Abstract
Objective: To explore association of patient characteristics and telehealth alert data with all-cause key medical events (KMEs) of emergency department (ED) visits and hospitalizations as well as cardiac-related KMEs of ED visits, hospitalizations, and medication changes. Materials and Methods: A 6-month retrospective study was conducted of electronic patient records of heart failure (HF) patients using telehealth services at a Massachusetts home health agency. Data collected included patient demographic, psychosocial, disease severity factors and telehealth vital signs alerts. Association between patient characteristics and KMEs was analyzed by Generalized Estimating Equations. Results: The sample comprised 168 patients with a mean age of 83 years, 56% females, and 96% white. Ninety-nine cardiac-related KMEs and 87 all-cause KMEs were recorded for the subjects. Odds of a cardiac-related KME increased by 161% with the presence of valvular co-morbidity (p=0.001) and 106% with increased number of telehealth alerts (adjusted p<0.0001). Odds of an all-cause KME increased by 124% (p=0.02), 127% (p=0.01), and 70% (adjusted p<0.0001) with the presence of cancer co-morbidity, anxiety, and increased number of telehealth alerts, respectively. Overall, only 3% of all telehealth alerts were associated with KMEs. Conclusions: The very low proportion of telehealth vital sign alerts associated with KMEs indicates that telehealth alerts alone cannot inform the need for intervention within the larger context of HF care delivery in the homecare setting. Patient-relevant data such as psychosocial and symptom status, involvement with HF self-management, and presence of co-morbidities could further inform the need for interventions for HF patients in the homecare setting.
Key words: cardiology/cardiovascular disease, home health monitoring, telehealth
Introduction
Heart failure (HF) is the most common chronic illness in home health, affecting almost 6 million Americans today.1 To assist HF patients manage their complex condition, home health agencies across North America are increasingly implementing new technologies, such as telehealth.2 However, an overlooked consequence of large numbers of patients with HF using telehealth is the resulting data explosion.3,4 Telehealth data overload has been considered a significant barrier to the sustained use of telehealth for managing HF.5,6 Clearly, the volume of telehealth-generated data could overwhelm the home health clinicians managing HF patients6 and impair efficient responses to telehealth alerts.11 Recent researchers have found a lack of association of telehealth with improved health utilization outcomes.7–10 Decision support models utilizing relevant patient parameters may inform prioritization of care and home health interventions in response to telehealth alerts, which may help improve efficiency of telehealth for HF management.
This study explored the association of patient characteristics and telehealth alert data with key medical events (KMEs) experienced by patients with HF receiving telehealth services from a home health agency. KMEs are events that resulted in a change in the level of care delivered by home health nurses to their HF patients. Cardiac-related KME in this study was defined as a cardiac-related emergency department (ED) visit, hospitalization, or medication changes, and all-cause KME was defined as an all-cause ED visit or hospitalization experienced by the HF patient.
Materials and Methods
This study was a retrospective chart review of electronic patient records from a Massachusetts home health agency. This agency has been using electronic documentation for nursing services and telehealth for over 10 years. In this study, telehealth consisted of an electronic device in the patient's home through which the patient daily transmitted his or her physiological data (weight, blood pressure, heart rate [HR], and oxygen saturation [O2 sat]) and questionnaire on specific daily symptoms. The telehealth nurse was responsible for telehealth alerts, and the visiting nurse followed up on abnormal telehealth vital signs by visiting the HF patient and/or contacting the referring physicians or cardiologists to consult on patient care.
The sample included Medicare patients 65 years of age or older admitted to the home health agency with HF as a diagnosis and who had used telehealth between March and September 2011. HF patients with telehealth data transmitted for less than 3 days or missing any of the five vital sign parameters (weight, heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and pulse oximetry [O2 sat]) were excluded from analysis.
