Abstract
Purpose
To compare the pharmacokinetics (PKs) of intravitreally injected bevacizumab in vitrectomized versus nonvitrectomized control rabbit eyes.
Methods
Twenty-five-gauge pars plana vitrectomy without lensectomy was performed in 17 right rabbit eyes (V) and 18 nonvitrectomized right rabbit eyes served as controls (C). After 1.25 mg/0.05 mL intravitreal bevacizumab (IVB) injections, eyes were enucleated at 1 h, 1, 2, 5, 14, and 30 days after the injection and immediately frozen at −80°C. Bevacizumab concentrations were determined after separation of frozen vitreous and aqueous humor (AH) compartments using indirect enzyme-linked immunosorbent assay. Bevacizumab concentration–time data were analyzed to obtain PK data.
Results
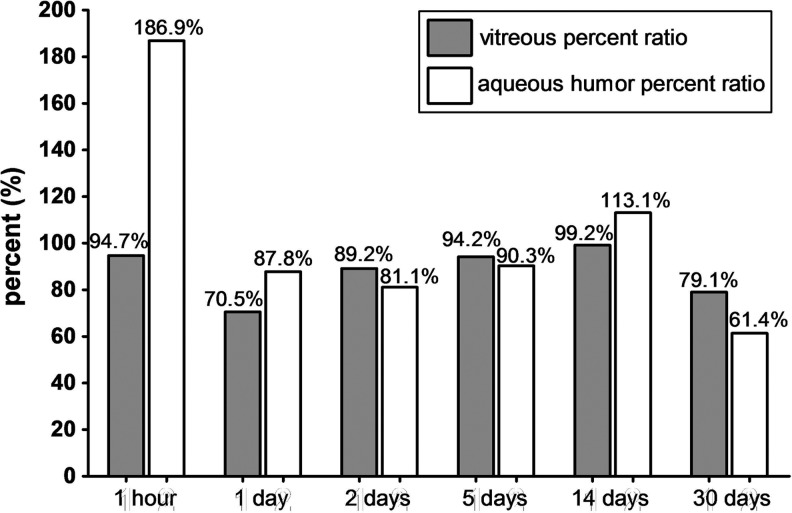
Vitreous clearance of IVB consisted of 2 phases, the first fast distribution and second slow elimination phase. Clearance of IVB was accelerated in V eyes only during the first phase and not in the second phase. The vitreous concentration percent ratios between V and C eyes were 94.7% (1 h), 70.5% (1 day), 89.2% (2 days), 94.2% (5 days), 99.2% (14 days), and 79.1% (30 days). Overall vitreous half-lives were 6.99 and 7.06 days for V and C eyes, respectively (1.6-h difference).
Conclusion
Overall IVB PKs in rabbit eyes after vitrectomy without lensectomy are not substantially different from nonvitrectomized control eyes.
Introduction
Since the implication of the vascular endothelial growth factor (VEGF) as the key mediator of numerous sight-threatening diseases such as exudative age-related macular degeneration (AMD), macular edema secondary to retinal vein occlusion or diabetic retinopathy, proliferative diabetic retinopathy, and neovascular glaucoma, anti-VEGF agents have become the mainstay of treatment in numerous retinal diseases, revolutionizing the treatment paradigm.1–5 Bevacizumab, a recombinant monoclonal antibody that binds to all subtypes of VEGF, has been widely used in the aforementioned VEGF-mediated diseases off-label, due to its relatively low cost. A recent prospective clinical trial, which demonstrated the noninferior efficacy of bevacizumab relative to ranibizumab, indicates the on-going wide use of bevacizumab for exudative AMD and other retinal diseases.6
Anti-VEGF agents are injected directly into the vitreous cavity to achieve most effective therapeutic drug concentrations in the posterior segment owing to unique intraocular drug delivery barriers present to confer the eye with sterile, immune privileged status. Due to the underlying chronic and progressive nature of the diseases necessitating injections, frequent and periodic intravitreal injections are inevitably performed. Pharmacokinetic (PK) profiles of intravitreally injected drugs are crucial in determining the optimal dosing frequency to achieve the maximum therapeutic intraocular concentration with the least number of injections. Several researchers analyzed the PK parameters of intravitreally injected bevacizumab in rabbit eyes. Bakri et al. reported the vitreous T1/2 of intravitreal bevacizumab (IVB) as 4.32 days, while Nomoto et al. and Sinapis et al. found the T1/2 to be 6–6.61 days in rabbit eyes.7–9
In eyes undergoing bevacizumab treatment, clinicians are frequently challenged with conditions necessitating surgical intervention, such as vitreous hemorrhage, vitreous opacity, epiretinal membrane, or macular hole. Up to now, based on past animal studies and some supportive clinical evidence, drug clearance has been generally assumed to increase and clinical drug effectiveness decrease, in vitrectomized eyes.10–15 However, there is scarce data on the PK of intravitreally injected bevacizumab in vitrectomy only (without lensectomy) eyes and scant evidence to ascertain the most effective dosing schedule for IVB injection in vitrectomized eyes.16
Hence, this study was performed to comparatively analyze the PK profiles of intravitreally injected bevacizumab in vitrectomized (V) eyes versus nonvitrectomized control (C) eyes and to ultimately suggest the most adequate treatment regimen for IVB injection in vitrectomized patients. Through this study, the role of the vitreous gel in the clearance of IVB could also be elucidated.
