Abstract
The natural history of type 2 diabetes mellitus (T2DM) is a relentless progression of β-cell failure and dysregulation of β-cell function with increasing metabolic derangement. Insulin remains the only glucose-lowering therapy that is efficacious throughout this continuum. However, the timing of introduction and the choice of insulin therapy remain contentious because of the heterogeneity of T2DM and the well-recognized behavioral and therapeutic challenges associated with this mode of therapy. Nevertheless, the early initiation of basal insulin has been shown to improve glycemic control and affect long-term outcomes in people with T2DM and is a treatment strategy supported by international guidelines as part of an individualized approach to chronic disease management. The rationale for early initiation of insulin is based on evidence demonstrating multifaceted benefits, including overcoming the glucotoxic effects of hyperglycemia, thereby facilitating “β-cell rest,” and preserving β-cell mass and function, while also improving insulin sensitivity. Independent of its effects on glycemic control, insulin possesses anti-inflammatory and antioxidant properties that may help protect against endothelial dysfunction and damage resulting in vascular disease. Insulin therapy and the achievement of good glycemic control earlier in T2DM provide long-term protection to end organs via “metabolic memory” regardless of subsequent treatments and degree of glycemic control. This is evidenced from long-term observations continuing from trials such as the United Kingdom Prospective Diabetes Study. As such, early initiation of insulin therapy may not only help to avoid the effects of prolonged glycemic burden, but may also positively alter the course of disease progression.
Introduction
The epoch-making discovery of insulin has saved the lives of countless numbers of people with diabetes mellitus since pancreatic extracts were first used in the early 1920s.1–5 Despite the early and dramatic fall in total deaths due to diabetic coma following the introduction of insulin,6 diabetes emerged over the subsequent decades as a chronic disease with accelerated degenerative complications. In the 1930s, Himsworth and Kerr7 described the two main categories of diabetes: insulin-sensitive and insulin-insensitive (or insulin-resistant) diabetes. Currently, these are referred to as type 1 and type 2 diabetes mellitus (T2DM). In the 1950s, the advent of oral antidiabetic drugs (OADs), such as the insulin secretagogues (sulfonylureas) and the biguanides (phenformin and metformin), provided additional therapeutic opportunities for the management of T2DM. Since then, further generations of sulfonylureas have become available, and phenformin has been discontinued. Furthermore, newer therapeutic modalities have been introduced, including the α-glucosidase inhibitors, thiazolidinediones, and, more recently, the incretin class of agents. Many more therapeutics are under development in an attempt to address the widespread pathophysiological deficits relating to pancreatic β-cell function and insulin resistance.
Clinical inertia, noncompliance, and adverse effects often result in prolonged glycemic burden for individuals with T2DM receiving OADs.8 There is too often a delay in advancing therapy when glycemic control is inadequate, with insulin supplementation being commenced when complications are already evident due to the inability to achieve target glycemic control.9,10 However, the timing of introduction and the choice of insulin remain inconsistent owing, in large part, to the heterogeneous nature of T2DM, but also to the unwillingness of the person with diabetes—and often the caregiver—to commence insulin therapy, which presents both a behavioral (lifestyle) and a therapeutic challenge. Several management guidelines and consensus statements have been developed in an attempt to provide a structured algorithmic approach that is both evidence-based and cost-effective. Despite many attempts, along with the development of numerous new therapies, the glycemic outcome for the majority of persons with T2DM remains unsatisfactory, whereas improvements in the control of hypertension and dyslipidemia are more evident.11,12 Recently, both the American Diabetes Association and the European Association for the Study of Diabetes issued position statements for the management of hyperglycemia in T2DM that emphasize a patient-centered approach.13,14 These guidelines review the properties of all currently available glucose-lowering agents to guide treatment choice by the clinician for individual patients, taking into consideration the patient's preferences, tolerance, needs, and values, representing an individualized approach to disease management.
The purpose of this article is to review the multifaceted benefits of insulin therapy in T2DM, as well as to provide an overview of the clinical evidence for insulin—administered either early after failure of OADs or as first-line therapy in certain clinical situations.13 Originally, before the development of OADs, insulin was always the first-line treatment for diabetes. However, data for this period are not examined in this review because of the many developments that have occurred relating to the diagnosis and management of diabetes since that time.
Rationale for Early Initiation of Insulin Therapy
Multifaceted benefit of insulin
Aside from glycemic control, insulin treatment can potentially provide additional benefits. The anti-inflammatory and antioxidant effects of insulin may contribute to protection against endothelial dysfunction and vascular disease. These effects include suppression of reactive oxygen species (ROS) and adhesion molecule expression.15–17 Insulin has also been demonstrated to induce endothelial nitric oxide synthase expression in endothelial cells, causing vascular dilatation due to increased production of nitric oxide.15–17
In general, T2DM is associated with progressive deterioration of β-cell mass and function, and one of the key goals of therapy is to preserve β-cells. Factors that are thought to promote β-cell loss include insulin resistance, glucotoxicity and lipotoxicity, inflammation, and obesity. It has been known for nearly 40 years that insulin therapy improves β-cell function as determined by an enhanced insulin response to glucose.18 This was first demonstrated in a small study of seven insulin-naive individuals with T2DM. In their analysis, Turner et al.18 described the vicious circle evident in diabetes, in which defective β-cells lead to hyperglycemia, which subsequently stresses β-cell function further. The authors postulated that overcoming glucotoxicity through insulin use facilitates “β-cell rest,” which in turn allows a store of readily available endogenous insulin to be accumulated for early release to a nutrient challenge, resulting in improvement in β-cell function.