Home Health Electronic Health Record Data Sources
Initially, the electronic health record (EHR) database of the home health agency was queried using the ICD-9-CM codes 428.0, 428.2, 428.3, 428.43, 428.9, and 402 and telehealth alert data to identify eligible subjects with a diagnosis of HF and who had used telehealth services. The search returned a list of medical record numbers of eligible subjects in Microsoft® (Redmond, WA) Excel sheet format, which was used for data analysis. The medical record numbers of the subjects, which are unique to the home health agency, were entered as the search criterion in the home health agency electronic documentation system to find corresponding study data for the subjects. Study data included Medicare-mandated Outcome and Assessment Information Set (OASIS) patient data as well as nursing documentation notes and telehealth logs.
Data on patient demographics (age, race, and gender), psychosocial status (anxiety, depression, and living situation [assessed by presence or absence of caregivers]), disease characteristics (dyspnea, type [primary/secondary] of admitting HF diagnosis), number and types of co-morbidities, number of medications, type of cardiac medications, and status of HF diagnosis (new/chronic) were obtained from OASIS and nursing documentation notes. Telehealth alert characteristic data (type of telehealth vital sign alert [weight, SBP, DBP, HR, or O2 sat]) were obtained from the telehealth logs for all the days that the HF patients were using telehealth during the study period. Telehealth questionnaire data on symptoms experienced by patients (for example, did you experience shortness of breath in the past 24 h, how many pillows did you use while sleeping, etc.) were not available consistently for all subjects in this study and hence could not be collected for this study.
The electronic nursing documentation notes also provided documentation of home health nursing interventions and medication change events in response to the telehealth alerts. An event was coded as a medication change KME if the HF patient's medication was changed in response to symptoms experienced while receiving home health nursing service. OASIS and nursing documentation notes on ED visits, re-admissions to inpatient facilities, and resumption of home health were used to collect data on outcome variables of all-cause and/or cardiac-related ED visits and hospitalizations and were coded as KMEs. An ED visit followed by a hospitalization was coded as a single KME. Within the study period, data for each subject were collected for an entire episode of telehealth (from admission to discharge) for the HF patient at the home healthcare agency. Institutional Review Board approval to conduct this study was obtained from the academic institution affiliated with the researchers.
Data Analysis
Descriptive statistics were calculated for all patient characteristic, telehealth alert data, and outcome variables. To account for the within-subject correlation among the time-varying predictors, Generalized Estimating Equations (GEE) was thought to be an appropriate analysis method. GEE was used to analyze patient characteristics of demographic factors, psychosocial factors, disease severity factors, and telehealth vital signs alerts (type and number) with KMEs of (1) cardiac-related medication changes, ED visits, and hospitalization and (2) all-cause ED visit and hospitalization.
Univariate analysis was used to eliminate variables with associations at a significance level >0.25 with outcome variables.12 Then all variables with association of p<0.25 were examined for inclusion in the initial GEE model. Variables were eliminated in an iterative fashion until the final GEE model included variables with association of p<0.1. The effects of potential effect modifiers and confounders (covariates without significance in prior steps) were investigated, and, if found significant, they were included in the final model. The best GEE model was determined through lowest value of quasi-likelihood information criterion (QIC) goodness-of-fit statistics.13 SPSS version 20 (SPSS, Inc., Chicago, IL) was used for the GEE analysis. An autoregressive correlation structure indicates that two observations taken close in time within an individual tend to be more highly correlated than two observations taken far apart in time from the same individual.14,15 As the telehealth vital sign values of the patients tend to be similar when they are closer in time, an autoregressive correlation matrix was thought appropriate for the GEE analysis.
Presence of individual telehealth vital sign alert of SBP, DBP, HR, O2 sat, and weight on a day was coded as 1, and absence of that telehealth alert on a day was coded as 0. Then, the median value of the individual telehealth alert for that week was calculated for an individual patient. For example, if an SBP telehealth alert was generated for 4 of 7 days in a week, that variable was coded as 1 for that week. Median of number of all telehealth alerts generated that week was also calculated, and the presence of a KME that week was noted from the EHR log. Longitudinal telehealth data were condensed to a weekly basis as opposed to daily telehealth data because (1) daily telehealth data were considered too sparse for analysis and (2) time lag of a day or two was evident between generated telehealth alerts and home health nurse and physician responses to the alerts, as is typical in a home health setting. Compared with daily telehealth data, weekly telehealth data enabled us to appropriately capture the sequence of generation of telehealth alert, associated home health interventions, and corresponding KME, if any, within a single data point.
