Abstract
Memory impairment is one of the most significant residual deficits following traumatic brain injury (TBI) and is among the most frequent complaints heard from patients and their relatives. It has been reported that the hippocampus is particularly vulnerable to TBI, which results in hippocampus-dependent cognitive impairment. There are different regions in the hippocampus, and each region is composed of different cell types, which might respond differently to TBI. However, regional and cell type-specific neuronal death following TBI is not well described. Here, we examined the distribution of degenerating neurons in the hippocampus of the mouse brain following controlled cortical impact (CCI) and found that the majority of degenerating neurons observed were in the dentate gyrus after moderate (0.5 mm cortical deformation) CCI-TBI. In contrast, there were only a few degenerating neurons observed in the hilus, and we did not observe any degenerating neurons in the CA3 or CA1 regions. Among those degenerating cells in the dentate gyrus, about 80% of them were found in the inner granular neuron layer. Analysis with cell type-specific markers showed that most of the degenerating neurons in the inner granular neuron layer are newborn immature neurons. Further quantitative analysis shows that the number of newborn immature neurons in the dentate gyrus is dramatically decreased in the ipsilateral hemisphere compared with the contralateral side. Collectively, our data demonstrate the selective death of newborn immature neurons in the hippocampal dentate gyrus following moderate injury with CCI in mice. This selective vulnerability of newborn immature dentate neurons may contribute to the persistent impairment of learning and memory post-TBI and provide an innovative target for neuroprotective treatment strategies.
Keywords: traumatic brain injury, controlled cortical impact, cell death, dentate gyrus, Fluoro-Jade B
Traumatic brain injury (TBI) is the leading cause of death in children and young adults (McCarthy et al., 2005; Vakil, 2005) and represents a significant socioeconomic burden (Langlois et al., 2005; Minino et al., 2006). In the United States, there are 1.4 million TBI incidences annually, which cost $60 billion in 2000 (Langlois et al., 2005; Minino et al., 2006), leaving many patients with substantial cognitive impairment (Hamm et al., 1992; Salmond and Sahakian, 2005) and epilepsy (Jiang et al., 2004; D’Ambrosio et al., 2005; Gupta and Gupta, 2006). TBI not only results in immediate central nervous system (CNS) tissue disruption (primary injury) but also damages the surviving cells secondarily by complex mechanisms triggered by the primary event (Fujimoto et al., 2004; Hall et al., 2004; Saatman et al., 2006; Singh et al., 2006), leading to persistent cognitive, sensory, and motor dysfunction (Fujimoto et al., 2004). The common neuropathological changes in the hippocampus in human closed-head injury and experimental models of TBI suggest that the hippocampus is particularly vulnerable following TBI (Isoniemi et al., 2006; Pullela et al., 2006; Tran et al., 2006). Disturbance in hippocampal function (Pullela et al., 2006; Statler, 2006; Tasker, 2006) plays a leading role in these pathologies, insofar as this structure is implicated in higher cognitive function (Pullela et al., 2006; Zhang et al., 2006) and is a frequent focus of seizure generation (Gupta and Gupta, 2006; Pitkanen and McIntosh, 2006).
The controlled cortical impact (CCI) injury model is a model of contusive brain injury that replicates focal abnormalities seen in many human TBI cases. The model was first introduced by Lighthall (1988) for use in the ferret and was later adapted to the rat (Dixon et al., 1991) and mouse (Smith et al., 1995). The mouse CCI model is being used with increasing frequency to investigate posttraumatic cell death (Ariza et al., 2006; Pullela et al., 2006). Multiple laboratories have shown that there is a prominent loss of granular neurons in the hippocampal dentate gyrus in the mouse brain after CCI (Ariza et al., 2006; Pullela et al., 2006). However, there are several types of cells in the hippocampal dentate gyrus, including neural stem cells (NSCs) in the subgranular zone (SGZ) and newborn immature neurons, developed from NSCs and located above the SGZ. These newborn neurons will further migrate into the granular layer and usually reside at the inner one-third of the granular layer, with mature granular neurons composing the outer two-thirds of the granular layer in the adult hippocampal dentate gyrus. These cells are distinct cell types with different characteristics, including morphologies, expression of cell type-specific markers, electrophysiological activities, and functions (Mizumori and Leutgeb, 2001; Kempermann et al., 2004a; Traub et al., 2004; Somogyi and Klausberger, 2005; Forster et al., 2006). It is still unknown whether the cell death is evenly distributed through such cell types or whether there is any cell type-specific vulnerability within the dentate gyrus following TBI. Analyzing the cell type specificity of post-traumatic cell death may provide a clear target and suggest potential therapeutic approaches to prevent cell death after TBI. In this study, we used the CCI model and examined cell death in the hippocampus by using Fluoro-Jade B (FJB; Schmued et al., 1997; Schmued and Hopkins, 2000) combined with cell type-specific markers. We found that newborn immature neurons in the hippocampal dentate gyrus are the most vulnerable cell type to the insults of CCI injury in the mouse brain.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (The Jackson Laboratories) were group housed with a 12/12-hr light/dark cycle and access to food and water ad libitum. They were used in experiments at the age of 4 weeks. All procedures were performed under protocols approved by the Animal Care and Use Committee of the University of Kentucky.
Controlled Cortical Impact Traumatic Brain Injury
C57BL/6 male mice 4 weeks old were subjected to moderate CCI injury as previously described (Sullivan et al., 1999a,b; Hall et al., 2004, 2005; Saatman et al., 2006). Briefly, the mice were anesthetized with 2.5–4% isoflurane and placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA) prior to TBI. Using sterile procedures, the skin was retracted, and a 4-mm craniotomy centered between the lambda and bregma sutures was performed. A point was identified midway between the lambda and bregma sutures and midway between the central suture and the temporalis muscle laterally. The skullcap was carefully removed without disruption of the underlying dura. Prior to the injury, the head of the animal was angled on a medial to lateral plane so that the impacting tip was perpendicular to the exposed cortical surface. This was accomplished by rotating the entire stereotaxic frame in the transverse plane while leaving the nose bar at 5.0. The mouse CCI model uses a pneumatic impactor with which the experimenter can independently control the contact velocity and the level of cortical deformation, thus altering the severity of the injury. In these experiments, the contact velocity was set at 3.5 m/sec and the amount of deformation was set at 0.5 mm, which results in an injury of moderate severity. After injury, a 4-mm disk made from dental cement (Dentsply Trubyte) and Surgicel (Johnson and Johnson, Arlington, TX) was laid over the craniotomy site and adhered to the skull using cyanoacrylate and allowed to dry before the wound was stapled closed. During all surgical procedures and recovery, the core body temperature of the animals was maintained at 36–37°C using a heating pad and a Hova-Bator incubator (37°C; model 1583; Randall Burkey Co.). Sham (noninjured) animals received craniotomy, but no CCI injury.
Tissue Processing
At 4, 24, and 72 hr after TBI surgery, animals were deeply anesthetized with an overdose of sodium pentobarbital and then perfused transcardially with cold 0.9% saline, followed by a fixative containing 4% paraformaldehyde (PFA) in PBS. The brains were removed and postfixed in PFA overnight, then cryoprotected with 30% sucrose for 48 hr. Serial coronal sections (30 μm thick) were cut using a cryostat (Microm HM 500 M) and stored at −20°C. The sections were then processed for FJB and immunohistochemical analysis.
