Abstract
Objective
Insulin-like growth factor-1 (IGF1) and its most abundant binding protein, insulin-like growth factor binding protein-3 (IGFBP3), have been implicated in fibrotic lung diseases and persistent acute respiratory distress syndrome (ARDS) because of profibrogenic and antiapoptotic activity. Whether levels of circulating IGF1 and IGFBP3 are altered in ARDS, and whether they predict progression of and survival from ARDS remains unknown. This study aims to characterize circulating levels of IGF1 and IGFBP3 in patients at risk for ARDS in relation to (1) development of ARDS, and (2) mortality among ARDS cases.
Design
In this case-cohort study, consecutive patients with risk factors for ARDS admitted to the intensive care unit (ICU) were enrolled and followed prospectively for development of ARDS. Cases were followed for all-cause mortality through Day 60. Of 2397 patients enrolled in the parent study, plasma samples were available in 531 (22%) patients (356 controls, 175 cases) from early in presentation. Total plasma IGF1 and IGFBP3 were measured.
Results
After adjusting for relevant clinical covariates including severity of illness, IGF1 and IGFBP3 levels were significantly lower in ARDS cases than controls (odds ratio [OR], 0.58; P =0.006; OR, 0.57; P=0.0015, respectively). Among ARDS cases, IGF1 and IGFBP3 levels were significantly lower in the 78 (45%) non-survivors (hazard ratio [HR], 0.70; P =0.024; HR, 0.69; P=0.021, respectively).
Conclusions
Lower levels of circulating IGF1 and IGFBP3 were independently associated with ARDS case status. Furthermore, lower levels were associated with mortality among ARDS cases. This data supports a role of the IGF pathway in ARDS.
Keywords: Adult Respiratory Distress Syndrome, Insulin-Like Growth Factor-1, Insulin-Like Growth Factor Binding Protein-3, Molecular Epidemiology, Serum Biomarkers
INTRODUCTION
Insulin-like growth factor-1 (IGF1) is a strongly profibrogenic peptide with mitogenic and antiapoptotic effects (1). It has been identified as a potential mediator of fibrotic lung diseases such as sarcoidosis and idiopathic pulmonary fibrosis (2–5). IGF1 bioavailability is tightly regulated by at least six high-affinity IGF binding proteins (IGFBPs) that directly modify the cellular actions of IGF1. IGFBP3 is most abundant in serum and has been shown to increase collagen and fibronectin synthesis by fibroblasts in vitro, even independent of IGF1 (6). Studies of the IGF pathway in fibrotic lung disease have shown increases in lung levels of IGF1 and IGFBP3. IGF1 also plays a critical role in fetal lung development, and IGF-mediated postnatal lung growth is associated with increased alveolar numbers and size (7).
IGF1 is also an endocrine mediator of growth hormone (GH)-induced metabolic and anabolic actions, and it has paracrine and autocrine functions. Circulating IGF1 is mainly controlled by growth hormone secretion, and levels are also predicted by age, gender, smoking status, and dietary intake (8,9). Cytokines and genetic factors may also influence both circulating levels and local levels in the lung (10). In general, acute critical illness is characterized by an increase in circulating GH levels and a decrease in IGF1 levels, indicative of relative GH resistance (11). This GH resistance is thought to be at least partially responsible for the negative nitrogen balance seen in critical illness that affects organ function, including that of the respiratory muscles (12).
The acute respiratory distress syndrome (ARDS) is an inflammatory insult to the lung that can progress to a persistent, or fibroproliferative, phase of ongoing inflammation on a backdrop of fibrosis. Given the role of the IGF pathway in fibroblast activation and collagen synthesis, there is some evidence for a role in the pathogenesis of ARDS. Increased IGFBP3 levels have been identified in bronchoalveolar lavage (BAL) fluid of ARDS patients and patients with risk factors for ARDS (13). Enhanced immunostaining for IGF1 and its receptor (IGF1R) has been identified in lung biopsy specimens from patients with fibroproliferative ARDS (14). Systemically, low serum IGF1 has been associated with bronchopulmonary dysplasia in premature infants, a disease with phenotypic similarities to ARDS, including exuberant inflammation, abnormal healing, and fibrosis (10). There are no studies, however, examining circulating IGF1 and IGFBP3 in ARDS specifically. This syndrome is a unique overlap of the systemic alterations of critical illness with injury and repair within the lung. Given previous studies of the GH pathway in critical illness, along with additional studies of the role of IGF1 in lung disease, we hypothesize that serum levels of IGF1 and IGFBP3 will be decreased in patients with ARDS compared to at-risk controls. Furthermore, we hypothesize that lower IGF1 and IGFBP3 levels will be associated with the outcome of mortality among ARDS cases.
SUBJECTS AND METHODS
Study Population and Design
This study is a case-cohort study that is part of the larger Molecular Epidemiology of ARDS Study for which study design and exclusion criteria have been thoroughly described previously (15). The Human Subjects Committees at Massachusetts General Hospital, Beth Israel-Deaconess Medical Center, the Harvard School of Public Health, and the Yale School of Medicine approved this study. Recruitment of adult intensive care unit (ICU) admissions at MGH (Boston, MA) began in January 1999, and at BIDMC (Boston, MA) in January 2007. Enrollment continued through March 2009. Admissions were screened daily for clinical risk factors for ARDS: pneumonia, sepsis or septic shock, aspiration, massive transfusions, pulmonary contusion or multiple fractures. Patients with ARDS risk factors were followed prospectively for the development of ARDS. Controls were at-risk patients who did not develop ARDS. Patients were defined as having ARDS if they met the American-European Consensus Conference (AECC) criteria for ARDS and required intubation and mechanical ventilation (16). Day 0 of ARDS was defined as the day on which the patient first met all AECC criteria simultaneously. The primary outcome was the development of ARDS. The secondary outcome among ARDS cases was all-cause 60-day mortality.
