfGCN5 complex, including PfADA2 and two PHD-domain proteins, are also heavily acetylated. This study identified the highest level of acetylation in a putative KAT complex.
Proteins are subjected to a wide array of posttranslational modifications and cross-talk is evident among these modifications (Yang and Seto, 2008; Soufi et al., 2012). The same proteins could bear different modifications with potentially different functional consequences. For example, of the 230 proteins identified in our acetylome, 133 (58%) were also detected in a screen for phosphoproteins (Treeck et al., 2011). In addition, 17 of the 73 ubiquitylated proteins identified in P. falciparum were identified in our acetylome (Ponts et al., 2008). Similarly, 29 of the 120 sumoylated proteins were also found to be acetylated in T. gondii (Jeffers and Sullivan, 2012). This contrasts with only one protein found to be both acetylated and sumoylated in P. falciparum, probably due to the small number of sumoylated proteins (23) identified in this parasite (Lopez-Rubio et al., 2007). Since ubiquitylation, sumoylation, and acetylation all modify lysine residues, the same lysine may be opted for one of these modifications, which will have drastically different influences on the functions of the proteins (Yang and Seto, 2008; Denuc and Marfany, 2010).
In conclusion, the Plasmodium parasites have evolved an extensive protein lysine acetylation network. Yet, the dataset reported here and most comparisons are only qualitative rather than quantitative. The biological consequence of protein lysine acetylation in these parasites still awaits future characterization.
Experimental procedures
Parasite Culture
P. falciparum 3D7 was cultured as described previously (Trager and Jensen, 1976). Synchronization of asexual stages was performed by two rounds of sorbitol treatment at the ring stage. For the time course studies, schizonts were purified by Percoll gradient centrifugation (Miao and Cui, 2011) and mixed with fresh RBCs. Parasites were harvested at 10, 20, 30, and 40 h later to represent ring, early trophozoite, late trophozoite and schizont stages, respectively.
Manipulation of HAT and HDAC Activities
To determine the effect of manipulation of KAT activities on overall acetylation of parasite proteins, we compared protein acetylation among three parasite lines: one with overexpression of a full-length active PfMYST (F1-GFP), on with overexpression of a truncated inactive version of PfMYST (F1C3-GFP) missing 81 amino acids in the C-terminus, and the GFP-control line (Miao et al., 2010). The transgenes were controlled by the hsp86 promoter and a single copy of the expression cassette was integrated at the attB locus of the 3D7attB strain (Miao et al., 2010). Parasites were cultured and harvested at 30 h post invasion. To determine the effect of HDAC inhibitors on protein lysine acetylation in the parasites, 3D7 parasites at ring stage (15 h post invasion) were treated with TSA at 1× to 4 × IC50 of TSA (16 to 64 nM) and nicotinamide at 5, 10 and 15 mM, which were determined earlier for P. falciparum in vitro culture (Prusty et al., 2008; Patel et al., 2009). After incubation for 15 h, parasites were harvested and protein lysates were prepared and used for Western analysis. To alter the levels of acetylation in PfMYST and PfGCN5, parasite lines with GFP-tagged endogenous PfMYST and PfGCN5 were cultured in the presence or absence of 16 nM TSA plus 5 mM nicotinamide for 15 h. Parasites were then harvested and GFP-tagged proteins were purified as described below. The HAT activities were measured by a previously described method using bovine core histones and nucleosomal histones as the substrates (Miao et al., 2010).
