Abstract
The human cutaneous circulation is an accessible and representative regional circulation for investigating mechanisms of microvascular dysfunction, a systemic disease process occurring early in the pathogenesis of atherosclerosis. Elevated concentrations of low-density lipoproteins ([LDL]) are highly atherogenic and independently associated with the severity of coronary atherosclerosis through their actions on the lectin-like oxidized LDL receptors (LOX-1). We hypothesized that cutaneous microvascular dysfunction, as measured by a decrement in endothelial nitric oxide- (NO-) dependent vasodilation during local heating, would be correlated with serum [LDL], oxidized [LDL], and soluble LOX-1 receptors [sLOX-1]. Intradermal microdialysis fibers were placed in the skin of 53 otherwise healthy men and women (aged 52±8 years) whose serum [LDL] ranged from 72 to 233 mg/dL. Skin blood flow was measured by laser Doppler flowmetry over a local forearm skin site as it was heated (42 °C) to induce sustained local vasodilation. After flux plateaued, L-NAME was infused to block endothelial NO synthase in order to determine the NO-dependent portion of the vasodilatory response. Data were normalized to maximal cutaneous vascular conductance (CVC). NO-dependent vasodilation was reduced as a linear function of [LDL] (R2=0.303, p<0.001), oxidized [LDL] (R2=0.214, p<0.001), and [sLOX-1] (R2=0.259, p=0.026) but was unrelated to high-density lipoprotein (HDL) concentration (R2=0.003, p=0.68). Hypercholesterolemia-induced microvascular dysfunction is related to various LDL markers and involves a reduction in NO-dependent vasodilation that appears to be a progressive process measurable in the skin microcirculation.
Introduction
Atherosclerosis is a systemic disease process (Abularrage et al., 2005b) characterized by inflammation, endothelial dysfunction, and vessel remodeling. Elevations in low-density lipoprotein (LDL) and its oxidized form (oxLDL) are major risk factors for the development of atherosclerosis (Toshima et al., 2000; Vasankari et al., 2001). OxLDLs are primary initiators of a deleterious cascade in the pathogenesis of atherosclerotic disease that begins with the activation of lectin-like oxLDL (LOX-1) receptors. LOX-1 stimulation activates multiple downstream signaling events resulting in a pro-oxidant environment and a loss of the crucial vasoprotective molecule, nitric oxide (NO) (Mitra et al., 2011), one of the earliest functional indicators of hyperocholesterolemic-associated vascular disease.
The human skin circulation is an accessible and generalizable vascular bed for the in vivo assessment of endothelial and smooth muscle vascular function (Abularrage et al., 2005a; Debbabi et al., 2010; Holowatz et al., 2007; Khan et al., 2008). Alterations in cutaneous vascular signaling are evident early in disease processes and are highly correlated with measures of conduit vessel function (Debbabi et al., 2010). This model has now been validated across several clinical populations and is highly correlated with measures of vascular dysfunction in the coronary (Khan et al., 2008) and the renal circulations (Coulon et al., 2012; Debbabi et al., 2010). Hypercholesterolemia-induced microvascular dysfunction involving a reduction in NO-dependent vasodilation is clearly evident in the cutaneous circulation (Binggeli et al., 2003; Holowatz and Kenney, 2011a, b; Holowatz et al., 2011; Rossi et al., 2009). Hypercholesterolemic humans exhibit a significantly attenuated cutaneous vasodilatory response to local skin heating, a stimulus known to induced vasodilation predominantly through the production of NO via endothelial nitric oxide synthase (eNOS) (Bruning et al., 2012; Kellogg et al., 2008).
We previously showed that cutaneous NO-dependent vasodilation in response to an eNOS-specific stimulus is attenuated in human subjects with clinically defined hypercholesterolemia (LDL>160 mg/dL). The reduction in NO-dependent vasodilation is mediated by: (1) upregulated arginase activity which limits substrate availability through eNOS (Holowatz et al., 2011), (2) an increase in ascorbate sensitive oxidant stress mechanisms (Holowatz and Kenney, 2011b), and (3) a reduction in the essential eNOS cofactor tetrahydrobiopterin (BH4) (Holowatz and Kenney, 2011a). Together, these mechanisms contribute to eNOS uncoupling and cutaneous microvascular dysfunction in humans with substantially elevated [LDL].