Results
The initial sample comprised 188 Medicare HF patients who used telehealth from March to September 2011. The final sample comprised 168 subjects after excluding subjects who had incomplete telehealth vital sign data or less than 3 days of telehealth data. One hundred forty-eight subjects (88%) had at least 1 week of incomplete transmitted telehealth data. Overall, 1,188 weeks of patient data were analyzed over the 6-month study period. Collection of weekly data and calculation of median values within a week were able to minimize the impact of missing daily telehealth data. Only the status of HF diagnosis (new or chronic) as an independent variable had missing data in 4 (2%) cases.
Sample Description
Subjects in the sample had a mean age of 83 (standard deviation 7.6) years, with 96% white and 56% females. HF was a primary admitting diagnosis in 54% of subjects. Only 14% were newly diagnosed with HF. Presence of dyspnea as reported in the OASIS data source was observed in 86% of subjects. Average number of co-morbidities was 5.5. Vascular disorders (70%), hypertension (64%), and musculoskeletal disorders (50%) were the most common co-morbidities (Table 1). Patients received telehealth service for an interval ranging from 1 to 25 weeks, with a mean duration of 7 weeks.
Table 1.
Descriptive Information of Patient Characteristics
| DEMOGRAPHICS | VALUE |
|---|---|
| Age (years) [mean (SD)] | 82.75 (7.6) |
| Race [n (%)] | |
| American Indian/Alaska Native | 0 |
| Asian | 0 |
| African American | 3 (1.8) |
| Hispanic or Latino | 4 (2.4) |
| Native Hawaiian/Pacific Islander | 0 |
| White | 161 (95.8) |
| Gender [n (%)] | |
| Male | 74 (44.0) |
| Female | 94 (56.0) |
| HF disease characteristicsa | |
| Type of HF diagnosis | |
| Primary | 91 (54) |
| Secondary | 77 (45.8) |
| Status of HF diagnosis | |
| Newly diagnosed | 23 (13.7) |
| Chronic HF | 141 (83.4) |
| Unknown | 4 (2.4) |
| Dyspnea (at admission) | |
| 0 (never) | 24 (14) |
| 1 (climbing stairs) | 57 (33.9) |
| 2 (with dressing, bathing) | 60 (35.7) |
| 3 (with eating, conversation) | 23 (13.7) |
| 4 (at rest) | 4 (2.4) |
| Co-morbidities (n types of co-morbidities) | 5.52 (1.9) [2–12] |
| Myocardial infarction | 26 (15.5) |
| Cardiac arrhythmia | 71 (47.0) |
| Valvular disorders | 12 (7.1) |
| Pulmonary disorders | 70 (40.5) |
| Vascular disorders | 122 (70.6) |
| Hypertension | 107 (63.7) |
| Neurological disorders | 21 (12.5) |
| Diabetes mellitus | 45 (26.8) |
| Thyroid disorders | 39 (23.2) |
| Renal disorders | 52 (31.0) |
| Gastrointestinal disorders | 25 (14.9) |
| Cancer | 14 (8.3) |
| Musculoskeletal disorders | 85 (50.6) |
| Dementia | 19 (11.3) |
| Psychiatric disorders | 42 (25) |
| Anxiety co-morbidity | 15 (8.9) |
| Anemia | 24 (14.3) |
| Obesity | 40 (23.8) |
| Infection | 31 (18.5) |
| Dermatological disorders | 25 (14.9) |
| Reproductive disorders | 19 (11.3) |
| Medications | |
| Cardiac medications | |
| ACEI/ARB | 79 (47) |
| Beta-blockers | 134 (79.8) |
| Diuretics | 144 (85.7) |
| Total n of medications | 12.87 (4.64) [4–30] |
| Psychosocial [n (%)] | |
| Living alone | 46 (27.4) |
| Anxiety | |
| 0 (no anxiety) | 102 (60.7) |
| 1 (less often than daily) | 40 (23.8) |
| 2 (daily, not constantly) | 25 (14.9) |
| 3 (all the time) | 1 (.6) |
| Depression | 27 (16.1) |
| Telehealth alert characteristics [n (%)] | |
| Total n telehealth alerts | 6,025 |
| Average telehealth alerts generated by subjects | 35.9 (30.8) [0–201] |
| Telehealth alerts for | |
| Weight | 2,285 (37.9% of all alerts) |
| SBP | 1,458 (24.2% of all alerts) |
| HR | 1,017 (16.9% of all alerts) |
| DBP | 990 (16.4% of all alerts) |
| O2 saturation | 275 (4.6% of all alerts) |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure; HF, heart failure; HR, heart rate; SBP, systolic blood pressure; SD, standard deviation.