Preparation of Protein Lysates
Parasites at the trophozoite stage were treated with 0.05% saponin to lyse the RBCs. Released parasites were pelleted by centrifugation and washed twice with cold phosphate-buffered saline (PBS). To prepare the cytoplasmic and nuclear fractions, parasite pellet was resuspended in 5 volumes of buffer A [10 mM Hepes, pH 7.8, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), 0.5 mM EDTA and protease inhibitor cocktail], incubated on ice for 15 min to allow cells to swell, and ground for 40 times using a Dounce homogenizer with a loose pestle. The homogenate was first centrifuged at 700 g for 20 min at 4°C, and the supernatant was centrifuged at 10,000 g for 10 min to obtain the cytoplasmic extract. The pellet was resuspended in 3 volumes of buffer B (20 mM Hepes, pH 7.8, 20% glycerol, 200 mM KCl, 0.5 mM DTT, 0.5 mM EDTA, 0.5% NP40 and protease inhibitor cocktail) and dounced for 40 times with a tight pestle. The homogenate was centrifuged at 10,000 g for 10 min to obtain the nuclear extract.
Preparation of Acetylated Peptides
Proteins in the cytoplasmic and nuclear lysates were precipitated by adding four volumes of ice-cold acetone. Precipitated proteins were re-dissolved in 6 M urea/2 M thiourea/10 mM Hepes, pH 8.0, reduced by 1 mM DTT, alkylated with 5.5 mM idoacetamide, and digested with endoproteinase Lys-C (1:100) first and trypsin (1:100) after 4-fold dilution in distilled water. The resulting peptides were purified using Sep-Pak C18 peptide purification cartridges (Waters Corporation, Milford, MA) and eluted in 50% acetonitrile. The eluates were dried and redissolved in an IP buffer (50 mM MOPS, pH 7.2, 10 mM sodium phosphate, 50 mM NaCl and 0.5% NP40), and incubated with agarose-conjugated anti-acetyllysine antibodies (ImmuneChem, Burnaby, British Columbia, Canada) overnight at 4°C. The peptide-bound agarose resin was washed four times with the IP buffer and twice with distilled water. The acetylated peptides bound to the agarose resin were eluted by 0.1 % TFA and the final eluates were dried to 5–10 μl using a speedvac.
MS Analysis and Data Processing
The purified acetylated peptide mixture was separated using a two-dimensional (2D) nano liquid chromatography (LC) system (Eksigent Technologies, Dublin, CA) with an Agilent Zorbax SB300-C8 trap and eluted by a reverse phase gradient onto a 0.075 mm × 120 mm emitter packed in-house with the Magic C18 material (5 μm, 300 A pore) (Michrom Bioresources, Auburn, CA). Mobile phase solution consisted of a water and 0.1% formic acid aqueous phase and a 0.1% formic acid in 50% acetonitrile:ethanol organic phase. The gradient ran from 1 to 60 min and from 10 to 35% organic phase with a 95% wash and eluted into a Thermo Finnigan LTQ mass spectrometer. In these LC/MS experiments, a full MS scan was acquired in parallel with data dependent MS/MS scans of the top five most abundant m/z peaks. MS/MS was performed with wideband activation, dynamic exclusion of 1 for 60 sec with a list of 300 m/z and a width of +/− 1.5/0.5 m/z, collision energy of 35%, and noise level of 3000 NL. The peak list was generated using Xcalibur (2.0.5) (Thermo Scientific). The LC/MS/MS data were searched with Bioworks 3.0 (Thermo Scientific) against a P. falciparum 3D7 database (3D7.version.2.0, P. falciparum Genome Sequencing Consortium at http://www.sanger.ac.uk/resources/downloads/protozoa/plasmodium-falciparum.html) with reversed protein decoys. Search parameters included trypsin digestion (C-terminal K and R cleavage) full cleavage with 4 missed sites (non-specific cleavages not permitted), amino acid length of 6 to 100 with tolerance of 1.4 Da, dynamic modifications of methionine methylation (+14 Da), cysteine carboxyamidomethylation (+57 Da) and lysine or arginine acetylation (+42 Da). Mass tolerance for fragment ions is +/− 0.5 m/z amu (atomic mass unit). Peptides were filtered by a Ranked Preliminary Score of 1, probability of 1e-3, and minimum 20% theoretical ions observed. The resulting dataset was searched against the human protein database to filter out potentially contaminating human proteins. In addition, since acetylation hinders trypsin digestion after the acetylated lysine residue, the dataset was further filtered to include peptides with only internally acetylated lysines. Finally, peptides of interest were independently identified manually. The raw spectrum of each acetylated peptide is available at http://enot.psu.edu/research/labs/liwang-cui.