However, because our previous investigations were limited to comparisons of subject groups with normal (LDL<100 mg/dL) and high (>160 mg/dL), little is known the presence and/or severity of microvascular dysfunction in humans with moderately elevated cholesterol or across a spectrum of cholesterol concentrations. Nor has the relation between microvascular dysfunction and LOX-1 receptors been examined. With respect to the latter point, LOX-1 receptor function can be assessed by measuring soluble LOX-1 (sLOX-1) receptors in blood samples. LOX-1 is shed and released into the plasma as sLOX-1 which is a specific and sensitive biomarker of atherosclerotic vascular disease in both apparently healthy men with risk factors (Uchida et al., 2011) and patients with acute coronary syndrome (Kume et al., 2010).
In the present study, we expand the scope of our previous work by characterizing a broader spectrum of LDL-induced microvascular dysfunction. We hypothesized that detectable cutaneous microvascular dysfunction as measured by a reduction in NO-dependent vasodilation during local skin heating would be present in a midrange (110<LDL<160) hypercholesterolemic cohort of human subjects. We further explored whether total LDL-cholesterol, oxLDL-cholesterol, or soluble LOX-1 receptors are significant predictors of cutaneous microvascular dysfunction in an otherwise healthy group of middle-aged subjects.
Methods
Subjects
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent was voluntarily obtained from all subjects prior to participation. Fifty-three healthy men and women (Table 1) participated in the study consisting of functional in vivo assessment of cutaneous NO-dependent vasodilatation during local skin heating. Subjects were nonobese, nonsmokers, nondiabetic, normally active (neither sedentary nor highly exercise trained), and not currently taking statins or other medications including aspirin, vitamins, or antioxidants.
Table 1.
Subject Characteristics (n=53; 26 men, 27 women).
| Mean±SD | Range | R2 | |
|---|---|---|---|
| Age (years) | 52±8 | 40–70 | 0.011 |
| Total Cholesterol (mg/dL) | 207±47 | 138–320 | 0.275* |
| HDL (mg/dL) | 54±15 | 31–99 | 0.003 |
| Total Cholesterol/HDL | 4.1±1.4 | 2.3–8.1 | 0.19* |
| LDL (mg/dL) | 131±40 | 72–233 | 0.303* |
| Oxidized LDL (U/L) | 64±23 | 21–131 | 0.214* |
| Soluble LOX-1 receptors (pg/mL) | 44±16 | 14–76 | 0.259* |
| Triglycerides (mg/dL) | 111±56 | 41–310 | 0.102* |
| Fasting glucose (mg/dL) | 93±8 | 78–114 | 0.007 |
| ADMA (μmol/L) | 0.45±0.14 | 0.21–0.76 | 0.031 |
| Framingham risk | 6.8±4.9 | 1.1–22.4 | 0.032 |
p<0.05 for the relation to functional cutaneous NO-dependent vasodilation.
For the main aim of the study, the subject sample was treated as a continuum of LDL markers. To provide additional insights, subjects were subsequently grouped as normocholesterolemic (NC; LDL=95± 11 mg/dL), midrange cholesterolemic (MC; LDL=123±11 mg/dL), and hypercholesterolemic (HC; LDL=179±24 mg/dL).
Blood analysis
Serum and plasma samples were obtained during the initial screening visit and stored at −80 °C for batched analysis of asymmetrical dimethyl L-arginine (ADMA: Alpco Immunoassay Salem, NH) and endogenous NOS inhibitor, and oxLDL (Mercodia Uppsala, Sweden) though enzyme-linked immunosorbent assays (ELISA). ADMA intra-and inter-assay coefficients of variation were 8.2% and 24.0%, respectively. oxLDL intra- and inter-assay coefficients of variation were 6.1% and 5.6%, respectively. Total cholesterol, triglycerides, high density lipoproteins, and fasting blood glucose were analyzed by an outside clinical laboratory (Quest Diagnostics, Pittsburgh PA). Serum low-density lipoproteins were calculated through the Friedewald formula (DeLong et al., 1986).
Soluble LOX-1 receptor measurements
Soluble LOX-1 receptor (sLOX-1) concentration was determined in serum obtained from a subset of 19 subjects for whom banked serum samples were available. Concentrations of sLOX-1 were measured using a cytometric bead array (BD Biosciences, San Jose, CA). This format employs multiple populations of microbeads, each with unique fluorescence characteristics. Each bead population is coated with capture antibodies raised against specific cytokines or soluble receptors. The beads are then mixed into the sample along with detection antibodies raised against each analyte that are labeled with compounds that fluoresce at a different wavelength than the beads. After incubation and washing, the beads are resuspended and analyzed with a BD FACSArray flow cytometer. Analytes are differentiated by bead fluorescence characteristics, and analyte concentration is measured by the fluorescence intensity of the labeled detection antibody, which is compared with standard curves generated with recombinant analytes. The sLOX-1 assay had detection limits (based on 95% confidence intervals over blank) of <5 pg/ml,.