Recognizing the importance of the insulin signaling pathway in β-cell growth, maintenance, and differentiation, Jetton et al.19 sought to determine the roles of insulin receptor substrate-2 and protein kinase B/Akt (Akt) in pancreatic regeneration following partial (60%) pancreatectomy in normally insulin-sensitive Sprague–Dawley rats. They demonstrated that insulin receptor substrate-2 plays an important role in pancreatic regeneration by mediating the proliferation of common duct cells and the differentiation of certain duct cells into β-cells, as well as by maintaining the phenotype of differentiated β-cells.19 In addition, they found that expression of activated Akt was restricted to cells of the common duct epithelium, suggesting that Akt may be involved in pancreatic regeneration by regulating postmitotic cell-specific gene expression and/or survival, possibly by signaling through insulin receptor substrate-2.19 Jetton et al.20 went on to examine whether these proteins are also involved in the β-cell growth response to insulin resistance, using the Zucker fatty rat (fa/fa) model (a model of insulin resistance, hyperlipidemia, and obesity caused by mutation of the leptin receptor gene). Their findings indicate that Akt plays a central role in early β-cell mass compensation by regulating new β-cell development and promoting survival (anti-apoptosis) of existing β-cells.20
In order to help elucidate whether the insulin signaling pathway is physiologically important for glucose sensing, Bouche et al.21 conducted experiments in healthy humans using combined isoglycemic–hyperinsulinemic and hyperglycemic clamps, compared with sham clamps, to assess the effects of pre-exposure to insulin on glucose-stimulated insulin secretion. Pre-exposure to insulin over 4 h under isoglycemic conditions was found to increase the β-cell secretory response by approximately 40%.21 These findings demonstrate that, in healthy, insulin-sensitive individuals, insulin potentiates the β-cell secretory response to glucose, indicating that the human β-cell is an insulin-responsive tissue. Thus, insulin regulation of glucose-stimulated insulin secretion might be altered in persons with insulin resistance or T2DM and might contribute to the progressive loss of β-cell function that occurs in T2DM.21
Following on from the findings of Turner et al.,18 a study by Garvey et al.22 of short courses of intensive insulin therapy of 2–3 weeks in duration demonstrated multiple clinical benefits of insulin therapy in people with T2DM. Using intensive insulin therapy and reducing hyperglycemia enhanced insulin secretion (predominantly second phase) and improved insulin sensitivity.22 Furthermore, suppression of hepatic glucose production was observed, resulting in near normalization of basal hepatic glucose production.22
A study by Engerman and Kern23 examined four groups of dogs: dogs with alloxan-induced diabetes and poor glucose control for 5 years, good glucose control for 5 years, or poor glucose control for 2.5 years followed by good glucose control for 2.5 years, as well as a group of healthy controls. Retinopathy was seen to be worst in the group with poor control but was also seen to develop in the group with poor control followed by good control. In this group, retinopathy was seen to be absent or equal to that of the good control group at 2.5 years but developed during the 2.5 years of good control, despite improved treatment. The group with good control throughout did not experience retinopathy, showing that earlier intensive treatment to maintain good glycemic control is beneficial.23
Overall, these early results clearly demonstrate that intensive insulin therapy in people with poorly controlled T2DM provides multiple benefits beyond glucose control that may contribute to preservation of β-cell function and improvement in vascular endothelial health. Earlier initiation of such a treatment option, before the loss of further function, may be more effective in providing long-term efficacy against the complications of T2DM, as suggested by the long-term observations in the follow-up study of the United Kingdom Prospective Diabetes Study (UKPDS).10,24
Early intervention to change the course of disease progression in T2DM
Management of T2DM in the first decade of the 21st century was considered largely in the context of evidence from landmark studies, such as UKPDS, that clearly demonstrated the benefits of intensive glucose control therapy in newly diagnosed T2DM.25 In the analysis of individuals treated with a sulfonylurea and/or insulin (n=2,729), median glycated hemoglobin (HbA1c) levels over 10 years of treatment were significantly lower with intensive versus conventional (n=1,138; standard treatment with diet modification) therapy: 7.0% versus 7.9%, respectively. This difference was associated with a significant reduction in the relative risk for the intensive therapy group of 25% for any microvascular end point (P=0.0099), including a 21% reduction in the incidence of retinopathy (P=0.015) and a 33% reduction in microalbuminuria (P<0.0001). Furthermore, the relative risk of non-fatal myocardial infarction (MI) was 21% lower with intensive therapy, although this was not statistically significant (P=0.057). It is interesting that there was no significant difference in mortality rates, although there was a trend in favor of the intensive group, with a relative risk reduction of 10% for diabetes-related death and 6% for all-cause mortality.
The UKPDS follow-up 10 years after the end of the original study showed that a period of good glycemic control early in the course of the disease has a lasting benefit independent of subsequent glycemic control.24 The relative risks of non-fatal MI, death related to diabetes, and all-cause mortality were 15% (P=0.01), 17% (P=0.01), and 13% (P=0.007) lower in individuals originally treated with intensive therapy compared with conventional therapy. The reduction in the risk of microvascular complications with intensive therapy was also maintained (24%; P=0.001). These benefits were achieved despite the fact that the difference in HbA1c levels apparent at the end of intervention was lost within 1 year and that HbA1c levels were comparable over the remaining follow-up period.
The results of the UKPDS mandate that treatment of T2DM include aggressive efforts to lower blood glucose to as close to normal as possible early in the disease process.26 The lasting benefit could partly be due to metabolic memory, thereby reducing vascular stresses in diabetes despite deteriorating glycemic control.27 A legacy effect or “metabolic memory” is believed to be associated, in part, with the increased formation of advanced glycation end-products evident in T2DM. Advanced glycation end-products modify mitochondrial DNA respiratory proteins, resulting in excess ROS, which cause further mitochondrial DNA damage and functional respiratory chain decline. This process perpetuates ROS generation and overall cellular injury, maintaining oxidative stress signaling even after normoglycemia is achieved.27 Intensive insulin treatment is associated with significantly lower levels of advanced glycation end-products than conventional treatment,28 supporting the long-term benefits observed with an intensive management strategy.24
Owing to the favorable outcomes observed in the UKPDS study, several large-scale studies have been undertaken that assessed the effects of intensive treatment regimens on vascular outcomes in people with advanced T2DM. These include ACCORD (Action to Control Cardiovascular Risk in Diabetes),29 ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation),30 and VADT (Veterans Affairs Diabetes Trial).31 In all three of these studies, an intensive control regimen resulted in better glycemic outcomes, including significantly lower HbA1c, than standard therapy.
However, in these studies, at randomization, subjects were of an advanced age (mean of 62, 66, and 60 years, respectively), had long-standing disease (mean of 10.0, 8.0, and 11.5 years, respectively), and, particularly in VADT, had poorly controlled glycemia (mean HbA1c of 8.1%, 7.2%, and 9.4%, respectively), which may reduce the benefit observed with an intensive regimen. The benefits of intensive glycemic control in people with T2DM have been confirmed by several meta-analyses. These suggest that intensive glycemic control does not significantly affect all-cause mortality or cardiovascular (CV) death but is associated with significant reductions in the risk of non-fatal MI, with an increase in the risk of severe hypoglycemia, compared with standard treatment. Based on these observed benefits Duckworth et al.31 suggested that intensive glycemic control would provide maximal benefits if initiated earlier in the disease, particularly if severe hypoglycemia was avoided.
The INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycemia Treatment) trial was designed to assess whether early intervention with insulin glargine could enable people with T2DM who had high glucose levels and were on either no or suboptimal doses of OADs to safely achieve an HbA1c target of ≤6.5% more effectively than the conventional approach of optimizing OAD therapy.32 After 24 weeks, people treated with insulin glargine in addition to their existing treatment were significantly more likely to achieve two consecutive HbA1c levels of ≤6.5% than those treated with intensified OAD therapy; these people experienced significantly greater reductions in HbA1c and fasting plasma glucose (FPG) levels. Insulin glargine was associated with a significantly greater increase in weight than intensified OAD therapy, but no between-group differences in hypoglycemia were observed.32
The potential benefits of early intervention with insulin glargine were further investigated in the landmark ORIGIN (Outcome Reduction with an Initial Glargine Intervention) study, which assessed the use of insulin glargine to target normal FPG in people with early T2DM or prediabetes (impaired fasting glucose or impaired glucose tolerance; approximately 12% of the population) and a high risk of CV events.33 This long-term, prospective, large-scale, randomized controlled trial included 12,537 subjects who were followed for a median of 6.2 years. The coprimary outcomes were the incidence of non-fatal MI, non-fatal stroke, or death from CV causes, as well as these events plus the incidence of revascularization or hospitalization for heart failure. No increase or decrease in CV outcomes was found between the insulin and standard care groups, in terms of the coprimary outcomes and their components (hazard ratio [HR] for first coprimary outcome, 1.02; 95% confidence interval [CI], 0.94, 1.11; P=0.63; HR for second coprimary outcome, 1.04; 95% CI, 0.97, 1.11; P=0.27) (Fig. 1), although those in the insulin group achieved the target median FPG (≤94 mg/dL). There was also no difference between the insulin and standard care groups in the incidence of any cancer, or death from cancer (Fig. 1).33 As in the aforementioned INSIGHT trial, a low rate of hypoglycemia and a modest increase in weight were observed with the use of insulin glargine. It is interesting that despite this weight gain (which is a known risk factor for diabetes), those people with prediabetes treated with insulin were 28% less likely to develop diabetes from the time of randomization until the first oral glucose tolerance test than those assigned to standard care (odds ratio [OR], 0.2; 95% CI, 0.58, 0.91; P=0.006). Based on this finding, the ORIGIN authors supported further research into the effect of insulin regimens on endocrine pancreatic function.33
FIG. 1.
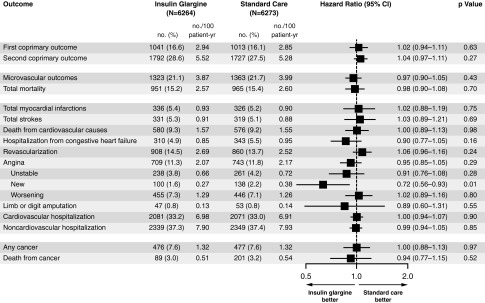
Hazard ratios for the coprimary and other outcomes in the ORIGIN study.33 Hazard ratios are adjusted for the factorial allocation, baseline diabetes status, and the presence or absence of a history of a cardiovascular event before randomization. CI, confidence interval.
The Glucose Reduction and Atherosclerosis Continuing Evaluation (GRACE) substudy of ORIGIN investigated whether treatment with insulin glargine within the ORIGIN trial, compared with standard care, affected the annualized rate of carotid intima-media thickness change over a median of 4.9 years.34 Carotid intima-media thickness was used as a surrogate end point for atherosclerosis. The ORIGIN-GRACE study included a subset of 1,184 subjects from the ORIGIN study who had a baseline and subsequent annual carotid ultrasound examinations. A modest, favorable, reduction in carotid intima-media thickness was observed in people treated with insulin glargine compared with standard care; however, this was not significant. It was suggested that extended follow-up is necessary to determine whether the differences in atherosclerosis between the study arms persists and also whether this translates into a reduction in clinical events.
The lack of a significant effect on CV outcomes with insulin glargine, despite its anti-inflammatory and antioxidant effects, in people who have prediabetes or early T2DM could be because long-term follow up is needed to determine the effects within this population. In earlier studies, for example, the UKPDS study, a legacy effect was observed with benefits seen during the extended follow-up. It could be that owing to the initiation of insulin early in the disease course, the number of CV events in both treatment arms is likely to be low, resulting in the similar numbers in the two arms. It is, therefore, hoped that further analyses of the ORIGIN study, as well as its 2-year extension ORIGINALE (Outcome Reduction with an Initial Glargine Intervention and Legacy Effect), will provide further insights into potential long-term outcome reductions with insulin glargine treatment, including any CV benefits. However, at present there is no indication for the use of insulin in people with prediabetes.
Overall, data from these long-term studies highlight the potential benefit of early, aggressive treatment to normalize metabolic control and minimize long-term complications associated with T2DM.
Clinical Evidence for Earlier Initiation of Insulin
Early initiation of insulin in T2DM results in improved glycemic control and protects β-cell function
Several studies have assessed the potential benefits of early intensive insulin therapy and tried to elucidate the mechanisms by which acute intervention with intensive insulin therapy confers early and sustained normoglycemia beyond the acute study treatment period in people with newly diagnosed T2DM (Table 1).35–40 A study using continuous subcutaneous insulin infusion (CSII) as intensive therapy over 2 weeks led to long-term glycemic control, improvement in β-cell function, and restoration of first-phase insulin response, with many individuals achieving remission.36 Similarly, in a study by Ryan et al.,39 intensive insulin therapy using multiple daily injections (MDI) over 2–3 weeks resulted in 44% of people maintaining glycemic control for up to 1 year with diet therapy alone. When MDI was compared with OAD treatment over 12 months, improvements in HbA1c levels and higher proportions of individuals achieving HbA1c targets were observed with the intensive MDI insulin therapy.41 Furthermore, β-cell function was significantly improved with neutral protamine Hagedorn insulin therapy relative to treatment with OADs.41 Similar observations were reported by Weng et al.40 in a study in which early intensive insulin therapy (either CSII or MDI; treatment stopped when normoglycemia was maintained for 2 weeks) resulted in high remission rates of approximately 50% (defined by maintained optimal glycemic control for at least 12 months without medication) and improvements in β-cell function, as well as quicker achievement of glycemic control compared with OADs.
Table 1.