IP and Western Blots
IPs were performed to verify the acetylation of individual proteins. GFP monoclonal antibodies (Roche Diagnostics, Indianapolis, IN, USA) were used to pull down respective proteins from transgenic parasite lines carrying GFP-tagged PfGCN5 (Miao and Cui, 2011), PfMYST (Miao et al., 2010), and PFL1210w (Pfapi-IRS) (Istvan et al., 2011). Polyclonal antibodies against PfEF-1β (Mamoun and Goldberg, 2001) and PfPMT (Pessi et al., 2004) were used to pull down respective proteins in 3D7 parasites. For IP, ~5 × 109 trophozoites were harvested and lysed in five volumes of PA150 buffer (150 mM KCl, 20 mM Tris-HCl, pH 7.7, 3 mM MgCl2, 0.5 mM DTT, 0.1% Tween 20) containing a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). The lysate was centrifuged for 10 min at 20,000 × g and the supernatant was incubated with 5 μg of monoclonal anti-GFP antibody (Roche) or 30 μl of rabbit anti-EF-1β and PfPMT antisera on ice for 1 h with occasional mixing. Protein A agarose (30 μl packed bead volume) was added to the lysate and incubated for 1 h. The agarose beads were washed four times with PA150, and bound proteins were either eluted with elution buffer for analysis of activities or resuspended in SDS-PAGE loading buffer for immunoblotting. Eluted proteins in the loading buffer and parasites samples collected from the four time points during IDC were separated in a 12% SDS-PAGE gel. Proteins were transferred to a nitrocellulose membrane and probed with anti-acetyllysine antibodies (ImmuneChem) as primary antibodies in the presence or absence of acetylated BSA (Kim et al., 2006). Secondary horseradish peroxidase-conjugated antibodies were used at 1:3000. Proteins were visualized using an ECL Kit (Life Technologies, Grand Island, NY, USA).
Bioinformatic Analysis
Analysis of the amino acid sequences surrounding the acetylation sites was performed using WebLogo (Crooks et al., 2004). Acetylated proteins were classified according to the gene ontology (GO) annotation in PlasmoDB 9.0 (www.plasmodb.org) (Bahl et al., 2003) to include molecular function and cellular localization data. Domain structures of certain proteins were extracted from PlasmoDB 9.0. To determine the degree of evolutionary conservation of acetylation, we firstly used BLASTP to compare acetylated protein sequences of P. falciparum 3D7 against all protein sequences in EupathDB (www.eupathdb.org), which includes 6 Plasmodium species, 3 Amoeba species, 3 Cryptosporidium species, 3 Giardia species, 5 microsporidia species, 3 Piroplasma species, 5 Toxoplasma strains and 4 species. By applying a reciprocal best BLAST hit approach, we determined the orthologous proteins among these genomes. For each orthologous group, we used MUSCLE v3.8.31 to do multiple sequence alignment (Edgar, 2004). Then we determined the lysine conservation for each species by counting the total number of conserved acetylated lysines and the total number of conserved non-acetylated lysines. A lysine residue was considered to be conserved if both the P. falciparum 3D7 protein and the query protein in the multiple sequence alignment are lysine residues at the aligned position. All lysine residues of the proteins identified in this study were considered as control. Mean conservation of the acetylated and control lysines between P. falciparum sequences and sequences from other microorganisms in the EupathDB were plotted separately. P-values were calculated for each comparison using Fisher’s exact test. To determine the level of conservation of acetylation in the glycolysis pathway, sequences of 10 acetylated enzymes found in the survey were aligned using Clustal W with their known acetylated orthologs in nine organisms (http://www.ebi.ac.uk/Tools/msa/clustalo/). Acetylation in P. falciparum was considered conserved if the acetyllysine was also found in one of the orthologs at the aligned position.