Intradermal microdialysis
All protocols were performed in a thermoneutral laboratory with the subject semi-supine and the experimental arm at heart level. An intradermal microdialysis probe was inserted into the ventral forearm skin for localized delivery of pharmacological agents as previously described (Holowatz et al., 2011). The microdialysis site was initially perfused with a lactated Ringers solution.
Laser-Doppler flux
An index of skin blood flow was measured using integrated laser-Doppler flowmeter probes and local temperature was controlled with a local heater (MoorLAB, Temperature Monitor SH02, Moor Instruments, Devon, UK) placed directly above the microdialysis membrane. This multipoint probe monitored blood flow under an area approximately 2 mm directly over each microdialysis fiber. Arterial blood pressure was measured every 5 minutes using an automated brachial cuff (Cardiocap) which was verified with brachial auscultation. Cutaneous vascular conductance (CVC) was calculated as laser-Doppler flux divided by mean arterial pressure (MAP) and standardized to maximal CVC, i.e., %CVCmax.
Local heating protocol
After the resolution of the initial insertion trauma with local skin temperature clamped at 33 °C, a standardized local skin warming protocol was performed to induce eNOS-dependent vasodilatation (Bruning et al., 2012). The local heater temperature was increased from 33 °C to 42 °C at a rate of 0.1 °C per second and then clamped at 42 °C for the duration of the heating protocol. After skin blood flow reached an established plateau (30–40 minutes), 10 mM L-NAME was perfused to quantify NO-dependent vasodilatation. This concentration of L-NAME has been shown to be efficacious in younger, middle-aged (Bruning et al., 2012), hypercholesterolemic (Holowatz et al., 2011), and older age groups using the same intra-dermal microdialysis technique.
After post-L-NAME stabilization of skin blood flow, local temperature was increased to 43 °C and 28 mM sodium nitroprusside (SNP) was perfused to induce CVCmax (Bruning et al., 2012). In our previous work and in pilot work this combination of heat and high concentration of SNP has been shown to induce maximal vasodilation. Higher temperatures (44 °C) or increasing concentrations of SNP (50 mM) did not produce a further increase in absolute CVC.
Data and statistical analysis
All data from the local heating experiments were digitized at 40 Hz and stored for offline analysis with signal-processing software (Windaq, DATAQ Instruments). CVC data were averaged for a stable 5 minutes of baseline, plateau, post L-NAME plateau, and maximal vasodilatation. The vasodilatation due to NO was calculated from the difference between the plateau and the post-L-NAME plateau and represented as a percentage. Because the late plateau phase of the local heating response is primarily dependent on eNOS function whereas the early phase has contributions from both sensory nerves and NO, analysis and further discussion focus solely on the later phase of the cutaneous vasodilatory response.
Pearson correlations and linear regression analysis were used to examine the relations between continuous variables. Forward and backward stepwise regression analysis was also conducted. In addition to the regression analyses performed on the entire subject sample, student’s unpaired t tests were used to determine significant differences in subgroup (NC, MC, HC) characteristics. A mixed models two-way repeated measures ANOVA was conducted to detect differences in %CVCmax among the 3 subject groups for the different phases of the local warming response (SAS, version 9.1). Specific planned comparisons with Bonferoni correction were performed when appropriate to determine where differences between groups occurred. The level of significance was set at α=0.05. Values are presented as means ± SEM.
Results
Subject characteristics are presented in Table 1. The sample was selected to represent a wide range of serum cholesterol concentrations in an otherwise healthy middle-aged population. Representative skin blood flow responses during local heating are shown in Fig. 1 for one normocholesterolemic subject and one hypercholesterolemic subject. LDL and oxLDL values for these two subjects are shown on the figure. Vertical arrows are also depicted for each subject to illustrate how NO-dependent vasodilation was calculated from the drop in %CVCmax from the plateau value to the post-L-NAME value.
Fig. 1.