Analysis of Total IGF1 and IGFBP3 Levels in Plasma
The target window for blood draws was during the first 48 hours of ICU admission; for patients developing ARDS beyond the first 2 days in the ICU, blood draws were also targeted for the first 48 hours of meeting the ARDS case definition. For the current study, control samples were included if they were drawn on Day 0, 1, or 2 of ICU admission. Case samples were included if they were drawn within the 2 days before or after Day 0 of ARDS. The timing of these case samples was also analyzed to determine how many of these specimens also coincided with Day 0, 1, or 2 of ICU admission. Time-to-measurement was considered in days, relative to Day 0 as defined above. Patients whose blood samples did not conform to these parameters were excluded.
Blood samples were collected in 10mL vacuum tubes and centrifuged for 10 minutes. Plasma samples were stored at −80°C until analysis. Total IGF1 and IGFBP3 levels were measured using an automated IMMULITE assay on an IMMULITE 1000 instrument (Siemens, Malvern, PA). For IGF1 and IGFBP3, the assay sensitivity was 20 ng/mL and 0.1 µg/mL, the high dose hook effect was at 45,000 ng/mL and 426 µg/mL, and the intra-assay variability was 3.56% and 4.20% respectively. Samples were run in duplicates, and measurements with a coefficient of variation higher than 15% were rejected and the samples were reanalyzed. Lab personnel were blinded to case/control status and clinical information. Because this nested study was part of a larger genetic study, only Caucasians were included in this study. Caucasians comprised >90% of the parent cohort. GH is secreted in pulsatile fashion, and its levels display day-to-night rhythmicity. An accurate assessment of GH levels would require frequent (every 10 minute) sampling for 24 hours which was not feasible in the context of this study.
Statistical Analysis
All statistical analyses were performed using SAS Version 9.2 (SAS Inc., Cary, North Carolina). Demographic and clinical characteristics between groups were compared using χ2 tests for categorical variables, and Student t-tests and/or nonparametric tests for continuous variables. Correlations between serum IGF1 and IGFBP3 and clinical variables were estimated using Spearman correlation.
IGF1 and IGFBP3 were log-transformed to approximate normality, and log-transformed values were used in all models. Logistic regression models were used to assess the association of IGF1 and IGFBP3 with ARDS case status. Cox proportional hazards models were used to assess the association between IGF1 and IGFBP3 levels and survival. All multivariable models used a backward selection algorithm with p≤0.1 to stay; models were then refit with the surviving covariates. Multivariable models considered the following clinically relevant covariates: age, gender, APACHE III score, body mass index (BMI), risk factor for ARDS, smoking status, and current history of cirrhosis or diabetes. The risk factor for ARDS was represented by the following individual covariates: sepsis, septic shock, direct pulmonary injury (defined as pneumonia, aspiration, or pulmonary contusion), trauma, and need for red blood cell transfusions. In multivariate analyses of ARDS case status, the APACHE III score excluded PaO2/FiO2 to avoid collinearity (17). Given evidence that sex hormones may be an effect modifier, exogenous estrogen or progestin use at the time of hospital admission was also considered (7). Time-to-measurement of the biomarkers was also added to all multivariate models to assess for possible confounding.
RESULTS
Patient population and IGF1 and IGFBP3 measurements
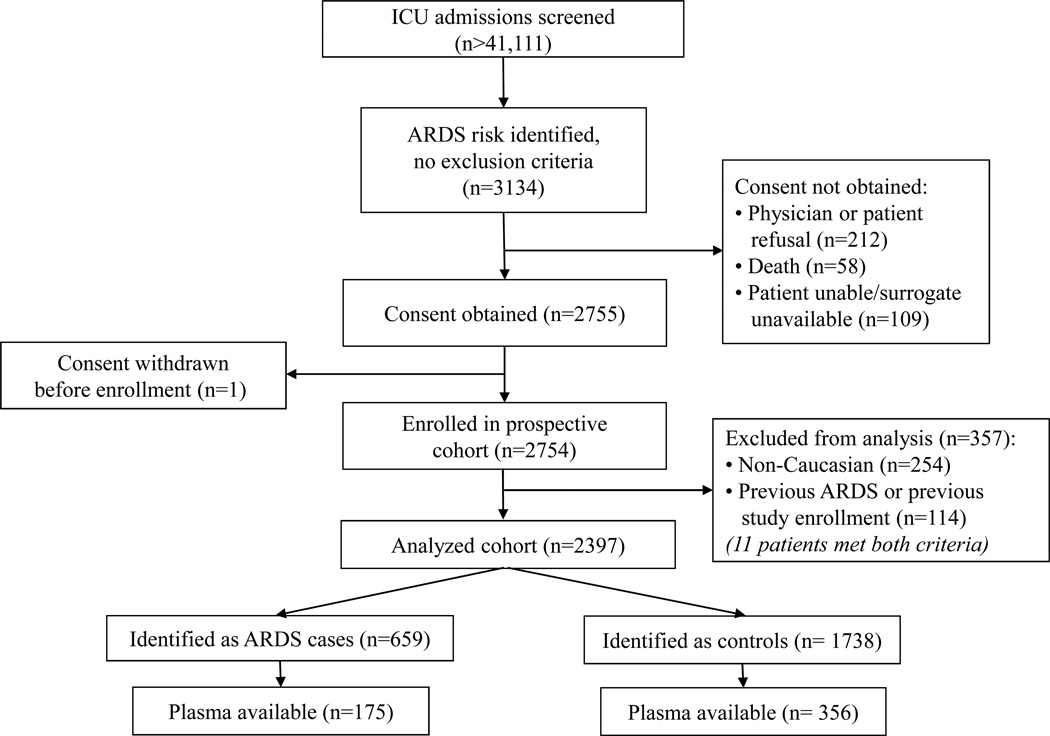