Statistical Analysis
Statistical tests for potential over- and under-representation of protein functional groups were conducted using the hypergeometric distribution (Khatri and Draghici, 2005). The significances of lysine conservation were analyzed by Fisher’s exact test for each species separately using the R software.
Supplementary Material
Acknowledgments
We are grateful to Dr. Daniel E. Goldberg for providing the tagged parasite line for the apicoplast isoleucyl-rRNA synthetase, and Dr. Choukri Ben Mamoun for the polyclonal antibodies against elongation factor 1 β (EF-1β) and phosphatidylethanolamine N-methyltransferase (PfPMT). This work was supported by NIH grants (AI064553 to LC, AI077502 to WJS).
Abbreviations
- 2D
two-dimensional
- DTT
dithiothreitol
- HDAC
histone deacetylase
- IDC
intraerythrocytic developmental cycle
- KAT
lysine acetyltransferase
- KDAC
lysine deacetylase
- LC
liquid chromatography
- MS
mass spectrometry
- PfPMT
phosphatidylethanolamine N-methyltransferase
- PK
Protein kinases
- PTEX
Plasmodium Translocon of EXported proteins
- RBC
red blood cell
- TCA
tricarboxylic cycle
- TSA
trichostatin A
Footnotes
The authors declare that they have no conflict of interest.
References
- Allfrey VG, Faulkner R, Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A. 1964;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas K, Misaghi S, Comeaux CA, Catic A, Spooner E, Duraisingh MT, Ploegh HL. Identification by functional proteomics of a deubiquitinating/deNeddylating enzyme in Plasmodium falciparum. Mol Microbiol. 2006;61:1187–1195. doi: 10.1111/j.1365-2958.2006.05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl A, Brunk B, Crabtree J, Fraunholz MJ, Gajria B, Grant GR, Ginsburg H, Gupta D, Kissinger JC, Labo P, Li L, Mailman MD, Milgram AJ, Pearson DS, Roos DS, Schug J, Stoeckert CJ, Jr, Whetzel P. PlasmoDB: the Plasmodium genome resource. A database integrating experimental and computational data. Nucleic Acids Res. 2003;31:212–215. doi: 10.1093/nar/gkg081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Rose KL, Zhang J, Beavis RC, Ueberheide B, Garcia BA, Chait B, Zhao Y, Hunt DF, Segal E, Allis CD, Hake SB. Proteome-wide prediction of acetylation substrates. Proc Natl Acad Sci U S A. 2009;106:13785–13790. doi: 10.1073/pnas.0906801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracchi-Ricard V, Moe D, Chakrabarti D. Two Plasmodium falciparum ribonucleotide reductase small subunits, PfR2 and PfR4, interact with each other and are components of the in vivo enzyme complex. J Mol Biol. 2005;347:749–758. doi: 10.1016/j.jmb.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Cassera MB, Zhang Y, Hazleton KZ, Schramm VL. Purine and Pyrimidine Pathways as Targets in Plasmodium falciparum. Curr Top Med Chem. 2011;11:2103–2115. doi: 10.2174/156802611796575948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Miao J, Furuya T, Li X, Su XZ, Cui L. PfGCN5-mediated histone H3 acetylation plays a key role in gene expression in Plasmodium falciparum. Eukaryot Cell. 2007;6:1219–1227. doi: 10.1128/EC.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning-Ward TF, Gilson PR, Boddey JA, Rug M, Smith BJ, Papenfuss AT, Sanders PR, Lundie RJ, Maier AG, Cowman AF, Crabb BS. A newly discovered protein export machine in malaria parasites. Nature. 2009;459:945–949. doi: 10.1038/nature08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denuc A, Marfany G. SUMO and ubiquitin paths converge. Biochem Soc Trans. 2010;38:34–39. doi: 10.1042/BST0380034. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Q, An L, Cui L. PfADA2, a Plasmodium falciparum homologue of the transcriptional coactivator ADA2 and its in vivo association with the histone acetyltransferase PfGCN5. Gene. 2004a;336:251–261. doi: 10.1016/j.gene.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Fan Q, An L, Cui L. Plasmodium falciparum histone acetyltransferase, a yeast GCN5 homologue involved in chromatin remodeling. Eukaryot Cell. 2004b;3:264–276. doi: 10.1128/EC.3.2.264-276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkemeier I, Laxa M, Miguet L, Howden AJ, Sweetlove LJ. Proteins of diverse function and subcellular location are lysine acetylated in Arabidopsis. Plant Physiol. 2011;155:1779–1790. doi: 10.1104/pp.110.171595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foth BJ, Zhang N, Chaal BK, Sze SK, Preiser PR, Bozdech Z. Quantitative Time-course Profiling of Parasite and Host Cell Proteins in the Human Malaria Parasite Plasmodium falciparum. Mol Cell Proteomics. 2011;10:M110 006411. doi: 10.1074/mcp.M110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen P, Wagner SA, Weinert BT, Sharma S, Bacinskaja G, Rehman M, Juffer AH, Walther TC, Lisby M, Choudhary C. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol Cell Proteomics. 2012 doi: 10.1074/mcp.M112.017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D, McNamara CW, Lim CS, Studer C, Riedl R, Aust T, McCormack SL, Plouffe DM, Meister S, Schuierer S, Plikat U, Hartmann N, Staedtler F, Cotesta S, Schmitt EK, Petersen F, Supek F, Glynne RJ, Tallarico JA, Porter JA, Fishman MC, Bodenreider C, Diagana TT, Movva NR, Winzeler EA. Selective and specific inhibition of the plasmodium falciparum lysyl-tRNA synthetase by the fungal secondary metabolite cladosporin. Cell Host Microbe. 2012;11:654–663. doi: 10.1016/j.chom.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrocks P, Wong E, Russell K, Emes RD. Control of gene expression in Plasmodium falciparum - ten years on. Mol Biochem Parasitol. 2009;164:9–25. doi: 10.1016/j.molbiopara.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Istvan ES, Dharia NV, Bopp SE, Gluzman I, Winzeler EA, Goldberg DE. Validation of isoleucine utilization targets in Plasmodium falciparum. Proc Natl Acad Sci U S A. 2011;108:1627–1632. doi: 10.1073/pnas.1011560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffers V, Sullivan WJ., Jr Lysine acetylation is widespread on proteins of diverse function and localization in the protozoan parasite Toxoplasma gondii. Eukaryot Cell. 2012;11:735–742. doi: 10.1128/EC.00088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P, Draghici S. Ontological analysis of gene expression data: current tools, limitations, and open problems. Bioinformatics. 2005;21:3587–3595. doi: 10.1093/bioinformatics/bti565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Sprung R, Chen Y, Xu Y, Ball H, Pei J, Cheng T, Kho Y, Xiao H, Xiao L, Grishin NV, White M, Yang XJ, Zhao Y. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Le Roch KG, Zhou Y, Blair PL, Grainger M, Moch JK, Haynes JD, De La Vega P, Holder AA, Batalov S, Carucci DJ, Winzeler EA. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- Leiva M, Moretti S, Soilihi H, Pallavicini I, Peres L, Mercurio C, Dal Zuffo R, Minucci S, de The H. Valproic acid induces differentiation and transient tumor regression, but spares leukemia-initiating activity in mouse models of APL. Leukemia. 2012;26:1630–1637. doi: 10.1038/leu.2012.39. [DOI] [PubMed] [Google Scholar]