Summary of Studies Assessing the Effects of Early Intensive Insulin Therapy (Adapted and Updated from Retnakaran and Drucker35)
| |
|
|
|
|
|
|
|
|
Patients with euglycemia (%) at |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Study | n | Mean age (years) | Mean BMI (kg/m2) | Duration of T2DM | Baseline HbA1c (%) | Type of therapy | Duration of therapy (days) | Patients who achieved euglycemia with therapy (%) | 6 months | 1 year |
| Li et al.36 | 138 | 49 | 25 | Newly diagnosed | 10.1 | CSII | 14 | 91 | 67 | 47 |
| Ilkova et al.37 | 13 | 50 | 26.9 | Newly diagnosed | 11.0 | CSII | 14 | 92 | 69 | NA |
| Park and Choi38 | 91 | 54 | NA | Mean 7.2 years | 13.2 | CSII | Mean 53.6 (SD 39) | 34 | ∼34 | ∼34 |
| Ryan et al.39 | 16 | 52 | 30.8 | Newly diagnosed | 11.8 | MDI | 14–21 | 88 | NA | 44 |
| Weng et al.40 | 382 | 51 | 25.0 | Newly diagnosed | ∼9.7 | CSII | 14–35 | 97 | NA | 51 |
| MDI | 14–35 | 95 | NA | 45 | ||||||
| OAD | 14–35 | 84 | NA | 27 | ||||||
| Chen et al.41 | 50 | ∼59 | ∼27.9 | Newly diagnosed | ∼11.6 | MDI, then insulin | 10–14 (MDI) | NA | 91 | 74 |
| MDI, then OADs | NA | 44 | 40 | |||||||
| Chandra et al.42 | 60 | ∼45.3 | ∼25.1 | Newly diagnosed | 10.4 | Premixed 30/70 | NA | NA | 80 | 62.5 |
| OAD | 3 | 5 | ||||||||
| Chon et al.43 | 61 | 48.3 | 25.0 | Newly diagnosed | 10.7 | Biphasic | Mean ∼201 | NA | 84 | 82 (groups combined) |
| Prandial±NPH or glargine | Mean ∼111 | 91.2 | ||||||||
BMI, body mass index; CSII, continuous subcutaneous insulin infusion; HbA1c, glycated hemoglobin; MDI, multiple daily injections; NA, not available; NPH, neutral protamine Hagedorn; OAD, oral antidiabetes drug; T2DM, type 2 diabetes mellitus.
A subanalysis of the Weng et al.40 study examined the effects of intensive insulin therapy on insulin sensitivity and β-cell function in people with newly diagnosed T2DM, compared with people with normal glucose tolerance (NGT) or impaired glucose tolerance (IGT).44 This substudy demonstrated that intensive insulin therapy not only partially restored β-cell function but also greatly improved insulin resistance. Before intensive insulin therapy, homeostasis model assessment for insulin resistance levels were significantly higher in people with T2DM than in people with IGT and NGT. Intensive insulin therapy was shown to significantly lower homeostasis model assessment for insulin resistance levels in people with T2DM who achieved glycemic remission, both immediately after treatment and after 12 months of follow-up (P<0.05), to a level comparable with those in the IGT and NGT groups. Intensive insulin therapy leading to glycemic remission also significantly improved homeostasis model assessment for β-cell function in people with T2DM, although function was still significantly lower than in people with NGT. The authors postulated that nearly normal restoration of insulin sensitivity could be an important beneficial mechanism for intensive insulin therapy-induced remission, as the decrease of insulin resistance would alleviate β-cell load.44
Another recent study, by Harrison et al.,45 conducted in 58 treatment-naive individuals with newly diagnosed T2DM, demonstrated the long-term benefits of early intensive therapy with premixed insulin on β-cell function. Following initial treatment with insulin plus metformin for 3 months, β-cell function was preserved for 3.5 years, regardless of whether subjects continued treatment with insulin plus metformin or switched to triple oral therapy with metformin, glyburide, and pioglitazone. Both regimens provided favorable glycemic control over the course of the study, with similar levels of modest weight gain and a significant decrease in the incidence of hypoglycemia.45 This study demonstrates that it is possible to preserve β-cell function long after the initial diagnosis of T2DM if insulin therapy is initiated in a timely and intensive manner. The authors suggested that this long-term evidence supports the use of an initial period of intensive insulin therapy in order to maximize β-cell recovery in patients with newly diagnosed T2DM, as opposed to a slower, stepwise treatment intensification in response to therapy failure.
Taken together, these clinical studies clearly highlight the impact of early intensive insulin treatment on reducing glucotoxicity and consequent improvements in insulin resistance and β-cell function, possibly through inducing β-cell rest.35 Even though OADs enable glycemic control, these trials demonstrate that the ability of insulin to rapidly return a greater number of people to near normoglycemia provides advantages, as people spend less time without good glycemic control, reducing the damage that is done during periods of poor glycemic control. This glycemic control has to be considered separately from other suggested protective mechanisms of insulin, based upon its antioxidative and anti-inflammatory properties.
Early versus late insulin therapy
Many of the studies demonstrating the benefits of early intensive insulin treatment used CSII or MDI to initiate insulin therapy (Table 1).36,40,41 As such regimens are expensive and complex—and therefore unlikely to be adopted in clinical practice—Zeng et al.46 recently conducted a randomized, open-label, parallel-group study to assess the effects of early insulin therapy using basal insulin monotherapy, compared with CSII, in 59 people with newly diagnosed T2DM. Following 2 weeks of treatment, both insulin regimens significantly improved glycemic control, β-cell function, and plasma lipid profiles compared with baseline, and there were no significant differences between the two groups, except that the time to achieve the fasting glycemic target (defined as a fasting capillary blood glucose level between 4.4 and 6.1 mmol/L, regardless of postprandial blood glucose levels) was significantly shorter with CSII than with insulin monotherapy (P<0.01).46 The results therefore suggest that basal insulin monotherapy might be a reasonable alternative to CSII for initial insulin therapy in people with newly diagnosed T2DM.
The importance of correcting basal insulin levels for diurnal control has long been recognized.47 The Treating-to-Target in Type 2 diabetes (4-T) study showed that initiating insulin-based treatment with a basal insulin (once/twice daily insulin detemir) provides considerable benefits over a prandial insulin (three times daily insulin aspart) or premixed insulin (twice daily biphasic insulin aspart) regimen in people with T2DM.48,49 At 3 years, a greater proportion of people randomized to basal or prandial insulin, versus premixed insulin, had reached an HbA1c target of ≤7.0%, whereas treatment with basal insulin detemir was associated with significantly lower rates of hypoglycemia and less weight gain compared with prandial and premixed insulin regimens.48 This study supports the principle that basal insulin provides a simple and convenient method of initiating insulin therapy, with other studies demonstrating that self-titration provides glycemic control that is equivalent to physician-led titration, with low rates of severe hypoglycemia.50
A study by Pennartz et al.51 in 14 people with T2DM and uncontrolled FPG levels on metformin found that chronic therapy (8 weeks of treatment) with add-on insulin glargine resulted in improved β-cell function, as determined by first- and second-phase insulin secretion. These data demonstrate that basal insulin therapy improves endogenous insulin secretion via pancreatic rest, potentially partly because of reductions in both glucose and lipotoxicity.