Materials and Methods
Animal experiment
After approval from the Seoul National University Bundang Hospital Institutional Animal Care and Use Committee, rabbit experiments were conducted with procedures adhering to the guidelines from the Association for Research in Vision and Ophthalmology for animal use in research. A total of 36 eyes of 36 healthy New Zealand white rabbits weighing 1.5 to 2 kg were initially used for the study. The rabbits were divided into the vitrectomy (V) group (18 right eyes) and the control (C) group (18 right eyes). Retinal detachment was noted in 1 rabbit of the V group on postvitrectomy eye examination and was excluded from the study. Subsequently, 35 eyes of 35 rabbits received IVB injections and were included for final analysis.
Rabbits in the V group were anesthetized with an intramuscular injection of 15 mg/kg of Zoletil (mixture of tiletamine hydrochloride and zolazepam hydrochloride; Virbac laboratories, CarrosCedex, France) and 5 mg/kg of xylazine hydrochloride. After dilation with phenylephrine hydrochloride and tropicamide eyedrops (Mydrin-P; Santen Pharmaceutical Co., Osaka, Japan), topical anesthesia was induced using 1% proparacaine hydrochloride ophthalmic eyedrops (Alcaine; Alcon laboratories, Inc., Fort Worth, TX). The eye was prepped for surgery by applying 5% povidone-iodine eyedrops on the conjunctiva and all procedures were conducted using the aseptic technique with surgical draping. After eye proptosis, 2 port 25-gauge sclerotomies were made with trocar insertion (Alcon Laboratories, Inc.); one port was used as the infusion cannula connected to a bottle of balanced salt solution (BSS) and the other port was used for the ocutome. A wide field fundus contact lens (Super Quad 160 lens; Volk, Mentor, OH) was placed after clear gel application on the cornea to enable wide view of the peripheral retina and light from the operating microscope served as the illumination source. Complete vitrectomy was done (Accurus Surgical System; Alcon Laboratories, Inc., Fort Worth, TX) with meticulous effort to remove as much vitreous as possible and fluid-air exchange was done to confirm removal of over 80% of the vitreous. At conclusion of surgery, the air was replaced with BSS. After cannula removal, the eyeball was palpated to ensure normal intraocular pressure and a broad-spectrum antibiotic ointment was applied to the eye.
After a recovery period of over 2 weeks for the V group, IVB injections were performed on 35 right eyes of 35 rabbits. For the intravitreal injections, 1% proparacaine and 5% povidone-iodine eyedrops were first applied to the right eye and using a 30-gauge needle, 1.25 mg/0.05 mL of bevacizumab (Avastin; Genetech, Inc., South San Francisco, CA) was injected intravitreally, 2 mm behind the limbus in the superotemporal quadrant.
Three rabbits in the V and C groups were sacrificed at each of the following 6 time points: 1 h, day 1, 2, 5, 14, and 30 after intravitreal injection, with the exception of 2 rabbits in the V group at 2 days after injection. The right eye was enucleated and immediately frozen at −80°C. The frozen vitreous and aqueous humor (AH) were subsequently separated from the eye as single compartments and kept at −80°C until the bevacizumab assay. Before analysis, the frozen samples were defrosted and solubilized in 1.0 ml 1% bovine serum albumin (BSA).