- Li T, Du Y, Wang L, Huang L, Li W, Lu M, Zhang X, Zhu WG. Characterization and prediction of lysine (K)-acetyl-transferase specific acetylation sites. Mol Cell Proteomics. 2012;11:M111 011080. doi: 10.1074/mcp.M111.011080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubio JJ, Gontijo AM, Nunes MC, Issar N, Hernandez Rivas R, Scherf A. 5′ flanking region of var genes nucleate histone modification patterns linked to phenotypic inheritance of virulence traits in malaria parasites. Mol Microbiol. 2007;66:1296–1305. doi: 10.1111/j.1365-2958.2007.06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luah YH, Chaal BK, Ong EZ, Bozdech Z. A moonlighting function of Plasmodium falciparum histone 3, mono-methylated at lysine 9? PLoS One. 2010;5:e10252. doi: 10.1371/journal.pone.0010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A, Lage K, Weinert BT, Bekker-Jensen DB, Secher A, Skovgaard T, Kelstrup CD, Dmytriyev A, Choudhary C, Lundby C, Olsen JV. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Rep. 2012;2:419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamoun CB, Goldberg DE. Plasmodium protein phosphatase 2C dephosphorylates translation elongation factor 1beta and inhibits its PKC-mediated nucleotide exchange activity in vitro. Mol Microbiol. 2001;39:973–981. doi: 10.1046/j.1365-2958.2001.02289.x. [DOI] [PubMed] [Google Scholar]
- Maurer-Stroh S, Dickens NJ, Hughes-Davies L, Kouzarides T, Eisenhaber F, Ponting CP. The Tudor domain ‘Royal Family’: Tudor, plant Agenet, Chromo, PWWP and MBT domains. Trends Biochem Sci. 2003;28:69–74. doi: 10.1016/S0968-0004(03)00004-5. [DOI] [PubMed] [Google Scholar]
- Merrick CJ, Duraisingh MT. Epigenetics in Plasmodium: what do we really know? Eukaryot Cell. 2010;9:1150–1158. doi: 10.1128/EC.00093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Fan Q, Cui L, Li J. The malaria parasite Plasmodium falciparum histones: organization, expression, and acetylation. Gene. 2006;369:53–65. doi: 10.1016/j.gene.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Miao J, Fan Q, Cui L, Li X, Wang H, Ning G, Reese JC. The MYST family histone acetyltransferase regulates gene expression and cell cycle in malaria parasite Plasmodium falciparum. Mol Microbiol. 2010;78:883–902. doi: 10.1111/j.1365-2958.2010.07371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Cui L. Rapid isolation of single malaria parasite-infected red blood cells by cell sorting. Nat Protoc. 2011;6:140–146. doi: 10.1038/nprot.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski KL, Llinas M. Central carbon metabolism of Plasmodium parasites. Mol Biochem Parasitol. 2011;175:95–103. doi: 10.1016/j.molbiopara.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Mazitschek R, Coleman B, Nguyen C, Urgaonkar S, Cortese J, Barker RH, Greenberg E, Tang W, Bradner JE, Schreiber SL, Duraisingh MT, Wirth DF, Clardy J. Identification and characterization of small molecule inhibitors of a class I histone deacetylase from Plasmodium falciparum. J Med Chem. 2009;52:2185–2187. doi: 10.1021/jm801654y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessi G, Kociubinski G, Mamoun CB. A pathway for phosphatidylcholine biosynthesis in Plasmodium falciparum involving phosphoethanolamine methylation. Proc Natl Acad Sci U S A. 2004;101:6206–6211. doi: 10.1073/pnas.0307742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponts N, Yang J, Chung DW, Prudhomme J, Girke T, Horrocks P, Le Roch KG. Deciphering the ubiquitin-mediated pathway in apicomplexan parasites: a potential strategy to interfere with parasite virulence. PLoS One. 2008;3:e2386. doi: 10.1371/journal.pone.0002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusty D, Mehra P, Srivastava S, Shivange AV, Gupta A, Roy N, Dhar SK. Nicotinamide inhibits Plasmodium falciparum Sir2 activity in vitro and parasite growth. FEMS Microbiol Lett. 2008;282:266–272. doi: 10.1111/j.1574-6968.2008.01135.x. [DOI] [PubMed] [Google Scholar]