Histology and Immunohistochemistry
Staining with FJB and immunohistochemical analysis with 4′,6-diamidino-2-phenylindole (DAPI) and cell type-specific markers was previously described (Schmued et al., 1997; Schmued and Hopkins, 2000). For FJB staining, sections were first incubated in a solution of 1% alkaline (NaOH) in 80% ethanol for 5 min and then were hydrated in graded ethanols (75%, 50%, and 25%; 5 min each) and distilled water. The sections were then incubated in a solution of 0.06% potassium permanganate for 10 min on a rotating stage, rinsed in distilled water for 2 min, and incubated in a 0.0004% solution of FJB (Histo-Chem Inc., Jefferson, AR) and 0.0004% DAPI (Sigma, St. Louis, MO) for 20 min. Sections were then rinsed in distilled water (2–3 min), air dried, and placed on a slide warmer until fully dry (5–10 min). The dry slides were cleared in xylene (2–5 min) and mounted with DPX (Fluka). For immunostaining, sections were rinsed in phosphate-buffered saline (PBS) three times and incubated in blocking solution (0.1% Triton X-100, 1% bovine serum albumin, 5% normal goat serum in PBS) for 1 hr at room temperature, followed by an overnight incubation with primary antibody at 4°C. Sections were then washed with PBS three times and incubated with the secondary antibody at room temperature for 2 hr. After being treated with DAPI for 2 min, the sections were washed with PBS three times and mounted with Fluorescentmount G. Primary antibodies and their final concentrations were as follows: antinestin antibody (1:200; rabbit; Covance, Berkeley, CA), anti-NeuN antibody (1:1,000; mouse; Chemicon, Temecula, CA), anti-GFAP antibody (1:1,000; mouse; Chemicon), anti-Doublecortin Dcx antibody (1:1,000; guinea pig; Chemicon). Secondary antibodies from Jackson Immunoresearch (West Grove, PA) were all applied with same dilution of 1:1,000.
Microscopy
The sections were analyzed by light microscopy at a primary magnification of ×10–63 with an inverted microscopy system (Zeiss Axiovert 200 M) interfaced with a digital camera (Zeiss Axio Cam MRc5) controlled by a computer. Images were captured in software (AxioVision, v4.0) and assembled and labeled in Photoshop 7.0 (Adobe Systems).
Quantification
The total number of FJB-positive neurons in each region in the hippocampus following TBI was determined through a blinded quantitative histological analysis (five mice for each time point). After sectioning, one in six sections of the entire extent of the hippocampal formation was selected for assessment with FJB staining or combined with fluorescent immunostaining. The anatomical boundaries of the individual hippocampal subregions (dentate gyrus granule cells, CA3 pyramidal cells, and CA1 pyramidal cells) were identified as described by Amaral and Witter (1989). To determine further the distribution of FJB-positive cells in hippocampal dentate gyrus, the dentate gyrus was divided into inner and outer layers, in which the granular neurons are generated from different origins during early hippocampal development (Altman and Bayer, 1990c). The density of the granular neurons in the inner granular neuron layer is much higher than that in the outer layer. Most of the newborn immature neurons were located in the inner one-third of the dentate gyrus. Most of the neurons in the outer two-thirds of the dentate gyrus are mature neurons. Every FJB-positive neuron was counted, and its localization, in the hilus, in the inner one-third of the dentate gyrus, in the outer two-thirds of the dentate gyrus, in CA1, or in CA3, was determined under a fluorescent microscope with a ×40 objective. The percentages of FJB-positive neurons in each subregion were calculated. For quantifying the Dcx-positive newborn neurons and NeuN-positive mature neurons presented in Figure 6, the immunostaining was performed with antibodies against Dcx and NeuN, following the procedure described above. Three sections (40-μm intervals) at the epicenter were chose from each animal (a total of three mice in each group) to quantify the Dcx-positive cells and NeuN-positive cells in the dentate gyrus. For quantifying the Dcx-positive cells, the total numbers of Dcx-positive cells in the ipislateral side or contralateral side of teach section were counted under a fluorescent microscope with a ×40 objective. For quantifying the NeuN-positive cells, the NeuN-positive cell number in the contralateral and ipsilateral sides of the dentate gyrus was estimated by using the optical fractionator method (Gundersen et al., 1988; West et al., 1991; Coggeshall and Lekan, 1996). A Bioquant stereology workstation (BioQuant Image Analysis Corp.) for stereological analyses was used. The step size across sections is 100 × 100 μm, which gives 10–15 intersections for the dentate gyrus. Dissector height was 20 μm, and a 5-μm zone at the uppermost part of the section was excluded from the analysis at every step as the upper guard zone. NeuN-positive cells in a counting frame of 50 × 50 μm were counted under a ×40 objective, which allowed accurate recognition. Each neuron was counted when it came into focus as the plane of focus was moved up and down through the height of the dissector (20 μm) following the unbiased counting rules for the optical dissector (West et al., 1991). The Cavalieri principle was used to estimate the volume of the dentate gyrus using the same stereology workstation. The number of Dcx-positive cell and NeuN-positive cell in the hippocampus was finally presented as cells/mm3. Statistical analysis was performed using either Student’s t-test or ANOVA, followed by post hoc comparison using the Fisher least squares difference test with significance set at P < 0.05.
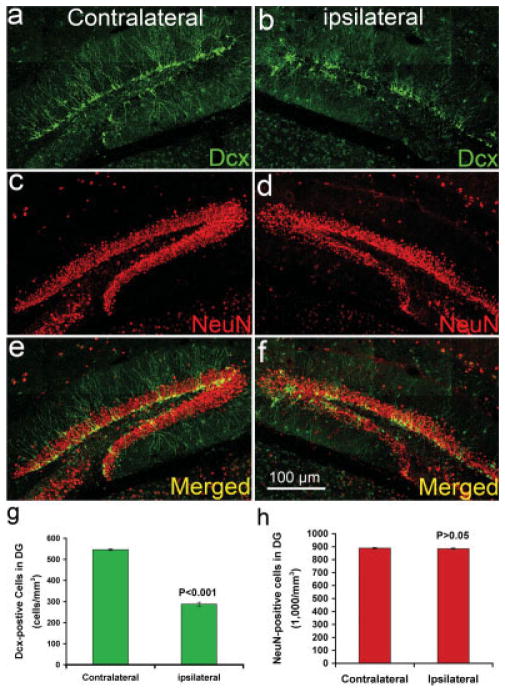
Fig. 6.