Data are n (%), mean (SD), or [range].
KME Outcomes
In total, 99 cardiac-related KMEs (37 cardiac ED visits or hospitalizations and 62 medication changes) and 87 all-cause ED visits or hospitalizations were recorded for the subjects in the study. Of the 1,188 weeks of data analyzed for this study, the presence of a KME was observed in 148 weeks, and the remaining 1,040 weeks did not have any incidence of KMEs.
Telehealth Alert Characteristics
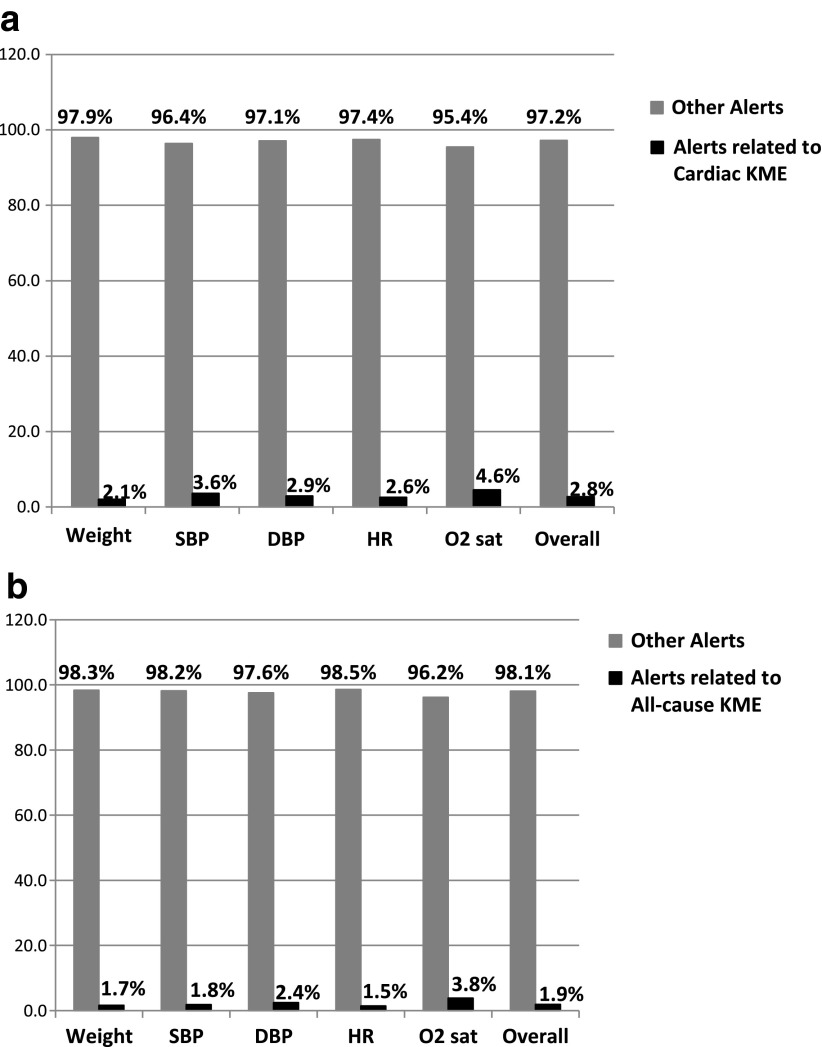
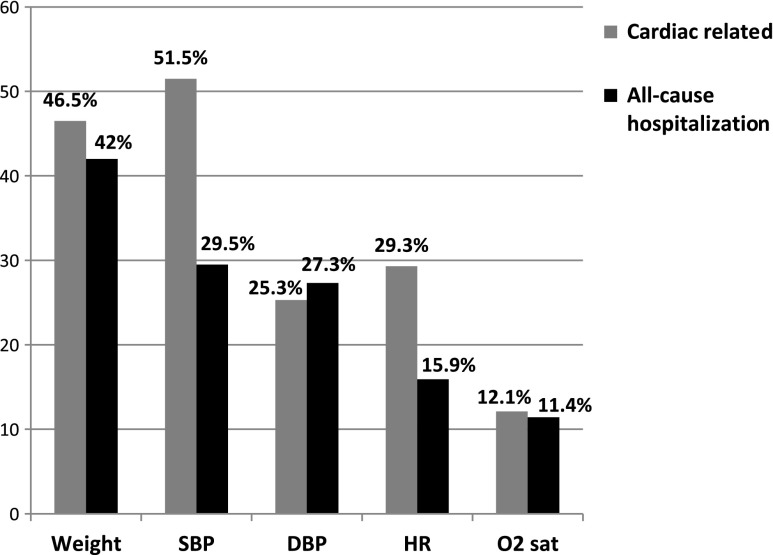
In the 1,188 weeks of patient data analyzed during the 6-month study period, in total, 6,025 telehealth alerts related with the physiological vital signs of weight, SBP, DBP, HR, and O2 sat were generated, with weight generating the highest number of telehealth alerts (38%), followed by SBP (24%). Overall, for 1,188 weeks of patient data, only 2.8% of all telehealth alerts were associated with cardiac-related KMEs (Fig. 1a) and 1.9% with all-cause KMEs (Fig. 1b). A telehealth alert was not generated for 22% of cardiac-related ED visits and hospitalizations. However, among telehealth alerts that were associated with cardiac-related KME, SBP had the highest proportion (52%), followed by weight (47%) (Fig. 2). The most common medication changes involved beta-blockers related to changes in SBP (51%), followed by diuretics related to changes in weight (42%).
Fig. 1.
Proportion of telehealth vital sign alerts that were related to (a) cardiac-related and (b) all-cause key medical events (KME). DBP, diastolic blood pressure; HR, heart rate; O2 sat, oxygen saturation; SBP, systolic blood pressure.