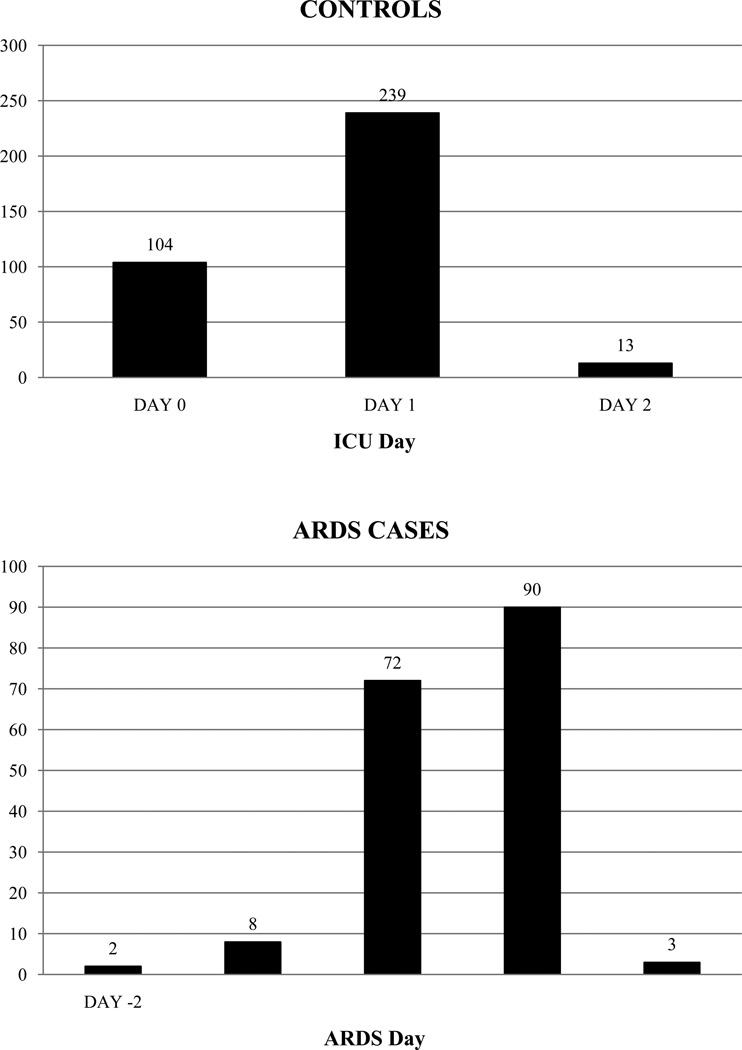
From January 1999 to March 2009, 44,111 consecutive ICU admissions were screened for possible inclusion (Figure 1). In total, 2397 patients were consented, enrolled, and analyzed for the current study. Five-hundred thirty-one patients (356 controls, 175 cases) had plasma drawn within the target windows around ICU admission or development of ARDS, as described above. Among the 356 controls, 104 (29%) had blood samples drawn on the day of admission to the ICU (Day 0); 239 (67%) had blood drawn on Day 1 of ICU admission (Figure 2, top). Among the 175 ARDS cases, 72 (41%) had blood samples drawn on Day 0 of ARDS; 90 (51%) had blood samples drawn on Day 1 of ARDS (Figure 2, bottom). Of these 175 case samples, 129 (73.7%) were also drawn within the first 2 days of ICU admission, as in the control group; the onset of ARDS was delayed from ICU admission in the remaining 46 case patients. Plasma measurement of IGF1 and IGFBP3 was successful in 520 and 526 patients, respectively.
Figure 1.
Study design and patient selection
ICU = intensive care unit; ARDS = acute respiratory distress syndrome.
Figure 2.
Timing of Plasma Samples Around ICU Admission in Controls (top) or ARDS Onset in Cases (bottom)
ICU = intensive care unit; ARDS = acute respiratory distress syndrome
Excluded patients were compared with tested patients across the spectrum of baseline variables (Table 1). Due to study design, ARDS cases were more likely to have plasma drawn within the target window, because those patients developing ARDS beyond ICU admission could be captured again with blood drawn around ARDS Day 0. Comparing tested and excluded patients, the groups differed significantly in age, BMI, and APACHE III score (Table 1). After stratifying by ARDS case status to adjust for the higher rate of ARDS cases among patients with available plasma, the differences in age and BMI were seen only in controls, and not among ARDS cases. However, the difference in APACHE III score persisted in both groups.
Table 1.
Baseline Characteristics of the Study Cohort
| Measure | All Patients (n=2397) |
Plasma Available (n=531) |
Plasma Unavailable (n=1866) |
Pa |
|---|---|---|---|---|
| Age, mean (SD) | 61.5 (17.5) | 63.7 (17.0) | 60.9 (17.6) | 0.001 |
| Female gender | 912 (38.1) | 207 (39.0) | 705 (37.8) | 0.61 |
| BMI, median (IQR) | 26.6 (8.0) | 26.1 (7.6) | 26.6 (7.9) | 0.03 |
| APACHE III score, mean (SD) | 60.8 (21.6) | 66.1 (22.4) | 59.3 (21.2) | <0.0001 |
| Sepsis syndrome | 766 (32.0) | 176 (33.2) | 590 (31.6) | 0.51 |
| Pulmonary source | 432 (56.4) | 107 (60.8) | 325 (55.1) | 0.19 |
| Septic shock | 1184 (49.4) | 277 (52.2) | 907 (48.6) | 0.15 |
| Pulmonary source | 654 (55.2) | 139 (50.2) | 515 (56.8) | 0.06 |
| Trauma | 196 (8.2) | 39 (7.3) | 157 (8.4) | 0.43 |
| Need for blood transfusion | 1143 (47.8) | 237 (44.6) | 906 (48.7) | 0.10 |
| Aspiration | 186 (7.8) | 40 (7.5) | 146 (7.8) | 0.82 |
| >1 risk factor for ARDS | 253 (10.6) | 52 (9.8) | 201 (10.8) | 0.52 |
| Direct pulmonary injury | 1356 (56.6) | 301 (56.7) | 1055 (56.5) | 0.95 |
| ARDS Cases | 659 (27.5) | 175 (33.0) | 484 (25.9) | 0.001 |
Results are No. (%) except as noted;
P-value reflects comparison between patients with and without available plasma
IGF1 and IGFBP3 levels were strongly positively correlated with each other (Spearman coefficient [ρ], 0.68, P<0.0001). IGF1 and IGFBP3 were both negatively correlated with age (ρ= −0.14, P =0.0010; ρ=−0.20, P<0.0001, respectively), consistent with the known parallel decline in growth hormone with age (9,18). Both IGF1 and IGFBP3 were also positively correlated with BMI (ρ=0.16, P =0.0006; ρ=0.20, P <0.0001, respectively).