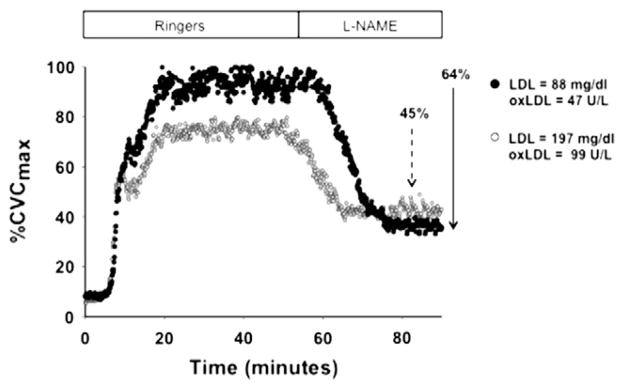
Representative tracings of the cutaneous vascular conductance (%CVCmax) response to local skin heating in a normocholesterolemic subject and a hypercholesterolemic subject. Both the plateau %CVCmax and the NO-dependent vasodilation (depicted by the vertical arrows showing the reduction in with NOS inhibition with L-NAME) were attenuated with elevated serum LDL concentrations. Arrows indicate the reduction in %CVCmax during NOS inhibition with L-NAME.
Fig. 2 illustrates mean values for local heating plateau %CVCmax and NO-dependent vasodilation for subject groups NC (LDL= 95±11 mg/dL), MC (LDL=123±11 mg/dL), and HC (LDL=179± 24 mg/dL). The inset figure to the right corresponds with the reduction in %CVCmax during NOS inhibition with L-NAME. The HC group reached a significantly lower plateau in CVC compared to the NC group, with the MC group intermediate (p=0.034 vs NC and p<0.001 vs HC). A similar stepwise pattern was seen for the NO-dependent portion of that plateau, with the HC group significantly lower than the NC group (p<0.05).
Fig. 2.
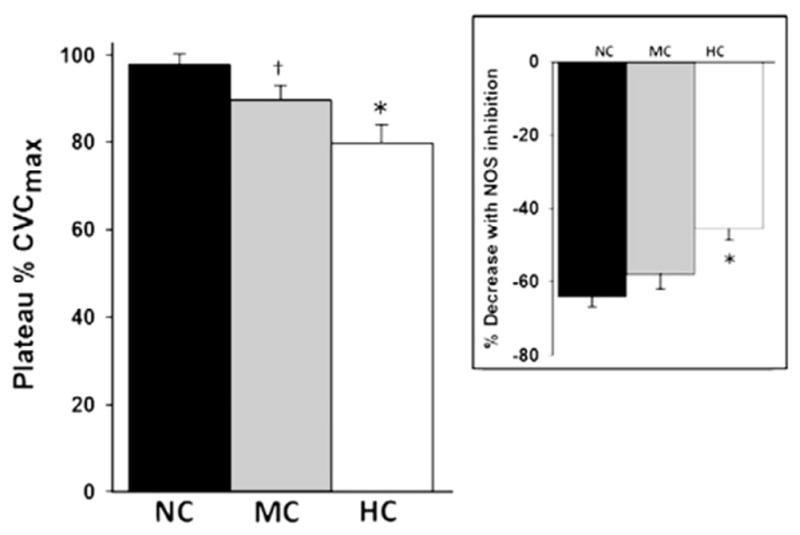
Mean (±SD) values for local heating plateau %CVCmax and NO-dependent vasodilation for subjects grouped as normocholesterolemic (NC; 95±11 mg/dL), mid-range cholesterolemic (MC; 123±11 mg/dL), and hyperocholesterolemic (HC; 179±24 mg/dL). *p<0.05 difference from the NC and MC groups. †p<0.05 difference from the NC group. The inset figure to the right corresponds with the reduction in %CVCmax during NOS inhibition with L-NAME illustrated by the vertical arrows shown in Fig. 1.