The benefit of earlier versus later insulin initiation is further illustrated by an evaluation of the addition of insulin glargine to none to two OADs in people with poorly controlled T2DM.52 Greater HbA1c reductions with lower risk of hypoglycemia were observed with insulin for individuals who previously failed therapy with none or one versus two OADs. Furthermore, mean reductions in HbA1c were greater in individuals previously receiving metformin alone versus a sulfonylurea alone or metformin plus a sulfonylurea. Despite a higher insulin dose in the prior metformin-only group, the incidence of hypoglycemia and weight gain was lowest in this group. Early insulin therapy has also demonstrated benefits over sulfonylurea-based treatment in terms of long-term (up to 4 years) glycemic control and endogenous insulin secretion.53,54
As previously outlined, the INSIGHT study demonstrated that adding insulin versus avoidance of insulin in people with T2DM receiving no or submaximal OAD therapy resulted in greater improvements in HbA1c and FPG levels and no difference in the occurrence of hypoglycemic events.32 More recently, the GLORY (Insulin Glargine First Line versus Metformin in Type 2 Diabetic Subjects) study randomized people with pharmacotherapy-naive T2DM to treatment with metformin or insulin glargine over 36 weeks and showed that first-line basal insulin therapy was associated with significant improvements in glycemic control and β-cell function compared with metformin.55 In this study, insulin did not increase the risk of symptomatic hypoglycemia versus metformin but was associated with a higher frequency of asymptomatic hypoglycemia and significant weight gain.
The efficacy and safety of early insulin therapy in a real-world clinical setting were recently demonstrated in an open-label observational study of 1,438 people with T2DM poorly controlled with the maximal dose of metformin (HbA1c, >7.5%). In total, 1,389 individuals were treated with insulin glargine as a second-step alternative to add-on OADs. After 24 weeks, mean HbA1c and fasting blood glucose were both significantly reduced (from 8.7% to 7.4% and from 181.7 mg/dL to 130.5 mg/dL, respectively; both P<0.01). Average body weight decreased by approximately 1 kg, and the number of hypoglycemic events did not increase significantly between Weeks 12 and 24, despite the increasing insulin dose. Overall, symptomatic hypoglycemia was reported in only 2.5% of people.56
Treatment inertia leads to high glycemic burden
As described, there is a wealth of evidence to suggest that earlier initiation of insulin therapy is likely to be of benefit for many people with T2DM; however, many individuals and their physicians are reluctant to start insulin therapy, owing predominantly to the perceived risk of hypoglycemia and weight gain. Several barriers to insulin initiation and intensification exist, both for people with T2DM and for physicians, including low motivation, lack of familiarity or experience with treatments, and time constraints.57 Treatment inertia may result in people treated with OADs experiencing a high glycemic burden for extended periods before initiation of insulin therapy.8 In a prospective, population-based study using retrospective observational data, Brown et al.8 demonstrated that people treated with OADs—metformin monotherapy, sulfonylurea monotherapy, and combination OAD regimens—accumulated nearly 5 HbA1c-years of excess glycemic burden >8.0% from diagnosis until starting insulin and about 10 HbA1c-years of burden >7.0%. Of the individuals initially treated with diet and exercise, the majority progressed to pharmacological treatment with OAD monotherapy (73.6%) and then subsequently to OAD combination therapy (approximately 91%). However, HbA1c levels reached, on average, ≥9.6% before combination therapy was initiated, which is considerably higher than recommended by international guidelines. Subsequently, almost 90% of individuals who received combination therapy eventually received insulin.8 This pattern of treatment is apparent in observational studies of clinical practice worldwide, which routinely report that insulin is initiated with a duration of T2DM of approximately 10 years and at a mean HbA1c level in excess of 9%. Inevitably, the number and extent of diabetes-associated complications in such a population are extensive.58 However, overcoming clinical inertia and intervening earlier with insulin therapy may reduce this burden.
The impact of early insulin therapy on quality of life
Quality of life (QoL) is an important measure of the success of T2DM treatment. There is some perception that insulin therapy can negatively impact QoL, and this may deter some physicians from initiating insulin therapy earlier. Studies including QoL outcomes with up to 4 years of follow-up in people with T2DM have demonstrated, however, that early initiation of insulin has no negative effect on QoL.53,54,59
Asche et al.60 conducted a systematic review of articles published between 2000 and 2010 that reported clinical and economic outcomes associated with early insulin initiation or intensification. Their analysis concluded that the addition of insulin to OADs did not significantly affect individuals' treatment satisfaction or QoL and that the improved glycemic control obtained by adding insulin to OADs had, in fact, a positive impact on QoL outcomes.60 A 24-week questionnaire-based study assessed treatment satisfaction and QoL for early insulinization at bedtime compared with adjusted oral therapy in 366 people with T2DM. QoL was measured by the Audit of Diabetes-Dependent Quality of Life (ADDQoL), which comprises 13 items relating to physical functioning, symptoms, psychological well-being, social well-being, role activities, and personal constructs. Evaluation of the ADDQoL demonstrated a significant improvement in QoL for individuals treated with early insulin at Week 12 (P=0.025) and Week 24 (P=0.024) compared with adjusted oral therapy.61
A more recent study used two QoL measures to evaluate the effect of short-term (4–8 weeks) intensive insulin therapy on QoL in 34 people with T2DM receiving none to two OADs. The Diabetes Quality of Life Measure demonstrated a significant improvement in QoL outcomes including global health perception (P=0.02), diabetes worry (P=0.006), and treatment satisfaction (P=0.007). The Diabetes Symptoms Checklist-Revised revealed a significant improvement in the diabetes-related total symptom score (P=0.01).62
Together, the results of these studies indicate that insulin therapy initiated early in the management of people with T2DM has no negative impact on QoL and may actually lead to improvements in QoL through improved glycemic control.
Conclusions
Clinical (treatment) inertia with OADs leads to excessively high and prolonged glycemic burden prior to the initiation of insulin therapy. Many people with T2DM remain poorly controlled on OAD treatment for extended periods, despite the observations that loss of β-cell function and mass are potentially preventable and that early intensive insulin treatment, enabling people to more rapidly obtain normoglycemia, may halt, or at least delay, progression of the disease. Basal insulin therapy confers improved glycemic control through suppression of hepatic glucose production and protection of β-cell function through reduced gluco- and lipotoxicity and consequently “pancreatic rest.” Clinical evidence demonstrates that early insulin therapy in T2DM maximizes the potential to nearly normalize glucose control, to prevent progression of glucose intolerance, to restore β-cell function, to improve metabolic memory, to offer long-term protection to end organs, and to enhance individuals' QoL. Recent clinical data from the ORIGIN trial confirm the efficacy and safety of early insulin therapy with only modest increases in hypoglycemia and weight gain. Treatment with insulin may also provide independent protection against vascular endothelial dysfunction through its anti-inflammatory and antioxidative stress actions; however, longer-term follow-up of trials is needed to determine whether this protection results in clinically relevant changes to outcomes.