Total IGF1 and IGFBP3 and Association with ARDS Case Status
Baseline characteristics are shown in Table 2. Median IGF1 concentration in the study population was 671.05 ng/mL (interquartile range [IQR], 595.88); median IGFBP3 concentration was 198.97 µg/mL (IQR, 161.60).
Table 2.
Baseline Characteristics of Cohort with Plasma Available by ARDS Status
| Characteristic | Controls (n=356) |
ARDS Cases (n=175) |
P valuea |
|---|---|---|---|
| Age, mean (SD) | 65.2 (16.5) | 60.7 (17.6) | 0.008 |
| Female gender | 134 (37.6) | 73 (41.7) | 0.39 |
| BMI, median (IQR) | 25.8 (7.8) | 26.6 (8.3) | 0.08 |
| APACHE III score, mean (SD) | 63.6 (22.3) | 71.3 (21.9) | <0.0001 |
| Sepsis syndrome | 131 (36.8) | 45 (25.7) | 0.01 |
| Pulmonary source | 72 (55.0) | 35 (77.8) | 0.008 |
| Septic shock | 166 (46.6) | 111 (63.4) | 0.0003 |
| Pulmonary source | 60 (36.1) | 79 (71.2) | <0.0001 |
| Trauma | 33 (9.3) | 6 (3.4) | 0.02 |
| Need for blood transfusion | 149 (41.9) | 88 (50.3) | 0.07 |
| Aspiration | 27 (7.6) | 13 (7.4) | 0.95 |
| >1 risk factor for ARDS | 33 (9.3) | 19 (10.9) | 0.56 |
| Direct pulmonary injury | 175 (49.2) | 126 (72.0) | <0.0001 |
| IGF-1 (ng/mL), median (IQR) | 726.3 (659.3) | 531.30 (522.0) | <0.0001 |
| IGFBP-3 (µg/mL), median (IQR) | 208.3 (159.6) | 170.69 (149.5) | 0.0009 |
IQR = interquartile range
Results are No. (%) except as note.
P-value reflects comparison between controls and cases
IGF1 and IGFBP3 levels were significantly lower in ARDS cases than controls (Table 2). Because of the strong correlation between IGF1 and IGFBP3 levels, there was evidence of collinearity when both were included in the same multivariable model. Thus, separate models were used to assess each biomarker to avoid the effect of collinearity on the parameter estimates. In unadjusted logistic regression models, higher IGF1 was associated with lower odds of ARDS (odds ratio [OR], 0.53; 95% confidence interval [CI], 0.41 to 0.70; P<0.0001). The same was true for IGFBP3 (OR, 0.58; 95% CI, 0.43 to 0.78; P=0.0003). In multivariable models, this relationship persisted for both IGF1 and IGFBP3 (Table 3). The other covariates significant in the models to the P<0.1 level were: age, APACHE III score, direct pulmonary injury, sepsis, trauma, diabetes, and the need for red cell transfusion. There was no significant association between the ratio of IGF1 to IGFBP3 (IGF1/IGFBP3) and ARDS case status in either unadjusted or adjusted models. Interaction terms for age and BMI with biomarker levels were not significant. Results were unchanged in the subset of patients with direct pulmonary injury as the etiologic risk factor ARDS. When time-to-measurement was included in the models, it was found to be significant (OR, 0.49; 95% CI, 0.34–0.71). This finding implies that the investigators were more successful in recruiting ARDS cases earlier in their course, as compared to controls. This is likely a corollary to the finding described above that, due to study design, patients with ARDS were more likely to have plasma samples available for analysis than controls. However, the inclusion of the time variable had no significant effect on the covariates found to be significant in the multivariable model, nor did it affect the magnitude of the parameter estimates or their P-values. Therefore, time-to-measurement was not thought to be a confounder and was not included in the final refit models.
Table 3.
Logistic Regression Models for Association of IGF-1 and IGFBP-3 Levels with ARDS Case Status
| Model 1: IGF-1 | Model 2: IGFBP-3 | |||||
|---|---|---|---|---|---|---|
| Measure | ORadj | 95% CI | P value | ORadj | 95% CI | P value |
| Biomarkera | 0.58 | 0.42–0.79 | 0.0006 | 0.57 | 0.40–0.81 | 0.0015 |
| Age | 0.97 | 0.96–0.98 | <0.0001 | 0.97 | 0.96–0.98 | <0.0001 |
| APACHE III scoreb | 1.02 | 1.01–1.03 | 0.0063 | 1.02 | 1.01–1.03 | 0.0006 |
| Direct pulmonary injuryc | 4.05 | 2.56–6.42 | <0.0001 | 3.84 | 2.44–6.05 | <0.0001 |
| Sepsisd | 0.54 | 0.33–0.87 | 0.011 | 0.53 | 0.33–0.85 | 0.0080 |
| Trauma | 0.25 | 0.086–0.70 | 0.0089 | 0.25 | 0.089–0.70 | 0.0086 |
| Current diabetes | 0.59 | 0.36–0.95 | 0.031 | 0.61 | 0.38–0.99 | 0.043 |
| Red cell transfusion | 1.91 | 1.23–2.96 | 0.0038 | 1.88 | 1.22–2.91 | 0.0044 |
Biomarker in Model 1 is logIGF-1; biomarker in Model 2 is logIGFBP-3
APACHE III with both age and PaO2/FiO2 removed
Direct pulmonary injury includes pneumonia, pulmonary contusion, and aspiration
Sepsis syndrome without septic shock
ORadj=Adjusted odds ratio in the multivariable model; Other covariates selected out of the models include gender, BMI, cirrhosis, estrogen/progesterone status, and septic shock
Total IGF1 and IGFBP3 and Association with Mortality in ARDS Cases
Baseline characteristics among ARDS cases are shown in Table 4. Of the 175 ARDS cases, 97 (55%) survived, and 78 (45%) did not. Survivors were significantly younger than non-survivors and had higher BMIs. Survivors also had significantly lower severity of illness. No patients died who had trauma as their risk factor for ARDS.