Fig. 2.
Proportion of individual telehealth vital sign alerts among telehealth alerts that are related to key medical events. DBP, diastolic blood pressure; HR, heart rate; O2 sat, oxygen saturation; SBP, systolic blood pressure.
Gee Model
Cardiac-related KMEs: ED visit, hospitalization, and medication change
Single predictor variables that were significant at the p<0.1 level included valvular, vascular, and gastrointestinal co-morbidities, median of weekly total telehealth alerts, and individual telehealth vital sign alerts of weight, HR, SBP, and DBP.
In the best GEE model constructed based on QIC goodness-of-fit statistics, median of weekly total telehealth alerts (adjusted p<0.0001) and valvular co-morbidity (adjusted p=0.001) emerged as statistically significant predictors of cardiac-related KMEs (ED visits, hospitalizations, and medication changes). The probability of a cardiac-related KME increased by 106% for every additional telehealth alert generated and increased by 161% with the presence of valvular co-morbidity (Table 2).
Table 2.
Generalized Estimating Equations Model for Prediction of Cardiac-Related Key Medical Events
| VARIABLE | COEFFICIENT (β) | STANDARD ERROR | ODDS RATIO (95% CI) | P VALUE |
|---|---|---|---|---|
| Median of weekly total telehealth flags | 0.73 | 0.13 | 2.06 (1.59, 2.68) | <0.0001 |
| Valvular co-morbidity | 0.96 | 0.29 | 2.61 (1.49, 4.57) | 0.001 |
CI, confidence interval.