Although it is widely recognized that achieving lower HbA1c levels earlier in the management of T2DM results in better long-term outcomes and lower risk of diabetes complications and mortality—with treatment guidelines generally recommending earlier insulin initiation as an option—this is not always reflected in clinical practice treatment patterns, as many individuals and physicians appear to be reluctant to start insulin therapy. With the advent of the incretin class of antidiabetes agents, continued efforts are required to further clarify the place of early insulin therapy in the treatment of T2DM.
Clinical Implications
As outlined in this review, there is clear evidence to support the efficacy and safety of early initiation of insulin therapy in the treatment of T2DM. Such an approach not only provides rapid and effective glycemic control, but also is associated with a range of additional effects that may have far-reaching benefits for the disease progression of individuals with T2DM.
Although many subjects with T2DM are likely to benefit from early insulin therapy, there are three groups of individuals who are particularly suitable for such a treatment approach. The first group is treatment-naive individuals who present with marked symptoms of hyperglycemia with an HbA1c level of >8.5%, in whom early insulin treatment not only provides rapid improvement in glycemic control but also restores β-cell function.40,41,44 Therapy in this population potentially has disease-modifying benefits, with up to 50% remaining in remission after 12 months on no therapy after the short-term intensive insulin therapy in an attempt to achieve normal glycemia. By directly combating the glucotoxicity, early intensive insulin treatment can induce “β-cell rest,” thereby optimizing the possibility of restoring β-cell function and delaying disease progression.35 Following early intensive insulin therapy, an individual could either continue with insulin-based therapy or switch to OAD therapy, as restoration of β-cell function by early insulin treatment appears to be preserved over the long term, regardless of which treatment approach is subsequently adopted.45
The second group likely to benefit from early insulin therapy is people with latent autoimmune diabetes of adults (LADA), also known as type 1.5 diabetes.63 LADA is not generally regarded as insulin-requiring; however, those with LADA have islet autoantibodies (most commonly, glutamic acid decarboxylase antibody) and relatively low C-peptide secretion, and the rate of progression to insulin dependency is faster than in people with T2DM.64,65 Although the optimal treatment strategy for people with LADA is currently unclear, evidence suggests that, by preserving β-cell function, early insulin treatment leads to better preservation of metabolic control and better long-term outcomes than conventional treatment with OADs.65,66 Initiating therapy with insulin may therefore be particularly beneficial for those individuals with LADA who have high titers of glutamic acid decarboxylase antibody (>20 U/mL).67
Third, as insulin treatment is known to have additional anti-inflammatory and antioxidant effects, which may contribute to protection against endothelial dysfunction and vascular disease,15–17 early insulin treatment is particularly suitable for individuals already at high risk for vascular complications, such as those with hyperlipidemia, hypertension, or both. In individuals with protracted disease who remain inadequately controlled on a multitude of other antidiabetic drugs, careful introduction of insulin is required to avoid hypoglycemia. In contrast, introduction of insulin in subjects at high CV risk early in the course of diabetes before they are on multiple OADs does not exaggerate the CV risk, with a minimal risk of hypoglycemia and only moderate weight gain. It is important that long-term studies, such as the 10-year follow-up to the UKPDS, have demonstrated that a period of good glycemic control early in the course of T2DM has a lasting benefit, independent of subsequent glycemic control.24 Similar CV benefits were seen in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study in persons with type 1 diabetes mellitus.68 The extension of the ORIGIN trial will provide additional information relating to the longer-term value of early insulin therapy in our subjects with T2DM.
The early use of insulin in these three populations needs to be investigated in clinical studies, so that treatment algorithms can be developed that allow for the optimal choice of diabetes management strategy in every individual with diabetes.
Acknowledgments
Editorial support was provided by Róisín O'Connor, PhD, Medicus International, and funded by Sanofi.
Author Disclosure Statement
D.R.O. has received lecture fees and honoraria from Sanofi and Roche Diagnostics. The author was responsible for the conception of the article and contributed to the writing, including critical review and editing of each draft, and approval of the submitted version.
References
- 1.Banting FG. Best CH. Pancreatic extracts. J Lab Clin Med. 1922;7:464–472. [PubMed] [Google Scholar]
- 2.Banting FG. Best CH. The internal secretion of pancreas. J Lab Clin Med. 1922;7:251–256. [Google Scholar]
- 3.Banting FG. Best CH. Collip JB. Campbell WR. Fletcher AA. Macleod JJR. Pancreatic extracts in the treatment of diabetes mellitus: preliminary report. Can Med Assoc J. 1922;12:141–146. [PMC free article] [PubMed] [Google Scholar]
- 4.Banting FG. Best CH. Collip JB. Macleod JJR. Noble EC. The effect of pancreatic extract (insulin) on normal rabbits. Am J Physiol. 1922;62:162–176. [Google Scholar]
- 5.Banting FG. Campbell WR. Fletcher AA. Further experience with insulin in the treatment of diabetes mellitus. Br Med J. 1923;i:8–12. doi: 10.1136/bmj.1.3236.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marble A. Insulin in the treatment of diabetes. In: Marble A, editor; White P, editor; Bradley RF, editor; Krall LP, editor. Joslin's Diabetes Mellitus. 11th. Philadelphia: Lea & Febiger; 1971. pp. 287–301. [Google Scholar]
- 7.Himsworth HP. Kerr RB. Insulin-sensitive and insulin-insensitive types of diabetes mellitus. Clin Sci. 1939;4:119. [Google Scholar]
- 8.Brown JB. Nichols GA. Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535–1540. doi: 10.2337/diacare.27.7.1535. [DOI] [PubMed] [Google Scholar]
- 9.Shichiri M. Kishikawa H. Ohkubo Y. Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–B29. [PubMed] [Google Scholar]
- 10.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Vital signs: prevalence, treatment, and control of hypertension—United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:103–108. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Vital signs: prevalence, treatment, and control of high levels of low-density lipoprotein cholesterol—United States, 1999–2002 and 2005–2008. MMWR Morb Mortal Wkly Rep. 2011;60:109–114. [PubMed] [Google Scholar]