Table 4.
Baseline Cohort Characteristics of ARDS Cases by 60-Day All-Cause Mortality
| Characteristic | Survivors (n=97) |
Non-survivors (n=78) |
P valuea |
|---|---|---|---|
| Age, mean (SD) | 55.1 (17.6) | 67.8 (14.9) | <0.0001 |
| Female gender | 42 (43.3) | 31 (39.7) | 0.64 |
| BMI, median (IQR) | 27.8 (8.7) | 25.4 (6.2) | 0.030 |
| APACHE III score, mean (SD) | 62.3 (20.0) | 82.3 (19.0) | <0.0001 |
| Sepsis syndrome | 28 (28.9) | 17 (21.8) | 0.29 |
| Pulmonary source | 21 (75.0) | 14 (82.4) | 0.57 |
| Septic shock | 59 (60.8) | 52 (66.7) | 0.43 |
| Pulmonary source | 45 (76.3) | 34 (65.4) | 0.21 |
| Trauma, n (%) | 6 (6.2) | 0 (0) | 0.025 |
| Need for blood transfusion | 44 (45.4) | 54 (56.4) | 0.15 |
| Aspiration | 4 (4.1) | 9 (10.3) | 0.063 |
| >1 risk factor for ARDS | 8 (8.3) | 11 (14.1) | 0.22 |
| Direct pulmonary injury | 73 (75.3) | 53 (68.0) | 0.28 |
| IGF-1 (ng/mL), median (IQR) | 600.6 (544.0) | 442.8 (391.5) | 0.0005 |
| IGFBP-3 (µg/mL), median (IQR) | 212.9 (146.5) | 132.8 (142.3) | 0.0016 |
IQR = interquartile range
Results are No. (%) except as note.
P-value reflects comparison between controls and cases
IGF1 and IGFBP3 levels were significantly lower in non-survivors than survivors (Table 4). In unadjusted Cox proportional hazards model, IGF1 was negatively associated with hazard of 60-day mortality (hazard ratio [HR], 0.62; 95% CI, 0.46 to 0.84; P =0.002). Similarly, IGFBP3 was negatively associated with hazard of death (HR, 0.63; 95% CI, 0.46 to 0.86; P =0.003). In multivariable models, this relationship persisted for both IGF1 and IGFBP3 (Table 5). Age and APACHE III score were also significant in the Cox models for both biomarkers. There was no significant association between IGF1/IGFBP3 and mortality. Interaction terms for age and BMI with biomarker levels were not significant. Results were unchanged in the subset of patients with direct pulmonary injury as the etiologic risk factor ARDS. When the time-to-measurement variable was included, it was not found to be significant in any models, nor did it appear to be a confounder.
Table 5.
Cox Proportional Hazards Models for Association of IGF-1 and IGFBP-3 Levels with Hazard of 60-Day Mortality
| Model 1: IGF-1 | Model 2: IGFBP-3 | |||||
|---|---|---|---|---|---|---|
| Measure | HRadj | 95% CI | P value | HRadj | 95% CI | P value |
| Biomarkera | 0.70 | 0.51–0.95 | 0.024 | 0.69 | 0.50–0.94 | 0.021 |
| Age | 1.03 | 1.01–1.04 | 0.0004 | 1.03 | 1.01–1.04 | 0.0006 |
| APACHE III scoreb | 1.03 | 1.01–1.04 | <0.0001 | 1.03 | 1.02–1.04 | <0.0001 |
Biomarker in Model 1 is logIGF-1; biomarker in Model 2 is logIGFBP-3
APACHE III revised to exclude age
HRadj=Adjusted hazards ratio in the multivariable model; Other covariates selected out of the models include gender, BMI, cirrhosis, current diabetes, estrogen/progesterone status, need for red cell transfusion, sepsis, septic shock, and trauma
DISCUSSION
We observed that among patients with clinical risk factors for ARDS, lower levels of IGF1 and IGFBP3 were independently associated with ARDS case status after controlling for severity of illness and other clinical covariates. Furthermore, lower levels of IGF1 and IGFBP3 early in ARDS were predictive of 60-day mortality. Circulating IGF1 is known to fall during acute illness due to relative GH resistance. However, our results show a differential effect in ARDS patients compared to critically ill controls, and the association with ARDS case status and mortality among ARDS cases was robust even after adjusting for etiology and severity of illness. This suggests a specific role for IGF1 and IGFBP3 in this disease state.
Many potential circulating biomarkers of inflammation and fibroproliferation have been examined in early ARDS. These have included hepatocyte growth factor, keratinocyte growth factor, type III procollagen peptide, matrix metalloproteinases, and tissue inhibitors of metalloproteinases (19–23). Although fibroproliferation in ARDS was long thought to be a late event based on clinical and histologic features, there has been increasing recognition that the initial activating events, including fibroblast activation and collagen turnover, occur very early in the course of ARDS (19,20). In several studies, circulating markers have shown parallel alterations with markers measured in BAL fluid. For instance, elevated levels of hepatocyte growth factor were measured in both plasma and pulmonary edema fluid early in the course of acute lung injury, and this correlated with mortality (19). Similarly, type III procollagen peptide was shown to be elevated in both serum and BAL fluid in acute lung injury and/or ARDS patients compared to controls (20,21).