Bevacizumab concentrations were measured using indirect enzyme linked immunosorbent assay (ELISA) as previously described.17 In brief, the 165 amino acid variant of human recombinant VEGF (R&D systems, Minneapolis, MN) was immobilized on 96-well flat bottom plates (Corning, Inc., Corning, NY). The human recombinant VEGF (rVEGF) was diluted to a concentration of 1.0 μg/mL in a 50 mM carbonate buffer, pH 9, and then aliquoted onto the 96-well plates at 100 μL/well. After incubating overnight at 4°C, the plates were washed with 1× PBS and blocked for 2–4 h at 4°C with 1% BSA in 1× PBS. After a final washing procedure, the plates were stored to dry at 4°C. Vitreous samples were diluted in 0.1% BSA, 1× PBS, aliquoted onto a VEGF plate at 100 μL/well, and then incubated overnight at 4°C. For each individual plate, a standard curve of known bevacizumab concentrations, ranging from 3.125 to 0.049 mg/mL, was included. Bevacizumab was detected with an anti-human immunoglobulin G horseradish peroxidase antibody (GE Healthcare, Pittsburg, PA). The immunoglobulin G antibody was incubated on the human rVEGF plate for 2 h, followed by a washing solution. The optical density was measured by detecting the absorbance after triggering the 3,3′,5,5′-tetramethyl benzidine substrate with hydrogen peroxide. Data analysis was done using SoftMax Pro software (Version 5.4.1, Molecular devices, Sunnyvale, CA).
PK data analysis
Two-phase PK analysis
All experimental concentration data were fit to the following 2-phase exponential decay equation with the SAAMII software (Version 1.2.1; Saam Institute, Seattle, WA):
C(t)=C1exp(−k1t)+C2exp(−k2t)
where C (μg/mL) denotes concentration at any time, t (hour). C1 (μg/mL) and C2 (μg/mL) are the back-extrapolated intercepts of the distribution and elimination phase, respectively. Both k1 (hour−1) and k2 (hour−1) represent rate constants at the distribution and elimination phase, respectively.
Overall (One-phase) PK analysis
All experimental concentration data were fit to the following single exponential decay expression after excluding the 1-h concentration data with the SAAMII software:
C(t)=C0exp(−kt)
where C (μg/mL) and C0 (μg/mL) denote concentration at any time, t (hour) and at t=0, respectively. k (hour−1) represents an elimination rate constant. The half-lives (T1/2) of bevacizumab in the V and C eyes were calculated with the following equation:
T1/2=0.693/k
Results
Data were collected from 35 eyes of 35 rabbits. There was no evidence of ocular inflammation or other adverse events.
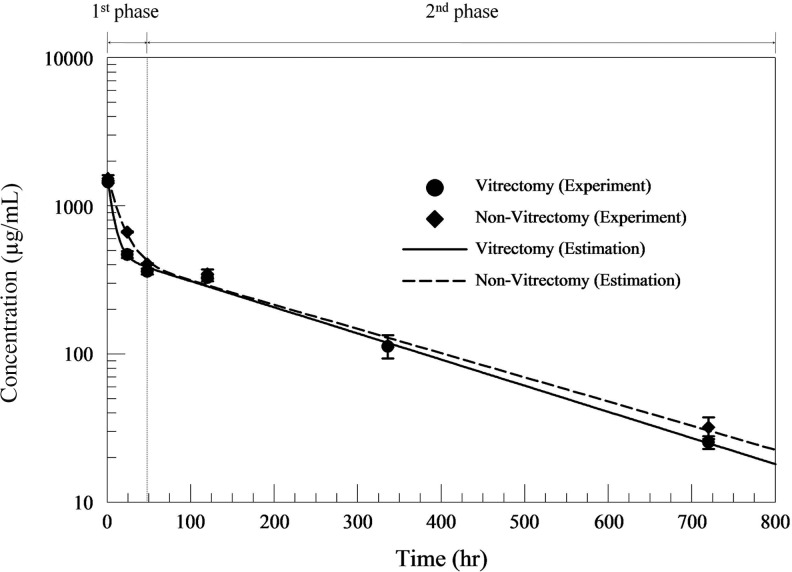
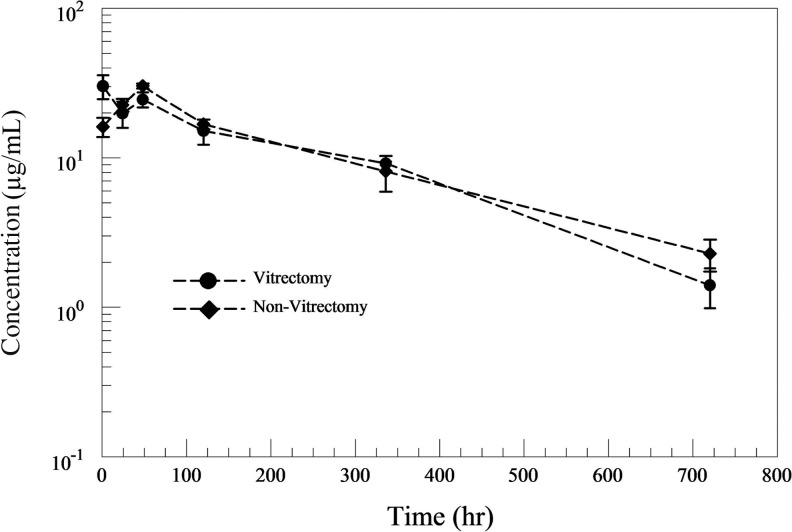