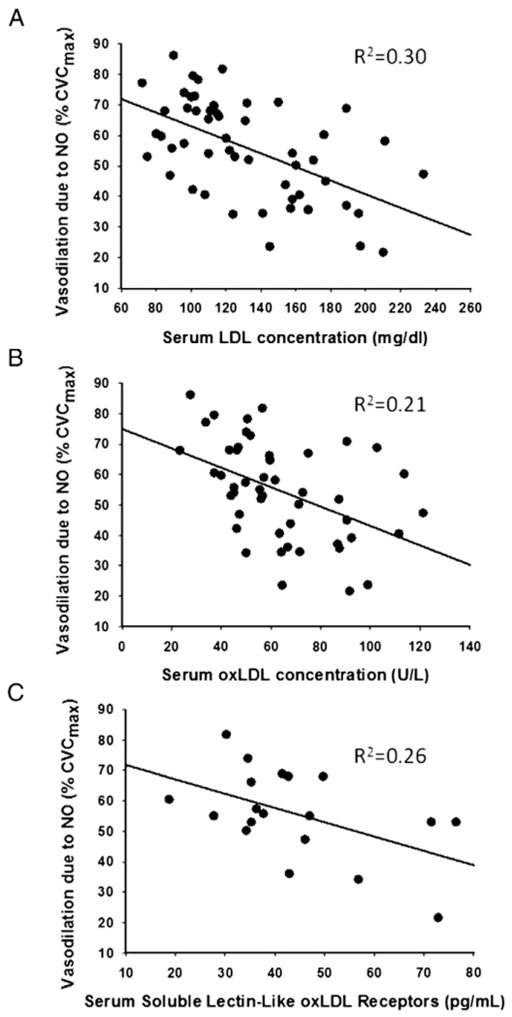
Fig. 3 is a scatterplot for the full range of data comparing the NO-dependent vasodilatory response as a function of (A) [LDL], (B) [oxLDL], and (C) [sLOX-1]. All relations are significant (p<0.0001 for [LDL] and [oxLDL]; p=0.026 for [sLOX-1]). When LDL was entered into a forward stepwise regression analysis, no additional variable significantly improved the relation. Although not shown, [oxLDL] was also highly correlated with the total cholesterol: HDL ratio (R2= 0.50; p<0.001); thus NO-dependent vasodilation was also correlated with this common clinical marker of cardiovascular risk (R2=0.19, p<0.001). NO-dependent vasodilation was also significantly, albeit weakly, related to serum triglyceride concentration (R2=0.10; p= 0.022), but not to calculated 10-year coronary heart disease risk as measured by the Framingham risk score (R2=0.03; p=0.20) (Table 1).
Fig. 3.
The relation between NO-dependent vasodilation and (A) serum [LDL], (B) serum [oxLDL], and (C) serum soluble LOX-1 receptors.
Discussion
The present study utilized the human skin as a model circulation for examining cholesterol-induced microvascular dysfunction in subjects with a broad spectrum of serum cholesterol concentrations. The principal new finding of the present study was that cutaneous vasodilation in response to a specific eNOS-dependent stimulus was reduced in a cohort of subjects with moderately elevated LDL cholesterol but not clinically defined as hypercholesterolemic. In that regard, changes in the skin microvasculature may precede and predict vascular dysfunction in larger systemic vessels. Further, there was a significant relation between NO-dependent vasodilation in the cutaneous microvasculature and serum LDL, oxLDL cholesterol, sLOX-1 receptors, and triglycerides. However, serum LDL cholesterol concentration explained a greater percentage of the variance in NO-dependent vasodilation than did oxLDL or the other variables.
Atherosclerosis is a complex systemic disease process involving inflammation, oxidant stress, and LDL uptake by macrophages into the vascular wall. High LDL concentrations are a major risk factor for the development of atherosclerotic vascular disease (Toshima et al., 2000), however it has also been suggested that oxLDL is more predictive of future CHD events (Meisinger et al., 2005), as oxLDL concentrations are representative of the generalized inflammatory pro-oxidant environment in the vessel. In the present study, we found that total serum LDL concentration was more strongly related to functional measures of microcirculatory dysfunction than oxLDL in an apparently healthy middle-aged population. Total LDL remained the only significant predictor of reduced NO-dependent vasodilation in a forward stepwise regression model. This is in contrast to what is observed in the conduit circulation where oxLDL and oxLDL/total LDL are more strongly associated with decrements in flow mediated vasodilatory responses in the brachial artery (van der Zwan et al., 2009) after adjusting for age, sex, glucose tolerance and Framingham risk score. These differences may be partially explained by the different circulations (macro vs. micro circulation) explored, the functional quantification of NO-dependent vasodilation with pharmacological antagonists, and the relatively healthy and homogenous subject group tested in the present study.
That microvascular dysfunction correlates most highly with LDL whereas macrovascular dysfunction is closely related to oxLDL may possibly reflect the progression of atherosclerotic disease, i.e., that microvascular dysfunction typically precedes functional changes in larger vessels. Changes in microvessels related to LDL may occur prior to any substantive shift toward a more pro-oxidant milieu. As the pro-oxidant state progresses, macrovascular dysfunction appears in addition to continuing microvascular changes as atherosclerosis progresses.