Previous studies have identified significant elevations in IGF1 and IGFBP3 as measured in BAL fluid very early in the course of ARDS (13). The current study, however, shows decreased levels of circulating IGF1 and IGFBP3 in early ARDS compared with controls. Thus, there may be an inverse association between circulating and lung compartment levels of IGF1 and IGFBP3. This mimics findings in bronchopulmonary dysplasia in which lung IGF1 levels are increased while circulating levels are decreased (10). Alternatively, IGF1 may be regulated entirely differently in the lung and systemic compartments, and decreased circulating levels of IGF1 and IGFBP3 as markers of GH resistance thus may merely be markers of protein and muscle breakdown with resulting increased susceptibility for infection or delay in recovery. Lower levels of circulating IGF1 and IGFBP3 have been observed in chronically-ill patients with cystic fibrosis and chronic obstructive pulmonary disease (COPD), and levels were positively correlated with measures of lung function (FEV1) and respiratory muscle function (maximal inspiratory pressure), respectively (23,24).
In a study of COPD patients hospitalized for acute exacerbation, plasma IGF1 levels on Day 1 of admission were found to be decreased compared to healthy elderly controls (25). Levels increased during hospitalization with treatment of the exacerbation, but levels on Day 15 were still significantly lower than in controls. IGF1 levels did not appear to be merely an alternative measure of inflammation, however, as levels of inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) were not correlated with IGF1 levels. One additional finding was that patients with emphysema had even lower plasma IGF1 levels than those COPD patients with chronic bronchitis. This may be relevant to the current study as it associates IGF1 with parenchymal lung disease, as opposed to airways disease and inflammation in the absence of significant parenchymal abnormalities.
The role of IGF1 in diaphragmatic muscle has also been studied in relation to acute respiratory failure in the setting of severe sepsis, as well as with the use of mechanical ventilation. (26,27)
In a murine model of lipopolysaccharide-induced sepsis, IGF1 expression in diaphragmatic myocytes was found to be increasingly suppressed from 48 to 96 hours after lipopolysaccharide injection (26). IGF1 levels also showed negative correlation with the amount of myofiber injury in the diaphragm, suggesting a protective effect of IGF1 on myofibers during sepsis (26). A second study compared IGF1 expression in the diaphragmatic muscle of rabbits treated with controlled mechanical ventilation (complete diaphragm muscle inactivity) vs. assisted mechanical ventilation (partial maintenance of neural activation and mechanical loading) (27). The authors observed significantly reduced IGF1 mRNA levels with controlled mechanical ventilation, decreasing expression by 65%. This corresponded to significant myofibrillar disarray. No change in IGF1 mRNA was seen with assisted mechanical ventilation alone.
In humans, studies in COPD patients have shown that local IGF1 production within muscle is important for stimulating muscle growth and repair, and decreased IGF1 may be an important factor in the development of skeletal muscle weakness (28, 29). Specifically, IGF1 levels were found to be similar in outpatients with stable COPD and COPD patients hospitalized for acute exacerbation (both compared to healthy elderly controls) (28). However, peripheral muscle force was positively correlated with systemic IGF1 levels and pulmonary functions, suggesting possible involvement of IGF1 in the development of peripheral muscle weakness (28). A similar study showed that IGF1 mRNA expression in vastus lateralis muscle biopsies was significantly decreased in COPD patients (29).
Despite the apparent diagnostic implications of our findings in ARDS, the data presented in the current study also raise the question of whether normalizing IGF1 and IGFBP3 could confer any direct therapeutic benefits and thus provide opportunities for further research in the field. Caution must be taken in making the therapeutic leap, however, given previous results of treating critically ill adults with recombinant human growth hormone (rhGH) in an effort to correct the negative nitrogen balance of critical illness (30). In this well-known study, mortality was significantly increased in patients receiving rhGH treatment, and multiple-organ failure and septic shock or uncontrolled infection were disproportionately represented as causes of death among the treated patients. Nitrogen balance was improved, however, but this did not correlate to decreased duration of mechanical ventilation, ICU stay, or hospital stay, nor did it correlate to increases in IGF1 concentrations. The low IGF1 and IGFBP3 levels seen in this study may reflect GH resistance and thus may explain the lack of efficacy of the rhGH treatment in the study. Although respiratory failure was one diagnosis for inclusion in the study, the remaining diagnostic groups were surgical, thus making this study poorly generalizable to the medical intensive care unit population.
Growth hormone levels on ICU admission were also found to predict mortality in a study of septic patients (31). Interestingly, in this study, IGF1 and IGFBP3 levels were also noted to be low, as in our study, but levels did not differ by severity of illness or between survivors and non-survivors, and levels were not predictive of mortality in multivariable models. The latter differs vastly from our study, particularly since sepsis and septic shock patients made up a large proportion of our study population. This again supports that IGF1 may have a specific role in ARDS, beyond that of general critical illness.
Finally, therapeutic use of IGF1 has been attempted in rats under hypoxic conditions, again relevant to the current study’s patient population (32). Based on the known anabolic effects of IGF1 demonstrated in animals in a catabolic state, recombinant human IGF1 (rhIGF1) was infused along with total parental nutrition (TPN) in rats under normoxic vs. hypoxic (FiO2 of 0.10) conditions, and nitrogen balance and body weight were compared to similar animals given TPN alone. Nitrogen balance was corrected in hypoxic rats receiving rhIGF1, but they had no significant change in body weight, as compared to hypoxic rats not receiving rhIGF1 who were observed to have a drop in body weight. In normoxic rats receiving rhIGF1, however, body weight increased significantly more than in the hypoxic rats. This study thus shows that IGF1 is indeed anabolic in normoxic rats, but this anabolic effect is tempered in animals made catabolic by exposure to hypoxia. This may be one reason why in the current study, the ARDS (and thus hypoxemic) patients with lower IGF1 levels had higher mortality than those with higher IGF1 levels.