- 13.Inzucchi SE. Bergenstal RM. Buse JB. Diamant M. Ferrannini E. Nauck M. Peters AL. Tsapas A. Wender R. Matthews DR American Diabetes Association, European Association for the Study of Diabetes. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan DM. Buse JB. Davidson MB. Ferrannini E. Holman RR. Sherwin R. Zinman B American Diabetes Association, European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dandona P. Chaudhuri A. Ghanim H. Mohanty P. Proinflammatory effects of glucose and anti-inflammatory effect of insulin: relevance to cardiovascular disease. Am J Cardiol. 2007;99:15B–26B. doi: 10.1016/j.amjcard.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Dandona P. Chaudhuri A. Mohanty P. Ghanim H. Anti-inflammatory effects of insulin. Curr Opin Clin Nutr Metab Care. 2007;10:511–517. doi: 10.1097/MCO.0b013e3281e38774. [DOI] [PubMed] [Google Scholar]
- 17.Dandona P. Mohanty P. Chaudhuri A. Garg R. Aljada A. Insulin infusion in acute illness. J Clin Invest. 2005;115:2069–2072. doi: 10.1172/JCI26045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner RC. McCarthy ST. Holman RR. Harris E. Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J. 1976;1:1252–1254. doi: 10.1136/bmj.1.6020.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jetton TL. Liu YQ. Trotman WE. Nevin PW. Sun XJ. Leahy JL. Enhanced expression of insulin receptor substrate-2 and activation of protein kinase B/Akt in regenerating pancreatic duct epithelium of 60%-partial pancreatectomy rats. Diabetologia. 2001;44:2056–2065. doi: 10.1007/s001250100011. [DOI] [PubMed] [Google Scholar]
- 20.Jetton TL. Lausier J. LaRock K. Trotman WE. Larmie B. Habibovic A. Peshavaria M. Leahy JL. Mechanisms of compensatory beta-cell growth in insulin-resistant rats: roles of Akt kinase. Diabetes. 2005;54:2294–2304. doi: 10.2337/diabetes.54.8.2294. [DOI] [PubMed] [Google Scholar]
- 21.Bouche C. Lopez X. Fleischman A. Cypess AM. O'Shea S. Stefanovski D. Bergman RN. Rogatsky E. Stein DT. Kahn CR. Kulkarni RN. Goldfine AB. Insulin enhances glucose-stimulated insulin secretion in healthy humans. Proc Natl Acad Sci U S A. 2010;107:4770–4775. doi: 10.1073/pnas.1000002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garvey WT. Olefsky JM. Griffin J. Hamman RF. Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 23.Engerman RL. Kern TS. Progression of incipent diabetic retinopathy during good glycemic control. Diabetes. 1987;36:808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- 24.Holman RR. Paul SK. Bethel MA. Matthews DR. Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 25.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 26.Stratton IM. Adler AI. Neil HA. Matthews DR. Manley SE. Cull CA. Hadden D. Turner RC. Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ceriello A. Ihnat MA. Thorpe JE. Clinical review 2: the “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab. 2009;94:410–415. doi: 10.1210/jc.2008-1824. [DOI] [PubMed] [Google Scholar]
- 28.Genuth S. Sun W. Cleary P. Sell DR. Dahms W. Malone J. Sivitz W. Monnier VM DCCT Skin Collagen Ancillary Study Group. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications participants with type 1 diabetes. Diabetes. 2005;54:3103–3111. doi: 10.2337/diabetes.54.11.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerstein HC. Miller ME. Byington RP. Goff DC., Jr Bigger JT. Buse JB. Cushman WC. Genuth S. Ismail-Beigi F. Grimm RH., Jr Probstfield JL. Simons-Morton DG. Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel A. MacMahon S. Chalmers J. Neal B. Billot L. Woodward M. Marre M. Cooper M. Glasziou P. Grobbee D. Hamet P. Harrap S. Heller S. Liu L. Mancia G. Mogensen CE. Pan C. Poulter N. Rodgers A. Williams B. Bompoint S. de Galan BE. Joshi R. Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 31.Duckworth W. Abraira C. Moritz T. Reda D. Emanuele N. Reaven PD. Zieve FJ. Marks J. Davis SN. Hayward R. Warren SR. Goldman S. McCarren M. Vitek ME. Henderson WG. Huang GD. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 32.Gerstein HC. Yale JF. Harris SB. Issa M. Stewart JA. Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with Type 2 diabetes on either no oral glucose-lowering agents or submaximal doses of metformin and/or sulphonylureas. The Canadian INSIGHT (Implementing New Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabet Med. 2006;23:736–742. doi: 10.1111/j.1464-5491.2006.01881.x. [DOI] [PubMed] [Google Scholar]
- 33.Origin Trial Investigators. Gerstein HC. Bosch J. Dagenais GR. Diaz R. Jung H. Maggioni AP. Pogue J. Probstfield J. Ramachandran A. Riddle MC. Ryden LE. Yusuf S. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–328. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 34.Lonn EM. Bosch J. Diaz R. Lopez-Jaramillo P. Ramachandran A. Hancu N. Hanefeld M. Krum H. Ryden L. Smith S. McQueen MJ. Dyal L. Yusuf S. Gerstein HC. GRACE and ORIGIN Investigators: Effect of insulin glargine and n-3FA on carotid intima-media thickness in people with dysglycemia at high risk for cardiovascular events: the Glucose Reduction and Atherosclerosis Continuing Evaluation Study (ORIGIN-GRACE. Diabetes Care. 2013 Apr 5; doi: 10.2337/dc12-2129. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Retnakaran R. Drucker DJ. Intensive insulin therapy in newly diagnosed type 2 diabetes. Lancet. 2008;371:1725–1726. doi: 10.1016/S0140-6736(08)60736-9. [DOI] [PubMed] [Google Scholar]
- 36.Li Y. Xu W. Liao Z. Yao B. Chen X. Huang Z. Hu G. Weng J. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27:2597–2602. doi: 10.2337/diacare.27.11.2597. [DOI] [PubMed] [Google Scholar]
- 37.Ilkova H. Glaser B. Tunckale A. Bagriacik N. Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 38.Park S. Choi SB. Induction of long-term normoglycemia without medication in Korean type 2 diabetes patients after continuous subcutaneous insulin infusion therapy. Diabetes Metab Res Rev. 2003;19:124–130. doi: 10.1002/dmrr.343. [DOI] [PubMed] [Google Scholar]
- 39.Ryan EA. Imes S. Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- 40.Weng J. Li Y. Xu W. Shi L. Zhang Q. Zhu D. Hu Y. Zhou Z. Yan X. Tian H. Ran X. Luo Z. Xian J. Yan L. Li F. Zeng L. Chen Y. Yang L. Yan S. Liu J. Li M. Fu Z. Cheng H. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 41.Chen HS. Wu TE. Jap TS. Hsiao LC. Lee SH. Lin HD. Beneficial effects of insulin on glycemic control and beta-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care. 2008;31:1927–1932. doi: 10.2337/dc08-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chandra ST. Priya G. Khurana ML. Jyotsna VP. Sreenivas V. Dwivedi S. Ammini AC. Comparison of gliclazide with insulin as initial treatment modality in newly diagnosed type 2 diabetes. Diabetes Technol Ther. 2008;10:363–368. doi: 10.1089/dia.2008.0045. [DOI] [PubMed] [Google Scholar]