One of the earliest and most critical events in the development of endothelial dysfunction in atherosclerosis is the interaction of oxLDL and the LOX-1 receptor. LOX-1 expression is induced under pathological conditions in a feed-forward mechanism whereby oxLDL upregulates LOX-1 expression in a time- and concentration-dependent manner (Mehta and Li, 1998; Mitra et al., 2011). In vascular cell types, LOX-1 stimulation activates multiple downstream signaling events leading to a decreasing in NO bioavailability including the activation of oxidant stress mechanisms through NADPH oxidases (Mitra et al., 2011) and the increase in arginase activity which utilizes the same substrate as eNOS and thereby contributes to NOS uncoupling (Ryoo et al., 2011). In the present study we measured serum sLOX-1 receptors as a surrogate measures of LOX-1 receptors. We found a significant relation between sLOX-1 receptors and reduced NO-dependent vasodilation. While this biomarker has not been explored in a hypercholesterolemic human model without overt vascular disease, sLOX-1 receptors are increased in patients with coronary heart disease (Hayashida et al., 2005). Additional research needs to be conducted to show a direct relation between the vascular LOX-1 receptors and downstream mediators of microvascular vasoconstrictor and vasodilator function.
We have utilized the cutaneous circulation to assess cutaneous microvascular dysfunction across a broad range of cholesterol concentrations. The cutaneous circulation is an accessible vascular bed for examining microcirculatory function and dysfunction and skin-specific thermal stimuli including local heating enable the pharmacodissection of important vascular signaling pathways. Local skin heating has been used in a number of clinical populations to evaluate decrements in microcirculatory function including hypertensives (Smith et al., 2011), primary aged (Minson et al., 2002), hyperhomcysteinemia (Abahji et al., 2007), renal disease (Stewart et al., 2004), type II diabetes (Sokolnicki et al., 2007), peripheral vascular disease (Rossi and Carpi, 2004), and systemic sclerosis (Boignard et al., 2005). In the present study we utilized local skin heating to induce eNOS-mediated vasodilation (Bruning et al., 2012) and we further directly quantified functional NO-dependent vasodilation within the same site pharmacologically through intradermal microdialysis. Interestingly, when comparing the predetermined cholesterol groups the mid-range group had a significant attenuation in the plateau skin blood flow response to local heating compared to the normocholesterolemic group, however there was greater variability for the within site quantification of NO-dependent vasodilation.
Our group has previously explored the mechanisms underlying the reduced NO-dependent vasodilation with hypercholesterolemia in the cutaneous microcirculation and the normalizing effects of an atorvastatin intervention. Hypercholesterolemia reduces the bio-availability of NO through a number of mechanisms including: (1) upregulated arginase activity which limits substrate availability through eNOS (Holowatz et al., 2011), (2) an increase in ascorbate sensitive oxidant stress mechanisms (Holowatz and Kenney, 2011b), and (3) a reduction in the essential eNOS cofactor tetrahydrobiopterin (BH4) (Holowatz and Kenney, 2011a). Increases in the endogenous NOS inhibitor assymetrical dimethyl-L-arginine (ADMA) do not contribute to hypercholesterolemic-microvascular dysfunction (Holowatz and Kenney, 2011a). While these precise eNOS uncoupling signaling pathways were not directly explored in the present study, it is likely that these are common underlying mechanisms of microvascular dysfunction. Interestingly, we found a relation between upstream mediators including the LOX-1 receptor and downstream functional measure of NO-dependent vasodilation. It may be that these mechanisms’ individual downstream targets of LOX-1 contribute differentially in the early pre-clinical stages of hypercholesterolemia and/or other compensatory mechanisms are upregulated and contribute to the variability in functional NO-dependent vasodilation observed.
In the present study, we characterized a broader spectrum of LDL-induced microvascular dysfunction and were able to determine that functionally detectable deficit in cutaneous microvascular function were present in a mid-range (110<LDL<160) hypercholesterol-emic cohort of human subjects. This study helps to further validate the cutaneous circulation as a model to assess microcirculatory dysfunction in pre-clinical disease states. Further it has been recently suggested that microcirculatory impairments, like those detected in the skin, predicate conduit vessel atherogenesis through inducing deleterious retrograde shear rate patterns (Padilla et al., 2011; Young et al., 2010).
Limitations
The regression analysis for determining the relation between LDL and cutaneous NO-dependent vasodilation was conducted using a calculated LDL value based on the Friedewald formula and not a direct measurement of LDL concentrations. Clinically LDL concentrations are commonly calculated and these values provide the basis for the various predictions equations for cardiovascular risk. We did not measure C-reactive protein or other generalized inflammatory markers in our subjects. Considering the healthy status of our population it is unlikely that we would have observed differences in these markers.