There are several limitations to this study. We did not assess GH in this study, but GH has been well-documented to be elevated in critical illness and the sepsis spectrum in particular, and may be independently associated with outcome, as described above (30,31). We focused on IGF1 and IGFBP3 that are downstream of GH, and low levels of that may reflect GH resistance in view of the elevated GH described in other studies of the critically ill. We measured biomarkers at a single point in time, early in the course of critical illness and ARDS, and thus we were not able to characterize changes in IGF1 and IGFBP3 over time. IGF1 and IGFBP3 do not exhibit significant diurnal variation, however, making the random timing of blood draws a negligible factor. We also did not adjust for dietary factors as we had no information on preadmission diet, and most intubated, critically ill patients are not fed at the time of ICU admission around which our samples were drawn. We did not obtain BAL fluid for biomarker analyses, and therefore we were unable to assess the simultaneous activity of the IGF pathway in circulation and lung.
Selection bias must be considered given the difficulty in enrolling all eligible patients within the first 48 hours of ICU admission or ARDS, thus resulting in only about a quarter of patients having plasma drawn during this window. This is a common difficulty encountered in clinical critical care research, and it is possible that patients with plasma drawn during this early window differ from those in whom plasma was not obtained. We analyzed clinical characteristics by participation (Table 1), and multivariable models adjusted for the variables that differed among participants. Given our robust findings, any residual selection bias would likely affect generalizability rather than the internal validity of the study.
This study has many strengths, most importantly that this is a large, well-characterized, prospectively enrolled cohort of patients at risk for ARDS. We used AECC guidelines for defining ARDS case status, a standard measure that limits misclassification in the absence of any “gold standard” for ARDS diagnosis short of surgical lung biopsy. Biomarker measurements were made using state-of-the-art methodology by personnel blinded to patient case status and to the underlying hypothesis of the study. Assay variability was minimal, and although assay errors cannot be excluded, such errors would result in random misclassification, biasing toward the null.
In summary, the findings in this study suggest an important role for the IGF pathway early in ARDS, as well as in predicting death among ARDS cases. Specifically, IGF1 and IGFBP3 may be protective in critical illness and development of ARDS. ARDS encompasses the systemic alterations of critical illness, and the injury and repair within the respiratory system. Thus, our findings may reflect a complex interplay of the effects of critical illness on the IGF/GH axis, the subsequent effects of critical illness and mechanical ventilation on IGF1 as it affects diaphragmatic function, and fibroproliferation in the lung compartment. Full elucidation of a potentially causal role of IGF1 and IGFBP3 would have important clinical implications, but this will require further mechanistic studies.
ACKNOWLEDGEMENTS
We thank Yael Tarshish, Hanae Fujii-Rios, Kezia Ellison, and Ian Taggart for patient recruitment; Janna Frelich, Julie DelPrato, and Marcia Chertok for data management; Andrea Shafer and Starr Sumpter for research administration; and Elizabeth Baker and Lauren Searl for sample preparation. We also thank Dr. Lester Kobzik and Phuong-Sun Nguyen for additional help with biomarker assays.
FUNDING: This work was supported by the National Institutes of Health [R01-HL60710 (NHLBI), ES00002 (NIEHS), T32-HL07874 (NHLBI)] and by the American Heart Association [10FTF3440007].
Footnotes
Disclaimer: This is not the definitive version of record of this article. This manuscript has been accepted for publication in European Journal of Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at doi: 10.1530/EJE-11-0778 2012 European Society of Endocrinology.
DECLARATION OF INTEREST: There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- 1.Clemmons DR. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Current Opinion in Pharmacology. 2006;6:620–625. doi: 10.1016/j.coph.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Krein PM, Winston BW. Roles for insulin-like growth factor 1 and transforming growth factor-β in fibrotic lung disease. Chest. 2002;122:289S–293S. doi: 10.1378/chest.122.6_suppl.289s. [DOI] [PubMed] [Google Scholar]
- 3.Allen JT, Bloor CA, Knight RA, Spiteri MA. Expression of insulin-like growth factor binding proteins in bronchoalveolar lavage fluid of patients with pulmonary sarcoidosis. American Journal of Respiratory Cell and Molecular Biology. 1998;19:250–258. doi: 10.1165/ajrcmb.19.2.3080. [DOI] [PubMed] [Google Scholar]
- 4.Aston C, Jagirdar J, Lee TC, Hur T, Hintz RL, Rom WN. Enhanced insulin-like growth factor molecules in idiopathic pulmonary fibrosis. American Journal of Respiratory and Critical Care Medicine. 1995;151:1597–1603. doi: 10.1164/ajrccm.151.5.7537587. [DOI] [PubMed] [Google Scholar]
- 5.Pala L, Giannini S, Rosi E, Cresci B, Scano G, Mohan S, Duranti R, Rotella CM. Direct measurement of IGF-1 and IGFBP-3 in bronchoalveolar lavage fluid from idiopathic pulmonary fibrosis. Journal of Endocrinological Investigation. 2001;24:856–864. doi: 10.1007/BF03343942. [DOI] [PubMed] [Google Scholar]
- 6.Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. American Journal of Pathology. 2005;166:399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser S, Friedrich N, Ewert R, Schaper C, Nauck M, Dorr M, Volzke H, Felix SB, Krebs A, Wallaschofski H, Koch B. Association between serum insulin-like growth factor (IGF) 1 and IGF binding protein-3 and lung function. Journal of Clinical Endocrinology and Metabolism. 2009;94:2452–2458. doi: 10.1210/jc.2008-2662. [DOI] [PubMed] [Google Scholar]