All-cause KMEs: ED visits and hospitalizations
Single predictor variables that were significant at the p<0.1 level included cancer and anxiety co-morbidity, median of weekly telehealth flags, and individual telehealth vital signs alerts of weight, HR, and SBP. In the best GEE model constructed based on lowest QIC goodness-of-fit statistics, cancer co-morbidity (adjusted p=0.026) and anxiety co-morbidity (adjusted p=0.008) emerged as statistically significant predictors of all-cause KME. The probability of all-cause KME increased by 124% and 127% with the presence of cancer and anxiety co-morbidities, respectively. The probability of all-cause KME also increased by 70% for every additional telehealth alert generated (Table 3).
Table 3.
Generalized Estimating Equations Model for Prediction of All-Cause Key Medical Events
| VARIABLE | COEFFICIENT (β) | STANDARD ERROR | ODDS RATIO (95% CI) | P VALUE |
|---|---|---|---|---|
| Median of weekly telehealth flags | 0.53 | 0.13 | 1.70 (1.33, 2.19) | <0.0001 |
| Anxiety co-morbidity | 0.82 | 0.32 | 2.27 (1.21, 4.25) | 0.01 |
| Cancer co-morbidity | 0.81 | 0.35 | 2.24 (1.14, 4.43) | 0.02 |
CI, confidence interval.
Discussion
Protocols at the home health agency require that telehealth nurses address every telehealth alert. The very low proportion of telehealth alerts (<5%) associated with KMEs (Fig. 1) demonstrates that telehealth nurses have to expend an inordinate amount of time following up on redundant alerts just so they do not miss the few meaningful alerts. One of the main reasons for the low proportion of meaningful telehealth alerts is the generation of high numbers of telehealth alerts not related to KME. Although the telehealth parameter of weight generated 38% of all telehealth alerts, 22% of cardiac-related ED visit and hospitalization did not have a single telehealth alert associated with them. In another study on telemonitoring for 168 HF patients, simple rule-of-thumb algorithms on weight gain such as 3 pounds in 1 day or 5 pounds in 3 days provided no discriminatory power in predicting worsening HF.17 Causes other than worsening HF were attributed for the weight fluctuations and the resultant alerts, such as the patient weighing with and without clothes from day-to-day, missed medication, or fluid retention caused by high dietary salt intake the previous day.17
In the current study, telehealth alerts that had to be responded to by the telehealth nurse were often false alarms that did not require any further intervention according to the telehealth nurse documentation in the telehealth logs. False alarms in this study were primarily due to inappropriate telehealth measurement techniques such as varying weight of clothes, unadjusted blood pressure cuff size, or family members weighing themselves on the telehealth devices. Telehealth alert thresholds based on initial home health admission vital signs caused many alerts to be generated as patient vital signs stabilized at a level different from the alert thresholds set earlier.
Median of weekly total telehealth alerts was positively correlated with KMEs in this study, which meant that higher the number of telehealth alerts, the higher were the odds of a KME. This finding was also observed in a study by Biddiss et al.,16 who analyzed the association of patient self-rated symptoms along with physiological telehealth alerts with KMEs for HF patients in a home health setting. However, in the current study, telehealth alerts only consisted of physiological alerts, and we explored the association of patient characteristics in addition to telehealth alerts with KMEs.
Anxiety, which was a key predictor of all-cause KMEs in this study, has consistently emerged as a predictor of high healthcare utilization for patients with HF using telehealth.16,18,19 Anxiety needs to be identified and treated in HF patients, as anxiety has been attributed as a barrier to HF self-management.20 The dual diagnosis of cancer and HF was a key predictor of all-cause KMEs in this study and was also associated with increased odds of withdrawal from telehealth services in another study on association of patient characteristics with home health resource utilization.19 Patients with the dual diagnosis of cancer and HF could potentially suffer from severe morbidity and sharp functional decline. Such patients may not be the best candidates for telehealth, and parameters other than telehealth vital sign changes need to be monitored for such patients.
Although not always indicative of KME, telehealth alerts provide home health nurses with the opportunity to reinforce HF self-management teaching in a contextually relevant manner, identify ways to improve patient satisfaction with care delivered, and assess the impact of co-morbidities for the HF patient. The alerts are indicative of patient health status trends and provide an understanding of how the individual patient reacts to HF treatment. Although telehealth alerts based on vital sign changes could potentially provide guidance on the direction of care, frequent telehealth alerts that do not require follow-up interventions may result in “alert fatigue”11 and decreased efficiency of telehealth interventions. The low proportion of meaningful telehealth alerts in this study indicates that telehealth processes could be implemented more efficiently for managing HF.