Despite variability in the data, our data show a significant correlation between NO-dependent vasodilation and various markers of LDL. However, because individuals are commonly grouped clinically as having normal, high, or moderately elevated cholesterol, we also examined the data as three distinct subgroups. When arbitrarily grouped in this manner (Fig. 2), the local heating-induced plateau — which reflects both NO-dependent and NO-independent vasodilation —changes in a graded fashion across groups. While the statistical comparisons related to the MC group (significantly lower plateau without the NO-dependent vasodilation achieving statistical significance) is likely a power issue, the possibility remains that the mildly reduced plateau in the MC group is unrelated to NO.
In summary, using a healthy cohort of human subjects with a broad range of serum LDL cholesterol concentrations, we found that that cutaneous vasodilation in response to a specific eNOS-dependent stimulus was reduced in subjects with only moderately elevated LDL cholesterol, i.e., not clinically defined as hypercholesterolemic. There was a significant relation between NO-dependent vasodilation in the cutaneous microvasculature and serum LDL-cholesterol, oxLDL cholesterol, sLOX-1 receptors, and triglycerides, but serum LDL cholesterol alone explained the greatest percentage of the variance in NO-dependent vasodilation. This study further validates the cutaneous circulation as a model for detecting microvascular dysfunction in pre-clinical disease states.
Acknowledgments
The authors would like to thank Jane Pierzga (PSU) and Gloria Sloan (GHSU) for their technical assistance. We would also like to thank Jessica Kutz, Anna Stanhewicz, and Caroline Smith for assistance with data collection. This work has been supported by R01-HL-089302 and M01-RR-10732.
Footnotes
Disclosures
The authors have nothing to disclose and no conflicts of interest to report.
References
- Abahji TN, et al. Acute hyperhomocysteinemia induces microvascular and macrovascular endothelial dysfunction. Arch Med Res. 2007;38:411–416. doi: 10.1016/j.arcmed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Abularrage CJ, et al. Evaluation of macrocirculatory endothelium-dependent and endothelium-independent vasoreactivity in vascular disease. Perspect Vasc Surg Endovasc Ther. 2005a;17:245–253. doi: 10.1177/153100350501700315. [DOI] [PubMed] [Google Scholar]
- Abularrage CJ, et al. Evaluation of the microcirculation in vascular disease. J Vasc Surg. 2005b;42:574–581. doi: 10.1016/j.jvs.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Binggeli C, et al. Statins enhance postischemic hyperemia in the skin circulation of hypercholesterolemic patients: a monitoring test of endothelial dysfunction for clinical practice? J Am Coll Cardiol. 2003;42:71–77. doi: 10.1016/s0735-1097(03)00505-9. [DOI] [PubMed] [Google Scholar]
- Boignard A, et al. Local hyperemia to heating is impaired in secondary Raynaud’s phenomenon. Arthritis Res Ther. 2005;7:R1103–R1112. doi: 10.1186/ar1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruning RS, et al. Endothelial nitric oxide synthase mediates cutaneous vasodilation during local heating and is attenuated in middle-aged human skin. J Appl Physiol. 2012;112:2019–2026. doi: 10.1152/japplphysiol.01354.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulon P, et al. Impairment of skin blood flow during postocclusive reactive hyperhemy assessed by laser Doppler flowmetry correlates with renal resistive index. J Hum Hypertens. 2012 Jan;26 (1):56–63. doi: 10.1038/jhh.2010.117. [DOI] [PubMed] [Google Scholar]
- Debbabi H, et al. Noninvasive assessment of endothelial function in the skin microcirculation. Am J Hypertens. 2010;23:541–546. doi: 10.1038/ajh.2010.10. [DOI] [PubMed] [Google Scholar]
- DeLong DM, et al. A comparison of methods for the estimation of plasma low-and very low-density lipoprotein cholesterol. The Lipid Research Clinics Prevalence Study. JAMA. 1986;256:2372–2377. [PubMed] [Google Scholar]
- Hayashida K, et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: a novel marker for early diagnosis. Circulation. 2005;112:812–818. doi: 10.1161/CIRCULATIONAHA.104.468397. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. J Physiol. 2011a;589:4787–4797. doi: 10.1113/jphysiol.2011.212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Kenney WL. Oral atorvastatin therapy increases nitric oxide-dependent cutaneous vasodilation in humans by decreasing ascorbate-sensitive oxidants. Am J Physiol Regul Integr Comp Physiol. 2011b;301:R763–R768. doi: 10.1152/ajpregu.00220.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, et al. The cutaneous circulation as a model of generalized microvascular function. J Appl Physiol. 2007 Jul;105 (1):370–372. doi: 10.1152/japplphysiol.00858.2007. [DOI] [PubMed] [Google Scholar]