- 8.Kaklamani VG, Linos A, Kaklamani E, Markaki I, Mantzoros CS. Age, sex, and smoking are predictors of circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3. Journal of Clinical Oncology. 1999;17:813–817. doi: 10.1200/JCO.1999.17.3.813. [DOI] [PubMed] [Google Scholar]
- 9.Kaklamani VG, Linos A, Kaklamani E, Markaki I, Koumantaki Y, Mantzoros CS. Dietary fat and carbohydrates are independently associated with circulating insulin-like growth factor 1 and insulin-like growth factor-binding protein 3 concentrations in healthy adults. Journal of Clinical Oncology. 1999;17:3291–3298. doi: 10.1200/JCO.1999.17.10.3291. [DOI] [PubMed] [Google Scholar]
- 10.Capoluongo E, Ameglio F, Zuppi C. Insulin-like growth factor-1 and complications of prematurity: a focus on bronchopulmonary dysplasia. Clinical Chemistry and Laboratory Medicine. 2008;46:1061–1066. doi: 10.1515/CCLM.2008.211. [DOI] [PubMed] [Google Scholar]
- 11.Mesotten D, Van den Berghe G. Changes within the GH/IGF-1/IGFBP axis in critical illness. Critical Care Clinics. 2006;22:17–28. doi: 10.1016/j.ccc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. New England Journal of Medicine. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 13.Schnapp LM, Donohoe S, Chen J, Sunde DA, Kelly PM, Ruzinski J, Martin T, Goodlett DR. Mining the acute respiratory distress syndrome proteome: identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. American Journal of Pathology. 2006;169:86–95. doi: 10.2353/ajpath.2006.050612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krein PM, Sabatini JB, Tinmouth W, Green FHY, Winston BW. Localization of insulin-like growth factor-I in lung tissues of patients with fibroproliferative acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2003;167:83–90. doi: 10.1164/rccm.2201012. [DOI] [PubMed] [Google Scholar]
- 15.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Critical Care Medicine. 2005;33:1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 16.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. American Journal of Respiratory and Critical Care Medicine. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 17.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A, Harrell FE. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–1636. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 18.Lam CS, Chen MH, Lacey SM, Yang Q, Sullivan LM, Xanthakis V, Safa R, Smith HM, Peng X, Sawyer DB, Vasan RS. Circulating insulin-like growth factor-1 and its binding protein-3: Metabolic and genetic correlates in the community. Thrombosis and Vascular Biology. 2010;30:1479–1484. doi: 10.1161/ATVBAHA.110.203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verghese GM, McCormick-Shannon K, Mason RJ, Matthay MA. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. American Journal of Respiratory and Critical Care Medicine. 1998;158:386–394. doi: 10.1164/ajrccm.158.2.9711111. [DOI] [PubMed] [Google Scholar]
- 20.Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, Laurent GJ. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. American Journal of Respiratory and Critical Care Medicine. 2000;162:1783–1788. doi: 10.1164/ajrccm.162.5.2001061. [DOI] [PubMed] [Google Scholar]
- 21.Chestnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. American Journal of Respiratory and Critical Care Medicine. 1997;156:840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 22.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer JM. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 1996;154:346–352. doi: 10.1164/ajrccm.154.2.8756805. [DOI] [PubMed] [Google Scholar]
- 23.Lebl J, Zahradnikova M, Bartosova J, Zemkova D, Pechova M, Vavrova V. Insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in cystic fibrosis: a positive effect of antibiotic therapy and hyperalimentation. Acta Paediatrica. 2001;20:868–872. [PubMed] [Google Scholar]
- 24.Pape GS, Friedman M, Underwood LE, Clemmons DR. The effect of growth hormone on weight gain and pulmonary function in patients with chronic obstructive lung disease. Chest. 1991;99:1495–1500. doi: 10.1378/chest.99.6.1495. [DOI] [PubMed] [Google Scholar]
- 25.Kythreotis P, Kokkini A, Avgeropoulou S, Hadjioannou A, Anastasakou E, Rasidakis A, Bakakos P. Plasma leptin and insulin-like growth factor 1 levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulmonary Medicine. 2009;9:11. doi: 10.1186/1471-2466-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin MC, Leung SY, Fang WF, Chin CH, Chung KF. Down-regulation of insulin-like growth factor I (IGF-I) in the mouse diaphragm during sepsis. Chang Gung Medical Journal. 2010;33:501–508. [PubMed] [Google Scholar]
- 27.Sassoon CSH, Zhu E, Fang L, Ramar K, Jiao GY, Caiozzo VJ. Interactive effects of corticosteroid and mechanical ventilation on diaphragm muscle function. Muscle and Nerve. 2011;43:103–111. doi: 10.1002/mus.21821. [DOI] [PubMed] [Google Scholar]
- 28.Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan-Ramirez G, Bogaerts P, Bouillon R, Decramer M. Muscle force during an acute exacerbation if hospitalized patients with COPD and its relationship to CXCL8 and IGF-1. Thorax. 2003;58:752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crul T, Spruit MA, Gayan-Ramirez G, Quarck R, Gosselink R, Troosters T, Pitta, Decramer M. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. European Journal of Clinical Investigation. 2007;37:897–904. doi: 10.1111/j.1365-2362.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 30.Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. New England Journal of Medicine. 1999;341:785–792. doi: 10.1056/NEJM199909093411102. [DOI] [PubMed] [Google Scholar]
- 31.Schuetz P, Müller B, Nusbaumer C, Wieland M, Christ-Crain M. Circulating levels of GH predict mortality and complement prognostic scores in critically ill medical patients. European Journal of Endocrinology. 2009;160:157–163. doi: 10.1530/EJE-08-0786. [DOI] [PubMed] [Google Scholar]
- 32.Iioka Y, Tatsumi K, Sugito K, Moriya T, Kuriyama T. Effects of insulin-like growth factor on nitrogen balance during hypoxic exposure. European Respiratory Journal. 2002;20:293–299. doi: 10.1183/09031936.02.00234302. [DOI] [PubMed] [Google Scholar]