A decision support system for telehealth can help eliminate or filter out grossly inaccurate alerts so the telehealth nurse can expend his or her time more meaningfully.4 Alert thresholds need not be generic and could be tailored to each patient's contextual information, such as psychosocial status, involvement with HF self-management, symptom status, and HF disease severity, including presence of co-morbidities. Effectively engaging HF patients with the telehealth process and HF self-management could also potentially reduce the high number of telehealth false alarms due to poor measurement techniques and improve the overall efficiency of telehealth for HF management. Protocols of communication between physicians and home health clinicians that allow prompt revision of telehealth alert thresholds in response to newly stabilized vital signs as well as prompt changes in HF treatment regimen in response to abnormal telehealth vital signs could contribute to improving efficiency of telehealth for HF management.
Limitations
The study suffered from low power. Associations of some co-morbidity variables with KMEs may need to be interpreted cautiously because of wide confidence intervals with a small number of data end points available for analysis. However, this study is exploratory in nature and provided preliminary associations of characteristics of HF patients using telehealth with KMEs that can be tested more rigorously in future studies with a larger sample set.
As this study was a retrospective review and the dataset was restricted to an available sample, a racially diverse sample could not be obtained, which limits generalizability of the study findings. Also, HF severity measures such as ejection fraction or New York Heart Association class or presence of implanted cardiac devices could not be analyzed because of unavailability of such data in the home health setting. Other HF self-management variables such as medication adherence, diet, and physical activity modifications and HF symptom variables of daily shortness of breath, which might have been associated with KMEs, were not collected for this study.
Conclusions
The findings of this preliminary study provides an understanding of patient-related factors associated with KMEs experienced by HF patients using telehealth in a home health clinical setting. The very low proportion of telehealth vital sign alerts associated with KMEs indicates that telehealth vital signs alone cannot inform need for intervention. Other patient-relevant data such as psychosocial status, involvement with HF self-management, symptom status, and HF disease severity, including presence of co-morbidities, could further inform need for interventions for HF patients receiving home health services. Efficient use of telehealth for HF may also require effective patient and healthcare provider engagement with the telehealth process to enable better management of HF.
Acknowledgments
We thank Anne Zettek-Sumner, RN, MEd, Telehealth Program Coordinator, VNA Care Network & Hospice, Worcester, MA. This study was funded by grant T32NR009356, University of Pennsylvania NewCourtland Center for Transitions and Aging, the training program in Individualized Care for At Risk Older Adults.
Disclosure Statement
No competing financial interests exist.
References
- 1.Roger VL. Go AS. Lloyd-Jones DM. Benjamin EJ. Berry JD. Borden WB. Bravata DM. Dai S. Ford ES. Fox CS. Fullerton HJ. Gillespie C. Hailpern SM. Heit JA. Howard VJ. Kissela BM. Kittner SJ. Lackland DT. Lichtman JH. Lisabeth LD. Makuc DM. Marcus GM. Marelli A. Matchar DB. Moy CS. Mozaffarian D. Mussolino ME. Nichol G. Paynter NP. Soliman EZ. Sorlie PD. Sotoodehnia N. Turan TN. Virani SS. Wong ND. Woo D. Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. . Erratum in: Circulation 2012;125:e1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Averwater N. Burchfield D. No place like home: Telemonitoring can improve home care. Healthc Financ Manage. 2005;59:46–48. , 50, 52. [PubMed] [Google Scholar]