Changes in the vitreous bevacizumab concentration over time in both V and C eyes are illustrated in Fig. 1. Plotting of the vitreous bevacizumab concentration showed vitreous bevacizumab clearance to consist of 2 different phases; a first fast distribution phase (1 h–1 day) and a second slow elimination phase (1–30 days). Vitreous bevacizumab concentrations peaked at 967.27 and 1021.54 μg/mL, both 1 h after injection, in V and C eyes, respectively. Vitreous concentrations of 16.9 and 21.4 μg/mL were maintained in V and C eyes, respectively, 30 days after IVB injection. Changes in the AH bevacizumab concentration showed a different pattern of drug elimination between the 2 groups during the first 2 days. (Fig. 2) A maximum AH concentration of 121 μg/mL was reached in both V and C eyes, which was achieved after 1 h in V eyes, but after 2 days in C eyes.
FIG. 1.
Vitreous bevacizumab concentrations over time in both vitrectomized and nonvitrectomized eye following intravitreal injection (1.25 mg) in rabbit eyes. The lines represent 2-phase exponential decay equation fit to each of the 2 data sets.
FIG. 2.
Aqueous humor (AH) bevacizumab concentrations over time in both vitrectomized and nonvitrectomized eye following intravitreal injection (1.25 mg) in rabbit eyes.
The percent ratios of vitreous and AH bevacizumab concentrations between V and C eyes were calculated to compare concentration differences at each measured time points. (Fig. 3) For vitreous bevacizumab concentrations, a marked drop was noted at day 1 (70.5%), but the ratio gradually increased to 89.2% at day 2 and 99.2% at day 14, implying little difference in the vitreous bevacizumab concentration between V versus C eyes during day 2 and 14. AH concentrations showed a different pattern, with a peak ratio of 186.9% at 1 h, implying large efflux of bevacizumab into the AH during the first hour only in V eyes, resulting in nearly 2 times the AH concentration in C eyes.
FIG. 3.
The percent ratios of vitreous and AH bevacizumab concentrations calculated as the concentration in vitrectomized eyes as a percentage of the nonvitrectomized eyes.
Two-phase PK analysis
The rate constants of transfer could be calculated for the first and second phase in both V and C groups. (Table 1) When the rate constants of transfer were compared between V and C eyes, the elimination rate of vitreous bevacizumab in the first phase showed a 100% increase in V eyes, whereas in the second phase, there was only 11% increase.
Table 1.
Comparison of Vitreous Humor Pharmacokinetics in Vitrectomized Versus Nonvitrectomized Eyes Using 2-Phase Pharmacokinetic Analysis
| |
Vitrectomized eye |
Nonvitrectomized eye |
|
||
|---|---|---|---|---|---|
| Phase | kvit (1/h) | T1/2 (h) | knon-vit (1/h) | T1/2 (h) | Percentage increase in elimination (kvit/knon-vit-1,%) |
| First phase (1–24 h) | 0.1354±0.0578 | 5.1 (0.2 days) | 0.0678±0.0241 | 10.2 (0.4 days) | 100% |
| Second phase (2–30 days) | 0.0041±0.0002 | 169.1 (7.0 days) | 0.0037±0.0003 | 187.3 (7.8 days) | 11% |
kvit, rate constant of transfer in vitrectomized eye; knon-vit, rate constant of transfer in nonvitrectomized eye; t1/2, half-life.