The number of newborn neurons in the adult hippocampal dentate gyrus dramatically decreased following CCI injury. Double immunostaning to show the newborn neurons with Dcx antibody and mature neurons with NeuN antibody in the contralateral (a,c,e) and ipsilateral (b,d,f) sides of the hippocampal dentate gyrus 24 hr after moderate CCI injury. a: Dcx-positive newborn neurons in the contralateral side of adult hippocampal dentate gyrus. b: Dcx-positive newborn neurons in the ipsilateral side of adult hippocampal dentate gyrus. c: NeuN-positive mature neurons in the contralateral side of adult hippocampal dentate gyrus. d: NeuN-positive mature neurons in the ipsilateral side of adult hippocampal dentate gyrus. e: Merged image of a and c. f: Merged imaged of b and d. g: Quantification of Dcx-positive newborn neurons in the contralateral and ipsilateral sides of the adult hippocampal dentate gyrus. h: Quantification of NeuN-positive mature neurons in the contralateral and ipsilateral sides of the adult hippocampal dentate gyrus.
BrdU Administration and Cell Counting
The mice were given 3-bromodeoxyuridine (BrdU) injections per day (50 mg/kg in 0.9% saline, i.p.; Sigma, St. Louis, MO) with 4 hr apart at different ages (4 weeks, n = 10; 6 weeks, n = 10). At the age of 8 weeks, mice were subjected to a moderate CCI TBI or sham treatment. Twenty-four hours after surgery, the mice were sacrificed, and the brains were fixed as described above. BrdU immunohistochemistry was performed simultaneously on sections from all intervals. Series of every sixth section (180 μm apart) through each hippocampus were processed. Free-floating sections were washed three times in PBS, incubated in 2 N HCl (30 min at 37°C), and rinsed in 0.1 M borate buffer, pH 8.4, for 10 min. Then, the immunostaining was performed following the procedure described above. For BrdU cell counting, dentate gyrus area contours were created, and volume was measured in Bioquant software (Nashville, TN). Every single BrdU-positive cell in the dentate gyrus were counted under a fluorescent microscope at ×40 through whole series of sections. BrdU-positive cells were expressed as average number/mm3.
RESULTS
Regional Distribution of Hippocampal Neuronal Death Following CCI Injury
In all animals in this study with a moderate level of CCI injury, we observed obvious FJB-positive neurons, which are degenerating neurons (Schmued et al., 1997; Schmued and Hopkins, 2000) in the hippocampus, beginning 4 hr after injury and extending to 72 hr. The FJB-positive neurons at 24 hr after TBI are shown in Figure 1. The somas of degenerating neurons are stained green by FJB. Some of the FJB-positive neurons still show clear dendritic processes (Fig. 1c,e), suggesting that FJB can label the degenerating neurons at a very early stage in the process of cell death. Furthermore, the FJB-positive neurons displayed distinct apoptotic nuclei with either condensed or fragmented morphology (Fig. 1f–i, white arrows) 24 hr after CCI injury. When we counted the number of FJB-positive neurons in each subregion in the hippocampus, including the hilus, the dentate gyrus, CA1, and CA3, we found the greatest number of FJB-positive cells (more than 98%) in the dentate gyrus and only a few FJB-positive neurons occasionally in the hilus (less than 2%). We did not observe any degenerating neurons at CA3 and CA1 in mice with moderate CCI injury (Fig. 1a,b). These results suggest that moderate CCI injury selectively caused secondary neuronal death in the hippocampal dentate gyrus.
Fig. 1.
Secondary neuronal death in the hippocampus following CCI injury. Fluoro-Jade B (FJB) staining to show the degenerating neurons in the mouse brain 24 hr following controlled cortical impact. a: Degenerating neurons are stained by FJB in green. The section was stained with DAPI in blue. b: Enlarged image from panel a to show the degenerating neurons in the hippocampal dentate gyrus. c: High-magnification view to show the morphology of degenerating neurons with FJB staining in green in the dentate gyrus. d: DAPI staining to show the nucleus. e: Merged image of c and d. f: Enlarged image from c to show the degenerating neurons. g: DAPI staining in blue to show the apoptotic neurons with condensed or cleaved nuclear morphologies, marked with a white arrow. h: Merged imaged of f and g. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Rostral to Caudal Distribution of Neuronal Death in the Hippocampal Dentate Gyrus Following CCI Injury
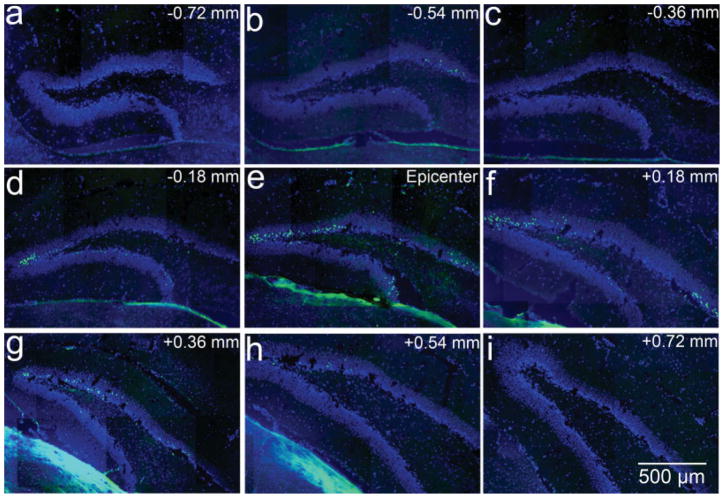
To characterize further the distribution of neuronal death in the hippocampal dentate gyrus in a rostral to caudal direction, we examined the FJB-positive neurons in the serial sections of the entire hippocampus 24 hr after TBI. We found the greatest numbers of the FJB-positive neurons in the section at the epicenter of the CCI injury. The numbers decreased gradually in either the rostral or the caudal direction (Fig. 2). The distribution of the FJB-positive neurons flanked about 1.44 mm across from the first section to the last section in which we observed the FJB-positive neurons. The most distal section with FJB-positive neurons was 0.72 mm away from epicenter (Fig. 2). The volume of the dentate gyrus that containing FJB-positive neurons is about half of the entire hippocampal dentate gyrus (Redwine, 2003).
Fig. 2.
Distribution of degenerating neurons in hippocampal dentate gyrus following moderate traumatic brain injury. FJB staining to show the degenerating neurons in green in the mouse brain following controlled cortical impact. The section was stained with DAPI in blue. a–d: Sections rostral to epicenter. e: Section at epicenter. f–i: Sections caudal to epicenter. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Layer-Specific Distribution of Neuronal Death in the Hippocampal Dentate Gyrus Following CCI Injury
The hippocampal dentate gyrus is composed of the granular zone, where the granular neurons are located, and the SGZ, where the NSCs are located. The granular zone can be further divided into an inner and an outer layer of granular neurons in which the granular neurons are generated from different origins during early hippocampal development (Altman and Bayer, 1990c). The density of the granular neurons in the inner granular neuron layer is much higher than that in the outer layer. Most of the newborn immature neurons were located in the inner one-third of the dentate gyrus. Most of the neurons in the outer two-thirds of the dentate gyrus are mature neurons. Because the granular neurons in the inner and outer layers are generated from different origins (Altman and Bayer, 1990a,b; Forster et al., 2006) and are at different maturation stages, we reasoned that their characteristics (Forster et al., 2006), functions (Forster et al., 2006), and responses to TBI (Tran et al., 2006) might be different as well. To characterize further the distribution of the FJB-positive neurons in the specific sublayer in the dentate gyrus, we counted the number of FJB-positive neurons in the inner one-third and outer two-thirds of the hippocampal granular cell layer from 4 hr to 72 hr after TBI (five mice for each time point and more than 20,000 FJB-posiitve cells were counted). All of the FJB-positive neurons in the dentate gyrus were counted. We found the greatest number (about 80%) of FJB-positive neurons in the inner granular cell layer. In contrast, there were many fewer FJB-positive neurons in the outer granular cell layer (less than 15%), and very rarely were FJB-positive cells detected in the SGZ of the hippocampal dentate gyrus (Fig. 3). These results further confirmed the selective cell death of neurons in the inner granular cell layer in the dentate gyrus following moderate CCI injury.