- 43.Chon S. Oh S. Kim SW. Kim JW. Kim YS. Woo JT. The effect of early insulin therapy on pancreatic beta-cell function and long-term glycemic control in newly diagnosed type 2 diabetic patients. Korean J Intern Med. 2010;25:273–281. doi: 10.3904/kjim.2010.25.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu Y. Li L. Xu Y. Yu T. Tong G. Huang H. Bi Y. Weng J. Zhu D. Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and beta-cell function in subjects with long-term remission. Diabetes Care. 2011;34:1848–1853. doi: 10.2337/dc10-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison LB. Adams-Huet B. Raskin P. Lingvay I. Beta-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care. 2012;35:1406–1412. doi: 10.2337/dc11-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeng L. Lu H. Deng H. Mu P. Li X. Wang M. Noninferiority effects on glycemic control and beta-cell function improvement in newly diagnosed type 2 diabetes patients: basal insulin monotherapy versus continuous subcutaneous insulin infusion treatment. Diabetes Technol Ther. 2012;14:35–42. doi: 10.1089/dia.2011.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner R. Stratton I. Horton V. Manley S. Zimmet P. Mackay IR. Shattock M. Bottazzo GF. Holman R. UKPDS 25: autoantibodies to islet-cell cytoplasm, glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. UK Prospective Diabetes Study Group. Lancet. 1997;350:1288–1293. doi: 10.1016/s0140-6736(97)03062-6. [DOI] [PubMed] [Google Scholar]
- 48.Holman RR. Farmer AJ. Davies MJ. Levy JC. Darbyshire JL. Keenan JF. Paul SK. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 49.Holman RR. Thorne KI. Farmer AJ. Davies MJ. Keenan JF. Paul S. Levy JC. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 50.Davies M. Storms F. Shutler S. Bianchi-Biscay M. Gomis R. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282–1288. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- 51.Pennartz C. Schenker N. Menge BA. Schmidt WE. Nauck MA. Meier JJ. Chronic reduction of fasting glycemia with insulin glargine improves first- and second-phase insulin secretion in patients with type 2 diabetes. Diabetes Care. 2011;34:2048–2053. doi: 10.2337/dc11-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fonseca V. Gill J. Zhou R. Leahy J. An analysis of early insulin glargine added to metformin with or without sulfonylurea: impact on glycaemic control and hypoglycaemia. Diabetes Obes Metab. 2011;13:814–822. doi: 10.1111/j.1463-1326.2011.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarsson M. Sundkvist G. Lager I. Berntorp K. Fernqvist-Forbes E. Steen L. Orn T. Holberg MA. Kirksaether N. Grill V. Effects of insulin vs. glibenclamide in recently diagnosed patients with type 2 diabetes: a 4-year follow-up. Diabetes Obes Metab. 2008;10:421–429. doi: 10.1111/j.1463-1326.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 54.Alvarsson M. Sundkvist G. Lager I. Henricsson M. Berntorp K. Fernqvist-Forbes E. Steen L. Westermark G. Westermark P. Orn T. Grill V. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231–2237. doi: 10.2337/diacare.26.8.2231. [DOI] [PubMed] [Google Scholar]
- 55.Pistrosch F. Köhler C. Schaper F. Landgraf W. Forst T. Hanefeld M. Effects of insulin glargine versus metformin on glycemic variability, microvascular, beta-cell function in early type 2 diabetes. Acta Diabetol. 2013 Feb 21; doi: 10.1007/s00592-012-0451-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hanefeld M. Fleischmann H. Landgraf W. Postrosch F. EARLY study: early basal insulin therapy under real-life conditions in type 2 diabetics. Diabetes Stoffwechsel Herz. 2012;21:91–97. [Google Scholar]
- 57.Kunt T. Snoek FJ. Barriers to insulin initiation and intensification and how to overcome them. Int J Clin Pract Suppl. 2009;(164):6–10. doi: 10.1111/j.1742-1241.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 58.Ziloz AV. Yang W. Gonzalez-Galvez G. Home P. Jianwen C. Hasan M. Prevalence of complications of diabetes in people with type 2 diabetes: data from Asia, Europe and Latin America from the A1chieve study [abstract 2485-PO] Diabetes. 2011;60:A656. [Google Scholar]
- 59.Lingvay I. Legendre JL. Kaloyanova PF. Zhang S. Adams-Huet B. Raskin P. Insulin-based versus triple oral therapy for newly diagnosed type 2 diabetes: which is better? Diabetes Care. 2009;32:1789–1795. doi: 10.2337/dc09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asche CV. Bode B. Busk AK. Nair SR. The economic and clinical benefits of adequate insulin initiation and intensification in people with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:47–57. doi: 10.1111/j.1463-1326.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- 61.Houlden R. Ross S. Harris S. Yale JF. Sauriol L. Gerstein HC. Treatment satisfaction and quality of life using an early insulinization strategy with insulin glargine compared to an adjusted oral therapy in the management of Type 2 diabetes: the Canadian INSIGHT Study. Diabetes Res Clin Pract. 2007;78:254–258. doi: 10.1016/j.diabres.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 62.Opsteen C. Qi Y. Zinman B. Retnakaran R. Effect of short-term intensive insulin therapy on quality of life in type 2 diabetes. J Eval Clin Pract. 2012;18:256–261. doi: 10.1111/j.1365-2753.2010.01552.x. [DOI] [PubMed] [Google Scholar]
- 63.Juneja R. Palmer JP. Type 1 1/2 diabetes: myth or reality? Autoimmunity. 1999;29:65–83. doi: 10.3109/08916939908995974. [DOI] [PubMed] [Google Scholar]
- 64.Fourlanos S. Perry C. Stein MS. Stankovich J. Harrison LC. Colman PG. A clinical screening tool identifies autoimmune diabetes in adults. Diabetes Care. 2006;29:970–975. doi: 10.2337/diacare.295970. [DOI] [PubMed] [Google Scholar]
- 65.Poudel RR. Latent autoimmune diabetes of adults: from oral hypoglycemic agents to early insulin. Indian J Endocrinol Metab. 2012;16(Suppl 1):S41–S46. doi: 10.4103/2230-8210.94257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thunander M. Thorgeirsson H. Torn C. Petersson C. Landin-Olsson M. Beta-cell function and metabolic control in latent autoimmune diabetes in adults with early insulin versus conventional treatment: a 3-year follow-up. Eur J Endocrinol. 2011;164:239–245. doi: 10.1530/EJE-10-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosário PW. Reis JS. Fagundes TA. Calsolari MR. Amim R. Silva SC. Purisch S. Latent autoimmune diabetes in adults (LADA): usefulness of anti-GAD antibody titers and benefit of early insulinization. Arq Bras Endocrinol Metabol. 2007;51:52–58. doi: 10.1590/s0004-27302007000100009. [DOI] [PubMed] [Google Scholar]
- 68.Nathan DM. Cleary PA. Backlund JY. Genuth SM. Lachin JM. Orchard TJ. Raskin P. Zinman B Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]