- 3.Nangalia V. Prytherch DR. Smith GB. Health technology assessment review: Remote monitoring of vital signs—Current status and future challenges. Crit Care. 2010;14:233. doi: 10.1186/cc9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basilakis J. Lovell NH. Redmond SJ. Celler BG. Design of a decision-support architecture for management of remotely monitored patients. IEEE Trans Inf Technol Biomed. 2010;14:1216–1226. doi: 10.1109/TITB.2010.2055881. [DOI] [PubMed] [Google Scholar]
- 5.Byrne JM. Elliott S. Firek A. Initial experience with patient-clinician secure messaging at a VA medical center. J Am Med Inform Assoc. 2009;16:267–270. doi: 10.1197/jamia.M2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade M. Desai A. Spettell C. Snyder A. McGowan Stackewicz V. Kummer P. Maccoy MC. Krakauer RS. Telemonitoring with case management for seniors with heart failure. Am J Manag Care. 2011;17:e71–e79. [PubMed] [Google Scholar]
- 7.Pekmezaris R. Mitzner I. Pecinka KR. Nouryan CN. Lesser ML. Siegel M. Swiderski JW. Moise G. Younker R., Sr Smolich K. The impact of remote patient monitoring (telehealth) upon Medicare beneficiaries with heart failure. Telemed J E Health. 2012;18:101–108. doi: 10.1089/tmj.2011.0095. [DOI] [PubMed] [Google Scholar]
- 8.Bowles KH. Hanlon AL. Glick HA. Naylor MD. O'Connor M. Riegel B. Shih NW. Weiner MG. Clinical effectiveness, access to, and satisfaction with care using a telehomecare substitution intervention: A randomized controlled trial. Int J Telemed Appl. 2011;2011:540138. doi: 10.1155/2011/540138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhry SI. Mattera JA. Curtis JP. Spertus JA. Herrin J. Lin Z. Phillips CO. Hodshon BV. Cooper LS. Krumholz HM. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363:2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi PY. Hanson GJ. Pecina JL. Stroebel RJ. Chaudhry R. Shah ND. Naessens JM. A randomized controlled trial of telemonitoring in older adults with multiple chronic conditions: The Tele-ERA study. BMC Health Serv Res. 2010;10:255. doi: 10.1186/1472-6963-10-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai AS. Stevenson LW. Connecting the circle from home to heart-failure disease management. N Engl J Med. 2010;363:2364–2367. doi: 10.1056/NEJMe1011769. [DOI] [PubMed] [Google Scholar]
- 12.Tabachnick BG. Fidell LS. Using Multivariate Statistics. 4th. Needham Heights, MA: Allyn & Bacon; 2001. [Google Scholar]
- 13.Gardiner JC. Luo Z. Roman LA. Fixed effects, random effects and GEE: What are the differences? Stat Med. 2009;28:221–239. doi: 10.1002/sim.3478. [DOI] [PubMed] [Google Scholar]
- 14.Azzalini A. Estimation and hypothesis testing for collections of autoregressive time series. Biometrika. 1984;71:85–90. [Google Scholar]
- 15.Zeger SL. Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 16.Biddiss E. Brownsell S. Hawley M. Predicting need for intervention in individuals with congestive heart failure using a home-based telecare system. J Telemed Telecare. 2009;15:226–231. doi: 10.1258/jtt.2009.081203. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J. Goode KM. Cuddihy PE. Cleland JG. TEN-HMS Investigators. Predicting hospitalization due to worsening heart failure using daily weight measurement: Analysis of the Trans-European Network-Home-Care Management System (TEN-HMS) study. Eur J Heart Fail. 2009;11:420–427. doi: 10.1093/eurjhf/hfp033. [DOI] [PubMed] [Google Scholar]
- 18.Radhakrishnan K. Jacelon C. Roche J. Perceptions on the use of telehealth for heart failure by homecare nurses and patients: A mixed method study. Home Health Care Manage Pract. 2012;24:175–181. [Google Scholar]
- 19.Radhakrishnan K. Jacelon C. Bigelow C. Roche J. Marquard J. Bowles K. Association of comorbidities with homecare nursing utilization and withdrawal from telehealth by patients with heart failure. J Cardiovasc Nurs. 2013;28:216–227. doi: 10.1097/JCN.0b013e3182512331. [DOI] [PubMed] [Google Scholar]
- 20.Schnell-Hoehn KN. Naimark BJ. Tate RB. Determinants of self-care behaviors in community-dwelling patients with heart failure. J Cardiovasc Nurs. 2009;24:40–47. doi: 10.1097/01.JCN.0000317470.58048.7b. [DOI] [PubMed] [Google Scholar]