Fig. 3.
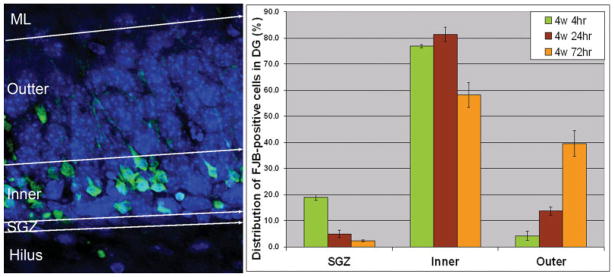
Layer-specific distribution of secondary neuronal death in the hippocampal dentate gyrus following CCI injury. FJB staining to show the degenerating neurons in green in the mouse brain following controlled cortical impact. DAPI was used to stain with DAPI in blue to show the structure of the hilus and the dentate gyrus. Left: Degenerating neurons were observed in the hilus, SGZ, inner granular layer, outer granular layer, and molecular layer (ML). Right: Quantification of degenerating neurons in the hippocampal dentate gyrus. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Cell Type Specificity of Neuronal Death in the Hippocampal Dentate Gyrus Following CCI Injury
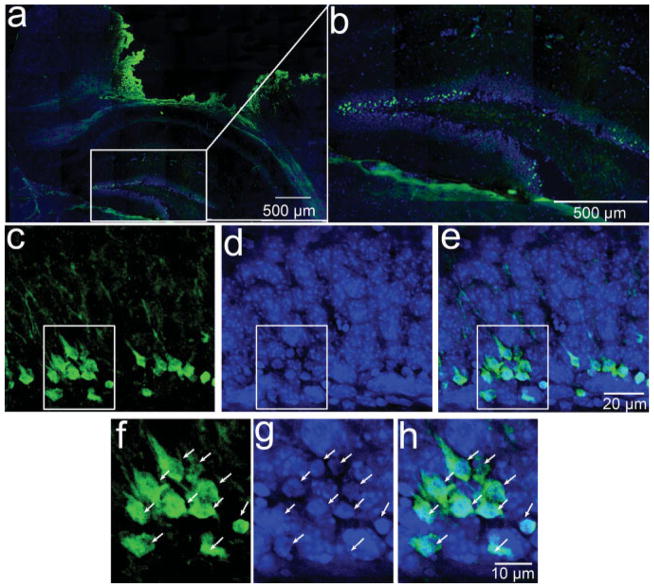
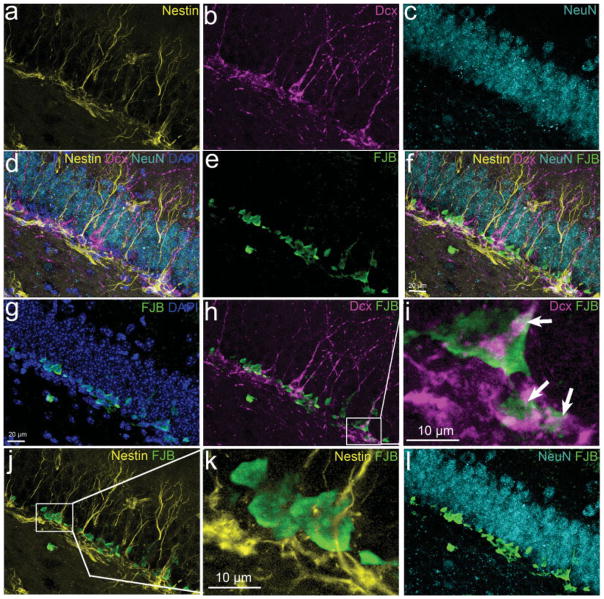
There are different cell types within the hippocampal dentate gyrus, including NSCs, newborn immature granular neurons, and mature granular neurons (Kempermann et al., 2004a; Traub et al., 2004; Forster et al., 2006). These cells originate from different embryonic sources during early hippocampal development (Altman and Bayer, 1990a,b; Forster et al., 2006) and are distinct cell types with different characteristics, including morphologies, expression of cell type-specific markers, electrophysiological activities, and functions. To determine the cell types of FJB-positive cells in the hippocampal dentate gyrus, we doubly labeled the sections with FJB and cell type-specific markers, such as nestin for NSCs (Lendahl et al., 1990; Dahlstrand et al., 1992; Kim et al., 2003), Dcx for newborn immature neurons (Gleeson et al., 1999; Chen et al., 2004; Koizumi et al., 2006), and NeuN for mature neurons (Mullen et al., 1992; Korzhevskii et al., 2006). The results from the brains 4 hr after TBI are shown in Figure 4. The results show that nestin-positive NSCs (Fig. 4a), Dcx-positive newborn immature neurons (Fig. 4b), and NeuN-positive mature neurons (Fig. 4c) are localized in three distinct layers (Fig. 4d). The FJB-positive degenerating neurons (Fig. 4e) are sharply localized in the inner one-third of the granular cell layer (Fig. 4g), confirming the results described above showing that the majority of FJB-positive cells are located in the inner granular cell layer. Double staining shows that FJB-positive cells were localized at the Dcx-positive newborn neuron layer (Fig. 4h), whereas they were located above the nestin-positive NPC layer (Fig. 4j) and below the NeuN-positive mature neuron layer (Fig. 4l). These results suggest that most of the FJB-positive cells might be newborn immature neurons. Indeed, the FJB-positive neurons are colabeled with Dcx (Fig. 4i). In contrast, we observed more than 1,000 JFB-positive cells and did not observe any FJB-positive cells colabeled with nestin (Fig. 4j,k) and only found two FJB-positive cells expressing weak NeuN (data not shown). We also performed confocal microscopy to confirm that FJB-positive cell expresses Dcx (Fig. 5). Together, these results suggest that CCI injury selectively induced newborn immature neuron death in the hippocampal dentate gyrus.
Fig. 4.
Cell type specificity of secondary neuronal death in the hippocampal dentate gyrus following CCI injury. a: Nestin antibody was used to show the neural stem cells in yellow in the hippocampal dentate gyrus. b: Dcx antibody to show newborn neurons in pink in the hippocampal dentate gyrus. c: NeuN antibody to show mature neurons in the hippocampal dentate gyrus. d: Merged image of a–c to show the layer of neural stem cells, newborn neurons, and mature neurons in the hippocampal dentate gyrus. e: Degenerating neurons were stained with FJB in green. f: Merged image of a–e to show the location of FJB-positive neurons between the nestin-positive cell layer and the NeuN-positive cell layer. g: The section was counterstained with DAPI in blue to show the location of FJB-positive cells in the hippocampal dentate gyrus. h: Separately merged images of Dcx staining and FJB staining to show the position of newborn neurons and degenerating neurons in the hippocampal dentate gyrus. i: Enlarged view of h to show the cells doubly stained with Dcx and FJB, marked by a white arrow. j: Separately merged images of nestin staining and FJB staining to show the position of neural stem cells and degenerating neurons in the hippocampal dentate gyrus. k: Enlarged view of j to show that there is no colocalization of Dcx with FJB. l: Separately merged images of NeuN staining and FJB staining to show the position of mature neuron and degenerating neurons in the hippocampal dentate gyrus.