- Ralph SA, van Dooren GG, Waller RF, Crawford MJ, Fraunholz MJ, Foth BJ, Tonkin CJ, Roos DS, McFadden GI. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat Rev Microbiol. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- Ramasamy G, Gupta D, Mohmmed A, Chauhan VS. Characterization and localization of Plasmodium falciparum homolog of prokaryotic ClpQ/HslV protease. Mol Biochem Parasitol. 2007;152:139–148. doi: 10.1016/j.molbiopara.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Salcedo-Amaya AM, van Driel MA, Alako BT, Trelle MB, van den Elzen AM, Cohen AM, Janssen-Megens EM, van de Vegte-Bolmer M, Selzer RR, Iniguez AL, Green RD, Sauerwein RW, Jensen ON, Stunnenberg HG. Dynamic histone H3 epigenome marking during the intraerythrocytic cycle of Plasmodium falciparum. Proc Natl Acad Sci U S A. 2009;106:9655–9660. doi: 10.1073/pnas.0902515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- Soufi B, Soares NC, Ravikumar V, Macek B. Proteomics reveals evidence of cross-talk between protein modifications in bacteria: focus on acetylation and phosphorylation. Curr Opin Microbiol. 2012;15:357–363. doi: 10.1016/j.mib.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Sun B, Guo S, Tang Q, Li C, Zeng R, Xiong Z, Zhong C, Ding J. Regulation of the histone acetyltransferase activity of hMOF via autoacetylation of Lys274. Cell Res. 2011;21:1262–1266. doi: 10.1038/cr.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Treeck M, Sanders JL, Elias JE, Boothroyd JC. The phosphoproteomes of Plasmodium falciparum and Toxoplasma gondii reveal unusual adaptations within and beyond the parasites’ boundaries. Cell Host Microbe. 2011;10:410–419. doi: 10.1016/j.chom.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdone L, Caserta M, Di Mauro E. Role of histone acetylation in the control of gene expression. Biochem Cell Biol. 2005;83:344–353. doi: 10.1139/o05-041. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert BT, Wagner SA, Horn H, Henriksen P, Liu WR, Olsen JV, Jensen LJ, Choudhary C. Proteome-wide mapping of the Drosophila acetylome demonstrates a high degree of conservation of lysine acetylation. Sci Signal. 2011;4:ra48. doi: 10.1126/scisignal.2001902. [DOI] [PubMed] [Google Scholar]
- Wilson T, Munro DS, Richard DR. Proguanil-resistance in Malayan strains of Plasmodium vivax. Br Med J. 1952;1:564–568. doi: 10.1136/bmj.1.4758.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Oh MH, Schwarz EM, Larue CT, Sivaguru M, Imai BS, Yau PM, Ort DR, Huber SC. Lysine acetylation is a widespread protein modification for diverse proteins in Arabidopsis. Plant Physiol. 2011;155:1769–1778. doi: 10.1104/pp.110.165852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XJ, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. The diversity of lysine-acetylated proteins in Escherichia coli. J Microbiol Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- Yuan H, Rossetto D, Mellert H, Dang W, Srinivasan M, Johnson J, Hodawadekar S, Ding EC, Speicher K, Abshiru N, Perry R, Wu J, Yang C, Zheng YG, Speicher DW, Thibault P, Verreault A, Johnson FB, Berger SL, Sternglanz R, McMahon SB, Cote J, Marmorstein R. MYST protein acetyltransferase activity requires active site lysine autoacetylation. EMBO J. 2012;31:58–70. doi: 10.1038/emboj.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, Liu CF, Grishin NV, Zhao Y. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol Cell Proteomics. 2009;8:215–225. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.