Overall (One-phase) PK analysis
To determine the overall PK properties of the 2 groups, we excluded the 1-h concentration data and performed one-phase PK analysis. The half-life (T1/2) of bevacizumab in the vitreous humor was 6.99 days in V eyes and 7.06 days in C eyes, with a negligible T1/2 difference of 1.64 h.
Discussion
In this study, the elimination of vitreous bevacizumab consisted of 2 distinct phases and there was no substantial difference in the overall (1–30 days) PK properties of IVB in V and C eyes, with a vitreous concentration half-life difference of mere 1.64 h. In addition, the IVB concentration in V eyes was maintained at about 90% of that in C eyes at 2–14 days after intravitreal injections.
Since the vitreous is a gel consisting of aligned collagen and glycosaminoglycan, it has been thought to act as a molecular barrier to drug diffusion. Earlier studies have consistently shown vitrectomy to shorten the half-life of intravitreally injected materials such as VEGF and triamcinolone acetonide.11,12 Lee et al. reported a 10 times faster elimination of VEGF in vitrectomized eyes than in nonvitrectomized eyes and the half-life was shortened from 2.46 h to 12.5 min.11 However, there are several concerns with regard to their experiment. Although the molecular weight of hVEGF165 is 42 kDa, equivalent to the size of ranibizumab (48 kDa), the vitreous half-life was 2.46 h, which was far short compared to that of ranibizumab (2.88 days)18 or even that of a very small molecule such as vancomycin (2.6 days, molecular weight 1449.3 Da).19 As VEGF is inherently present in the eye and the physiologic processing and clearance are controlled by complicated mechanisms, the half-life of intravitreally injected VEGF in vitrectomized eyes might not fully represent the effect of vitrectomy on the PKs of similar sized drugs. Triamcinolone acetonide showed faster elimination after vitrectomy and the half-life was shortened from 2.89 to 1.57 days.12,20 As triamcinolone acetonide aggregates to form visible crystals of microparticles in the vitreous, the PKs of triamcinolone might be different from that of soluble bevacizumab due to the larger sized triamcinolone particles.
Some studies performed vitrectomy combined with lensectomy to investigate PK properties of intravitreally injected drugs in vitrectomized eyes. The vitreous half-life of amphotericin-B was reduced from 9.1 to 1.4 days after vitrectomy and lensectomy.10 5-fluorouracil clearance increased 2 times in aphakic vitrectomized rabbit eyes compared to phakic vitrectomized eyes.13 A recent study by Kakinoki et al. also reported shortened AH half-life (1.5±0.6 days) of IVB in monkeys that underwent both lensectomy and vitrectomy compared to normal eyes (2.8±0.6 days).15
After removal of the lens, the 3 chambers of the eyeball become a single chamber. Thus, intravitreal injection of bevacizumab becomes equivalent to an intracameral injection, which is known to have different PK properties showing a shorter half-life.21 In addition, as AH half-life is influenced by the concentrations of the intravitreal drug, which is cleared into both the AH and retina, it is inappropriate to use AH data derived from aphakic eyes to estimate the vitreous PKs in phakic eyes. Therefore, the exact role of the vitreous gel and the effect of vitrectomy on the PKs of IVB might not be explained by their methodology and those studies cannot be directly compared with our data. Since we performed vitrectomy alone, saving the lens and maintaining the 3 chambers of the eyeball, our study is more appropriate to investigate the true effect of vitrectomy on IVB PKs.
Christoforidis et al. recently reported the decreased half-life of IVB from 4.22±0.07 to 2.30±0.09 days after vitrectomy only and 2.08±0.07 days after lensectomy only in rabbit eyes using radiolabeled bevacizumab and integrated positron emission tomography/computed tomography (PET/CT) to serially measure radioactivity emission and calculate the half-life of bevacizumab.16 Although they performed repetitive serial measurements of radiolabeled bevacizumab in a small number of rabbits following only vitrectomy, integrated PET/CT imaging is relatively novel and the measurements showed large variance, compared with our immunoassay methods. There may be additional limitations such as the uncertain decoupling of I-124 from the antibody in the vitreous cavity. Thus, we believe it is yet premature to directly compare their PET/CT data with the ELISA results of our study.