Fig. 5.
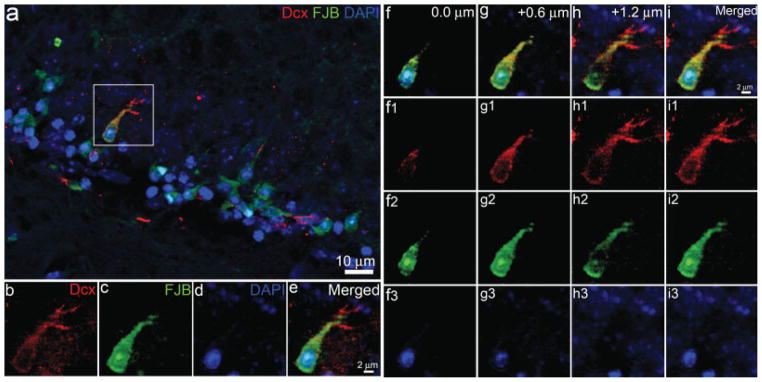
Confocal microscopy to confirm that FJB stained degenerating newborn neurons in the hippocampal dentate gyrus following TBI. a: Confocal image of FJB-positive cell expressing Dcx in the hippocampal dentate gyrus using FJB staining combined with immunostaining with antibody against Dcx. The section was counterstained with DAPI in blue. Enlarged view of FJB- and Dcx-positive cell in the white box is shown in separated channels in b–d. e: Merged image of b–d. Focal sections through the FJB- and Dcx-positive cell are shown in f–i. f: Merged image of f1–f3. g: Merged image of g1–g3. h: Merged image of h1–h3. i: Merged image of i1–i3.
Number of Newborn Neurons in the Adult Hippocampal Dentate Gyrus Dramatically Decreased Following CCI Injury
To confirm further the selective death of newborn neurons following CCI injury, we determined the numbers of Dcx-positive newborn neurons and NeuN-positive mature neurons in both the ipsilateral side and the contralateral side of the hippocampal dentate gyrus 24 hr after moderate CCI by using double immunostaining with antibody against Dcx and antibody against NeuN, a marker for mature neurons. The results show that the Dcx-positive newborn neurons are dramatically decreased in the ipsilateral side of hippocampal dentate gyrus (Fig. 6b) compared with the contralateral side (Fig. 6a). (three mice and >1,000 Dcx-positive cells were counted) The number of newborn neurons in the ipsilateral side is only about half (Fig. 6g) of the number of Dcx-positive newborn neurons in the contralateral side of hippocampal dentate gyrus. The statistical analysis shows that this difference is significant (P < 0.001). In contrast, the number of NeuN-positive mature neurons in the ipsilateral side of hippocampal dentate gyrus (Fig. 6d) is about the same (Fig. 6h) as the number of NeuN-positive mature neurons in the contralateral side (Fig. 6c). There is no statistically significant difference in the number of NeuN-positive mature neurons between the ipsilateral side and the contralateral side of hippocampal dentate gyrus following CCI injury (P > 0.05). These results confirm the selective death of newborn neurons in the adult hippocampal dentate gyrus following moderate CCI injury.
To determine further the selective death of newborn neurons in the hippocampal dentate gyrus following moderate CCI injury, we examined the ages of the neurons at the time when they were induced to die from CCI injury. It has been shown that newly generated neurons in the adult hippocampal dentate gyrus take about 4 weeks to express NeuN, a marker for mature neurons; at least 3 months to functionally mature; and more than 6 months to integrate into the existing network (Zhao et al., 2006; Toni et al., 2007). Therefore, neurons at age 2 weeks were considered as young immature neurons. Neurons 4 weeks old were considered as “mature” based on marker expression. To distinguish the type of cell death following CCI injury, we specifically labeled and traced the fates of young immature neurons and “mature” neurons following CCI injury. In this study, we observed a significant decrease of Dcx-positive cells 24 hr after moderate CCI in the adult hippocampal gyrus. Newborn neurons express Dcx from the time when they are generated and maintain the expression up to about 3 weeks. Therefore, most of these dead Dcx-positive newborn neurons should be generated before CCI injury. To label the young immature neurons at age 2 weeks and “mature” neurons at age 4 weeks at the time of CCI injury, we injected BrdU at 2 weeks or 4 weeks before the mice were subjected to CCI injury or sham treatment (n = 5 for each group). All mice were subjected to moderate CCI injury or sham treatment 24 hr after surgery and then were sacrificed to examine the BrdU-labeled cells in the hippocampal dentate gyrus. Immunostaining with anti-BrdU antibody was performed to identify BrdU-labeled cells. BrdU-positive cells in the dentate gyrus were quantified. For the mice that received BrdU 4 weeks before surgery, there were 238.3 ± 4.6/mm3 BrdU-positive cells in the dentate gyrus of sham-treated mice. Instead, there is 186.3 ± 11.9/mm3 of BrdU-positive cells in the dentate gyrus of TBI-injured mice. These results indicate that TBI injury increased death of 52.0 ± 16.4/mm3 BrdU-positive “mature” neurons in dentate gyrus. However, for the mice in which BrdU was injected 2 weeks before surgery, there were 357.6 ± 57.6/mm3 BrdU-positive cells in the dentate gyrus of sham-treated mice and 175.9 ± 24.0/mm3 BrdU-positive cells in the dentate gyrus of TBI-injured mice. These results indicate that TBI increased the death by 181.7 ± 34.3/mm3 BrdU-positive young immature neurons in the dentate gyrus. These results indicate that, 24 hr following TBI, 22.3% of dead cells are “mature” neurons and 77.7% are young immature neurons, suggesting the selective death of young immature neurons in the adult hippocampal dentate gyrus following CCI injury.
DISCUSSION
Despite the understanding that TBI results in neuropathological changes in the hippocampus that cause neurological dysfunction, i.e., cognitive impairment (Hamm et al., 1992; Scheff et al., 1997; Prigatano, 2005; Salmond and Sahakian, 2005) and epilepsy (Kelly, 2004; D’Ambrosio et al., 2005; Pitkanen and McIntosh, 2006), little is known about the cell type specificity of cell death, the mechanisms underlying selective cell death in the hippocampus, or the neuroprotective approaches that might prevent the specific type of cell death in the hippocampus following TBI. In the present study, we show that newborn immature neurons in the hippocampal dentate gyrus are the cell types perhaps most vulnerable to moderate CCI injury in the mouse brain. These results may provide insight into the mechanisms of selective cell death in the hippocampus and may also provide an innovative target for the development of potential treatment strategies following TBI.