- Holowatz LA, et al. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. J Physiol. 2011;589:2093–2103. doi: 10.1113/jphysiol.2010.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DL, Jr, et al. Endothelial nitric oxide synthase control mechanisms in the cutaneous vasculature of humans in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H123–H129. doi: 10.1152/ajpheart.00082.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan F, et al. Relationship between peripheral and coronary function using laser Doppler imaging and transthoracic echocardiography. Clin Sci (Lond) 2008;115:295–300. doi: 10.1042/CS20070431. [DOI] [PubMed] [Google Scholar]
- Kume N, et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts prognosis after acute coronary syndrome–a pilot study. Circ J. 2010;74:1399–1404. doi: 10.1253/circj.cj-09-0924. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Li DY. Identification and autoregulation of receptor for OX-LDL in cultured human coronary artery endothelial cells. Biochem Biophys Res Commun. 1998;248:511–514. doi: 10.1006/bbrc.1998.9004. [DOI] [PubMed] [Google Scholar]
- Meisinger C, et al. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation. 2005;112:651–657. doi: 10.1161/CIRCULATIONAHA.104.529297. [DOI] [PubMed] [Google Scholar]
- Minson CT, et al. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Mitra S, et al. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25:419–429. doi: 10.1007/s10557-011-6341-5. [DOI] [PubMed] [Google Scholar]
- Padilla J, et al. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension. 2011;57:484–489. doi: 10.1161/HYPERTENSIONAHA.110.165365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Carpi A. Skin microcirculation in peripheral arterial obliterative disease. Biomed Pharmacother. 2004;58:427–431. doi: 10.1016/j.biopha.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Rossi M, et al. Skin blood flowmotion and microvascular reactivity investigation in hypercholesterolemic patients without clinically manifest arterial diseases. Physiol Res. 2009;58:39–47. doi: 10.33549/physiolres.931351. [DOI] [PubMed] [Google Scholar]
- Ryoo S, et al. OxLDL-dependent activation of arginase II is dependent on the LOX-1 receptor and downstream RhoA signaling. Atherosclerosis. 2011;214:279–287. doi: 10.1016/j.atherosclerosis.2010.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, et al. Upregulation of Inducible Nitric Oxide Synthase Contributes to Attenuated Cutaneous Vasodilation in Essential Hypertensive Humans. Hypertension. 2011 Nov;58 (5):935–942. doi: 10.1161/HYPERTENSIONAHA.111.178129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolnicki LA, et al. Contribution of nitric oxide to cutaneous microvascular dilation in individuals with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2007 Jan;292 (1):E314–E318. doi: 10.1152/ajpendo.00365.2006. [DOI] [PubMed] [Google Scholar]
- Stewart J, et al. Noninvasive interrogation of microvasculature for signs of endothelial dysfunction in patients with chronic renal failure. Am J Physiol Heart Circ Physiol. 2004;287:H2687–H2696. doi: 10.1152/ajpheart.00287.2004. [DOI] [PubMed] [Google Scholar]
- Toshima S, et al. Circulating oxidized low density lipoprotein levels. A biochemical risk marker for coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:2243–2247. doi: 10.1161/01.atv.20.10.2243. [DOI] [PubMed] [Google Scholar]
- Uchida K, et al. Associations of atherosclerotic risk factors with oxidized low-density lipoprotein evaluated by LOX-1 ligand activity in healthy men. Clin Chim Acta. 2011;412:1643–1647. doi: 10.1016/j.cca.2011.05.022. [DOI] [PubMed] [Google Scholar]
- van der Zwan LP, et al. Circulating oxidized LDL: determinants and association with brachial flow-mediated dilation. J Lipid Res. 2009;50:342–349. doi: 10.1194/jlr.P800030-JLR200. [DOI] [PubMed] [Google Scholar]
- Vasankari T, et al. Oxidized LDL and thickness of carotid intima-media are associated with coronary atherosclerosis in middle-aged men: lower levels of oxidized LDL with statin therapy. Atherosclerosis. 2001;155:403–412. doi: 10.1016/s0021-9150(00)00573-6. [DOI] [PubMed] [Google Scholar]
- Young CN, et al. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–392. doi: 10.1016/j.atherosclerosis.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]