We examined secondary cell death in the hippocampus between 4 and 72 hr after CCI injury. We observed a great number of FJB-positive degenerating neurons 4 hr after CCI, suggesting that secondary cell death in the hippocampus happens soon after injury and that its inhibition has to be undertaken as early as possible.
It has been previously shown that the hippocampus is particularly vulnerable to TBI with various experimental injury models, including CCI (Lighthall, 1988; Scheff et al., 1997; Hall et al., 2005), fluid percussion (Thompson et al., 2005), and stretch injury (McCarthy, 2003; Mehrholz et al., 2005). The distribution and amount of cell death in the hippocampus vary in different injury models (Kelly, 2004), with various injury severities (Hellmich et al., 2005), and at different ages (Tong et al., 2002). In addition, the amounts are slightly different between research groups. Generally, in the CCI injury model (Anderson et al., 2005; Saatman et al., 2006), the hippocampal dentate gyrus shows the highest number of FJB-positive neurons. In contrast, in the fluid percussion injury model (Lowenstein et al., 1992; Cater et al., 2006; Morrison et al., 2006), the CA3 and CA1 regions show the highest numbers of FJB-positive neurons. Furthermore, in the in vitro hippocampal slice stretch injury model (DeRidder et al., 2006), CA3 and the dentate gyrus show the highest numbers of FJB-positive neurons. In addition, the severity of injury contributes to the distribution and amount of cell death in the hippocampus as well (Markgraf et al., 2001; Hellmich et al., 2005). The less severe the injury, the more restricted the cell death is in the specific regions mentioned above. With more severe injury, the distribution of cell death becomes less localized. In our present study, mice were subjected to moderate (0.5 mm) CCI injury, in which the majority of the degenerating neurons were located in the dentate gyrus. These data suggest the complexity of damage with different types of injury models. Understanding the different distribution and selective cell death with different injury models would provide critical information on guiding the potential therapeutic approaches.
The granular neurons in the dentate gyrus are divided into inner and outer layers, in which the granular neurons are generated from different sources during early hippocampal development (Altman and Bayer, 1990a,c). The granular neurons in the outer layer of the dentate gyrus originate from the densely packed, spindle-shaped cells of the secondary dentate matrix, which is a derivative of the primary dentate neuroepithelium (Altman and Bayer, 1990a,b). These neurons are generated mainly during the first week after birth (Kempermann et al., 2004a; Forster et al., 2006). In contrast, the granular neurons in the thicker inner layer of the dentate gyrus originate from an intrinsic tertiary germinal matrix. The granular neurons in the inner layer are born during the infantile and juvenile periods. The tertiary dentate matrix disappears between day P20 and P30, and the proliferating cells become largely confined to the SGZ at the base of the granular layer. This subset of cells proliferates actively after birth and begins to give rise to neurons located in the inner granular layer around the first week after birth (Kempermann et al., 2004a; Forster et al., 2006). Because the granular neurons in the inner and outer layers are generated from different origins (Altman and Bayer, 1990a,c), their characteristics (Forster et al., 2006), function (Forster et al., 2006), and response (Tran et al., 2006) to TBI might be different as well. When we examined the distribution of the FJB-positive neurons in the injured dentate gyrus, we found most of the FJB-positive neurons located in the inner granular layer, suggesting that the granular neurons in the inner layer are much more vulnerable to TBI than the granular neurons in the outer layer of the dentate gyrus.
There are different types of cells (Kempermann et al., 2004a) in the inner layer of the dentate gyrus, including newborn immature neurons and mature neurons. Newborn immature neurons are continually being generated from the SGZ, where the NSCs line the hilar side of the granular neuron layer (Gage et al., 1998; Kempermann and Gage, 2000; van Praag et al., 2002; Jagasia et al., 2006) and migrate into the granular zone of the dentate gyrus (Kempermann et al., 2004a). The newborn immature neurons transiently express a specific marker, Dcx (Gleeson et al., 1999; Chen et al., 2004), and are located deep within the inner granular neuron layer. The newborn immature neurons will migrate farther out within the granular layer and develop into mature neurons that express the specific marker NeuN (Mullen et al., 1992). We can determine, based on the position and cell type-specific markers, the different identities of the degenerating cells in the dentate gyrus. Accordingly, when we examined the cell type of FJB-positive degenerating neurons with FJB staining combined with double staining with cell type-specific markers, we found that the vast majority (more than 80%) of the degenerating neurons after moderate CCI injury are newborn immature neurons in the inner granular layer. In contrast, there was only a small portion of degenerating cells that were mature granular neurons. These results suggest that, among the highly vulnerable dentate gyral neurons, the newborn immature neurons are especially vulnerable following moderate CCI injury. Normally, these newborn neurons are continuously generated, integrated into the existing neural network in the hippocampus, and thought to function in the consolidation of new memories or learned behaviors and increased performance in behavioral tasks (Eriksson et al., 1998; Gage, 2002; Taupin and Gage, 2002; van Praag et al., 2002; Kempermann et al., 2004b; Kempermann et al., 2006). Therefore, the selective death of newborn neurons following TBI might be involved in the impairment of learning and memory following TBI. It has been shown that newly generated neurons in the adult hippocampal dentate gyrus take about 4 weeks to express NeuN, a marker for mature neurons, at least 3 months to mature functionally, and more than 6 months to integrate into the existing network (Zhao et al., 2006; Toni et al., 2007). Therefore, the death of newborn neurons might not significantly affect learning and memory at the acute phase following TBI, but it might be involved in the impairment of learning and memory in the chronic phase.
The mechanisms underneath this injury phenotype are still unknown. However, recent studies on the electrophysiological characteristics during development of newborn immature neurons in the dentate gyrus show that newborn immature neurons are electrophysiologically distinct from the mature granular neurons in the dentate gyrus (Liu et al., 1996; Tovar and Westbrook, 1999; Sipila et al., 2005; Ye et al., 2005; Ge et al., 2006; Giza et al., 2006; Larson et al., 2006; Overstreet-Wadiche et al., 2006). It has been agreed that the GABAA receptors are first expressed in migrating neuroblasts and migrating newborn neurons, followed by α-amino-5-hydroxy-3-methyl-4-isoxazole propionic acid (AMPA) receptors, and finally N-methyl-aspartate (NMDA) receptors expressed during newborn neuron maturation (Liu et al., 1996; Ye et al., 2005; Pandis et al., 2006; Thomas et al., 2006). The newborn granular neurons in the dentate gyrus appear to be the first to receive γ-aminobutyric acid (GABA)-ergic synaptic input at about 1 week after birth while migrating into the granular neuron layer (Overstreet-Wadiche and Westbrook, 2006). These newborn granular neurons then receive glutamatergic inputs by 2 weeks while undergoing transition from immature to mature neurons (Overstreet-Wadiche and Westbrook, 2006). Although, GABA is the main inhibitory neurotransmitter in the adult brain, GABAergic transmission is excitatory, rather than inhibitory, during early stages of development and postnatal periods because of elevated intracellular chloride and the relatively depolarized value of the GABA type A (GABAA) reversal potential (EGABA; Chen et al., 1996; Ben-Ari, 2002; Owens and Kriegstein, 2002; Payne et al., 2003; Represa and Ben-Ari, 2005; Rivera et al., 2005). In addition to the possibility of a GABAergic excitotoxic response of the newborn immature granule neurons, it is also known that the newborn immature neurons display exaggerated excitatory responses to glutamate receptor stimulation, possibly because of the transition of receptor expression from NR2B to NR2A while they are transitioning into mature granular neurons (Flint et al., 1997; Tovar and Westbrook, 1999; Ye et al., 2000; Snyder et al., 2001; Thomas et al., 2006). It will be very interesting to determine whether the differences in the location and excitabilities of GABA and glutamate receptors on immature granular neurons vs. mature granular neurons contribute to the greater vulnerability of the immature neurons following TBI. If so, then pharmacological approaches that inhibit glutamate release, or glutamatergic receptor activation, might specifically be applied to protect the highly vulnerable newborn hippocampal neurons.
Acknowledgments
Contract grant sponsor: Kentucky Spinal Cord and Head Injury Research Trust; Contract grant number: KSCHIRT 4-2 (to J.C.); Contract grant sponsor: NIH; Contract grant number: 1R01 NS046566 (to E.D.H.).
References
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990a;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990b;301:325–342. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Prolonged sojourn of developing pyramidal cells in the intermediate zone of the hippocampus and their settling in the stratum pyramidale. J Comp Neurol. 1990c;301:343–364. doi: 10.1002/cne.903010303. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Anderson KJ, Miller KM, Fugaccia I, Scheff SW. Regional distribution of fluoro-jade B staining in the hippocampus following traumatic brain injury. Exp Neurol. 2005;193:125–130. doi: 10.1016/j.expneurol.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Ariza M, Serra-Grabulosa JM, Junque C, Ramirez B, Mataro M, Poca A, Bargallo N, Sahuquillo J. Hippocampal head atrophy after traumatic brain injury. Neuropsychologia. 2006;44:1956–1961. doi: 10.1016/j.neuropsychologia.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Cater HL, Sundstrom LE, Morrison B., 3rd Temporal development of hippocampal cell death is dependent on tissue strain but not strain rate. J Biomech. 2006;39:2810–2818. doi: 10.1016/j.jbiomech.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Chen G, Trombley PQ, van den Pol AN. Excitatory actions of GABA in developing rat hypothalamic neurones. J Physiol. 1996;494:451–464. doi: 10.1113/jphysiol.1996.sp021505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Magavi SS, Macklis JD. Neurogenesis of corticospinal motor neurons extending spinal projections in adult mice. Proc Natl Acad Sci U S A. 2004;101:16357–16362. doi: 10.1073/pnas.0406795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103:589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Fender JS, Fairbanks JP, Simon EA, Born DE, Doyle DL, Miller JW. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 2005;128:174–188. doi: 10.1093/brain/awh337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRidder MN, Simon MJ, Siman R, Auberson YP, Raghupathi R, Meaney DF. Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition. Neurobiol Dis. 2006;22:165–176. doi: 10.1016/j.nbd.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Flint AC, Maisch US, Weishaupt JH, Kriegstein AR, Monyer H. NR2A subunit expression shortens NMDA receptor synaptic currents in developing neocortex. J Neurosci. 1997;17:2469–2476. doi: 10.1523/JNEUROSCI.17-07-02469.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat Rev Neurosci. 2006;7:259–267. doi: 10.1038/nrn1882. [DOI] [PubMed] [Google Scholar]
- Fujimoto ST, Longhi L, Saatman KE, Conte V, Stocchetti N, McIntosh TK. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci Biobehav Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza CC, Maria NS, Hovda DA. N-methyl-D-aspartate receptor subunit changes after traumatic injury to the developing brain. J Neurotrauma. 2006;23:950–961. doi: 10.1089/neu.2006.23.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis [review] Apmis. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Gupta M. Posttraumatic epilepsy: a review of scientific evidence. Indian J Physiol Pharmacol. 2006;50:7–16. [PubMed] [Google Scholar]
- Hall ED, Detloff MR, Johnson K, Kupina NC. Peroxynitrite-mediated protein nitration and lipid peroxidation in a mouse model of traumatic brain injury. J Neurotrauma. 2004;21:9–20. doi: 10.1089/089771504772695904. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Dixon CE, Gbadebo DM, Singha AK, Jenkins LW, Lyeth BG, Hayes RL. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- Hellmich HL, Capra B, Eidson K, Garcia J, Kennedy D, Uchida T, Parsley M, Cowart J, DeWitt DS, Prough DS. Dose-dependent neuronal injury after traumatic brain injury. Brain Res. 2005;1044:144–154. doi: 10.1016/j.brainres.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Isoniemi H, Kurki T, Tenovuo O, Kairisto V, Portin R. Hippocampal volume, brain atrophy, and APOE genotype after traumatic brain injury. Neurology. 2006;67:756–760. doi: 10.1212/01.wnl.0000234140.64954.12. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Song H, Gage FH, Lie DC. New regulators in adult neurogenesis and their potential role for repair. Trends Mol Med. 2006;12:400–405. doi: 10.1016/j.molmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Jiang T, Gao Y, Fu Y. Clinical research and surgical treatment of posttraumatic epilepsy. J Huazhong Univ Sci Technol Med Sci. 2004;24:392–395. doi: 10.1007/BF02861876. [DOI] [PubMed] [Google Scholar]
- Kelly KM. Modeling traumatic brain injury and posttraumatic epilepsy. Epilepsy Curr. 2004;4:160–161. doi: 10.1111/j.1535-7597.2004.44015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Neurogenesis in the adult hippocampus. Novartis Found Symp. 2000;231:220–235. discussion 235–241, 302–226. [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004a;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr Opin Neurobiol. 2004b;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Chesler EJ, Lu L, Williams RW, Gage FH. Natural variation and genetic covariance in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2006;103:780–785. doi: 10.1073/pnas.0510291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Panchision D, Kittappa R, McKay R. Generating CNS neurons from embryonic, fetal, and adult stem cells. Methods Enzymol. 2003;365:303–327. doi: 10.1016/s0076-6879(03)65022-6. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Korzhevskii DE, Gilerovich EG, Zin’kova NN, Grigor’ev IP, Otellin VA. Immunocytochemical detection of brain neurons using the selective marker NeuN. Neurosci Behav Physiol. 2006;36:857–859. doi: 10.1007/s11055-006-0098-5. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Thomas KE. The incidence of traumatic brain injury among children in the United States: differences by race. J Head Trauma Rehabil. 2005;20:229–238. doi: 10.1097/00001199-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Larson MJ, Perlstein WM, Demery JA, Stigge-Kaufman DA. Cognitive control impairments in traumatic brain injury. J Clin Exp Neuropsychol. 2006;28:968–986. doi: 10.1080/13803390600646860. [DOI] [PubMed] [Google Scholar]
- Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- Liu YB, Lio PA, Pasternak JF, Trommer BL. Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J Neurophysiol. 1996;76:1074–1088. doi: 10.1152/jn.1996.76.2.1074. [DOI] [PubMed] [Google Scholar]
- Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markgraf CG, Clifton GL, Aguirre M, Chaney SF, Knox-Du Bois C, Kennon K, Verma N. Injury severity and sensitivity to treatment after controlled cortical impact in rats. J Neurotrauma. 2001;18:175–186. doi: 10.1089/08977150150502604. [DOI] [PubMed] [Google Scholar]
- McCarthy ML, MacKenzie EJ, Durbin DR, Aitken ME, Jaffe KM, Paidas CN, Slomine BS, Dorsch AM, Berk RA, Christensen JR, Ding R. The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch Phys Med Rehabil. 2005;86:1901–1909. doi: 10.1016/j.apmr.2005.03.026. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Stretching the truth. Why hippocampal neurons are so vulnerable following traumatic brain injury. Exp Neurol. 2003;184:40–43. doi: 10.1016/j.expneurol.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Mehrholz J, Major Y, Meissner D, Sandi-Gahun S, Koch R, Pohl M. The influence of contractures and variation in measurement stretching velocity on the reliability of the Modified Ashworth Scale in patients with severe brain injury. Clin Rehabil. 2005;19:63–72. doi: 10.1191/0269215505cr824oa. [DOI] [PubMed] [Google Scholar]
- Minino AM, Anderson RN, Fingerhut LA, Boudreault MA, Warner M. Deaths: injuries, 2002. Natl Vital Stat Rep. 2006;54:1–124. [PubMed] [Google Scholar]
- Mizumori SJ, Leutgeb S. Directing place representation in the hippocampus. Rev Neurosci. 2001;12:347–363. doi: 10.1515/revneuro.2001.12.4.347. [DOI] [PubMed] [Google Scholar]
- Morrison B, 3rd, Cater HL, Benham CD, Sundstrom LE. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. J Neurosci Methods. 2006;150:192–201. doi: 10.1016/j.jneumeth.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–215. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- Overstreet-Wadiche LS, Bensen AL, Westbrook GL. Delayed development of adult-generated granule cells in dentate gyrus. J Neurosci. 2006;26:2326–2334. doi: 10.1523/JNEUROSCI.4111-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Developmental neurotransmitters? Neuron. 2002;36:989–991. doi: 10.1016/s0896-6273(02)01136-4. [DOI] [PubMed] [Google Scholar]
- Pandis C, Sotiriou E, Kouvaras E, Asprodini E, Papatheodoropoulos C, Angelatou F. Differential expression of NMDA and AMPA receptor subunits in rat dorsal and ventral hippocampus. Neuroscience. 2006;140:163–175. doi: 10.1016/j.neuroscience.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, McIntosh TK. Animal models of post-traumatic epilepsy. J Neurotrauma. 2006;23:241–261. doi: 10.1089/neu.2006.23.241. [DOI] [PubMed] [Google Scholar]
- Prigatano GP. Impaired self-awareness after moderately severe to severe traumatic brain injury. Acta Neurochir Suppl. 2005;93:39–42. doi: 10.1007/3-211-27577-0_5. [DOI] [PubMed] [Google Scholar]
- Pullela R, Raber J, Pfankuch T, Ferriero DM, Claus CP, Koh SE, Yamauchi T, Rola R, Fike JR, Noble-Haeusslein LJ. Traumatic injury to the immature brain results in progressive neuronal loss, hyper-activity and delayed cognitive impairments. Dev Neurosci. 2006;28:396–409. doi: 10.1159/000094166. [DOI] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Kaila K. Two developmental switches in GABAergic signalling: the K+-Cl− cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 2005;562:27–36. doi: 10.1113/jphysiol.2004.077495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Feeko KJ, Pape RL, Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Sahakian BJ. Cognitive outcome in traumatic brain injury survivors. Curr Opin Crit Care. 2005;11:111–116. doi: 10.1097/01.ccx.0000155358.31983.37. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Baldwin SA, Brown RW, Kraemer PJ. Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J Neurotrauma. 1997;14:615–627. doi: 10.1089/neu.1997.14.615. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Albertson C, Slikker W., Jr Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, Deng Y, Mbye LH, Hall ED. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26:1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Soltesz I, Voipio J, Kaila K. Depolarizing GABA acts on intrinsically bursting pyramidal neurons to drive giant depolarizing potentials in the immature hippocampus. J Neurosci. 2005;25:5280–5289. doi: 10.1523/JNEUROSCI.0378-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statler KD. Pediatric posttraumatic seizures: epidemiology, putative mechanisms of epileptogenesis and promising investigational progress. Dev Neurosci. 2006;28:354–363. doi: 10.1159/000094162. [DOI] [PubMed] [Google Scholar]
- Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-kappaB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999a;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PG, Thompson MB, Scheff SW. Cyclosporin A attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp Neurol. 1999b;160:226–234. doi: 10.1006/exnr.1999.7197. [DOI] [PubMed] [Google Scholar]
- Tasker RC. Changes in white matter late after severe traumatic brain injury in childhood. Dev Neurosci. 2006;28:302–308. doi: 10.1159/000094156. [DOI] [PubMed] [Google Scholar]
- Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69:745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL. Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol. 2006;95:1727–1734. doi: 10.1152/jn.00771.2005. [DOI] [PubMed] [Google Scholar]
- Thompson HJ, Lifshitz J, Marklund N, Grady MS, Graham DI, Hovda DA, McIntosh TK. Lateral fluid percussion brain injury: a 15-year review and evaluation. J Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Tong W, Igarashi T, Ferriero DM, Noble LJ. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp Neurol. 2002;176:105–116. doi: 10.1006/exnr.2002.7941. [DOI] [PubMed] [Google Scholar]
- Toni N, Teng EM, Bushong EA, Aimone JB, Zhao C, Consiglio A, van Praag H, Martone ME, Ellisman MH, Gage FH. Synapse formation on neurons born in the adult hippocampus. Nat Neurosci. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19:4180–4188. doi: 10.1523/JNEUROSCI.19-10-04180.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LD, Lifshitz J, Witgen BM, Schwarzbach E, Cohen AS, Grady MS. Response of the contralateral hippocampus to lateral fluid percussion brain injury. J Neurotrauma. 2006;23:1330–1342. doi: 10.1089/neu.2006.23.1330. [DOI] [PubMed] [Google Scholar]
- Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J Clin Exp Neuropsychol. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Ye GL, Song Liu X, Pasternak JF, Trommer BL. Maturation of glutamatergic neurotransmission in dentate gyrus granule cells. Brain Res Dev Brain Res. 2000;124:33–42. doi: 10.1016/s0165-3806(00)00103-6. [DOI] [PubMed] [Google Scholar]
- Ye GL, Yi S, Gamkrelidze G, Pasternak JF, Trommer BL. AMPA and NMDA receptor-mediated currents in developing dentate gyrus granule cells. Brain Res Dev Brain Res. 2005;155:26–32. doi: 10.1016/j.devbrainres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Zhang TY, Cho HJ, Lee S, Lee JH, Choi SH, Ryu V, Yoo SB, Lee JY, Kim DG, Jahng JW. Impairments in water maze learning of aged rats that received dextromethorphan repeatedly during adolescent period. Psychopharmacology. 2006 doi: 10.1007/s00213-006-0548-3. (in press) [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]