The reason for no substantial difference in overall PK of IVB after vitrectomy in our study may be explained as follows. In our study, vitrectomy mainly affected the first fast distribution phase, which lasts for about 1 day, while the second slow elimination phase showed minimal change in PK parameters. As the overall half-life of bevacizumab was determined by the second slow phase, there was no substantial difference in overall PK of IVB despite the early increase in IVB clearance. Second, the remnant vitreous after vitrectomy might have affected the clearance of IVB and attenuated the difference of half-lives between the 2 groups. Although we tried to remove as much vitreous as possible and confirmed vitreous removal using fluid-air exchange, the large lens and the firm adherence of the vitreous to the retina in rabbit eyes made complete vitrectomy impossible, requiring caution for clinical application in human eyes. However, subjective assessment of the removed vitreous and preliminary study data involving equal number of rabbits, which showed no differences in AH bevacizumab concentrations (unpublished data), made us confident that our data were robust. Up to now, there is very limited data on the changes of drug PK properties after vitrectomy alone. Experiments on ocular fluorescein kinetics of swine eyes showed that the drug elimination rate from the vitreous remains unchanged after vitrectomy, which supports our finding.22
One interesting finding is that bevacizumab clearance in the vitreous humor consists of 2 phases. There was an initial fast distribution phase in which, PK parameters differed largely according to vitrectomy and elimination was increased by 100% in V eyes during the first phase, but only 11% in the second phase. The relatively similar PK parameters in V and C eyes during the second elimination phase imply that vitrectomy significantly increased bevacizumab clearance only during the first rapid distribution phase and had a minimal effect on the second phase. Coincidently, the AH bevacizumab concentration in V eyes was markedly increased at 1 h after injection relative to that in C eyes, with a percent ratio of 186.9%, suggesting that efflux from the vitreous into the anterior chamber and subsequent absorption into the trabecular meshwork is the main elimination route of vitreous bevacizumab in the first distribution phase. Since the first fast distribution phase persists for only 1 day, any PK study without the 1-h data would most likely overlook the first phase. However, all prior studies measured the bevacizumab concentration in the vitreous 1 day after injection and showed only monoexponential elimination PKs.7–9,18 The 2 phases of drug elimination was previously reported in experiments of intravitreal vancomycin PKs.19 Thus, it is mandatory for future intravitreal drug PK studies to check the drug concentrations at earlier time points than 1 day after drug injection.
For a molecular probe placed in the vitreous, there are 2 major routes of elimination; through the retina or via the anterior route through the AH.23 Bakri et al. postulated a compartmental model of bevacizumab distribution after intravitreal injection and showed that distribution into the serum accounted for 96% of the total vitreous clearance, whereas distribution between the vitreous and aqueous chamber accounted for only a small fraction (<5%).9 Since the diffusional path through the vitreous to the retina is shorter than that to the anterior chamber, elimination through the posterior pathway has been believed to play the more major role in drug clearance.24 However, taking into account the fact that most of vitreous bevacizumab elimination occurred during the first 24 h in our study, and that bevacizumab could not be detected in the outer retina of monkey eyes 1 day after intravitreal injection,25 most of the vitreous bevacizumab clearance is supposed to occur by diffusion into the anterior chamber during the early period. However, the exact elimination route of the IVB in the second slow phase has not yet been determined. Our second phase results showing different PK profiles compared to that of the first phase indicate a different elimination pathway such as to the posterior segment through the retina.
Numerous studies analyzing the PK parameters of IVB in nonvitrectomized rabbit eyes have been already reported.7–9 Nomoto et al. and Sinapis et al. found T1/2 of bevacizumab in the vitreous humor after intravitreal injection to be 6–6.61 days in rabbit eyes.7,8 T1/2 of IVB in the vitreous humor of C eyes was 7.06 days in our study, which was consistent with previous reports.7,8 Considering that the molecular weight of bevacizumab (149 kDa) is 3 times larger compared with ranibizumab (48 kDa), the half-life of 6 to 7 days for bevacizumab is reasonable in comparison to ranibizumab (2.88–2.9 days).18,26 As the injection site is associated with the early distribution of bevacizumab,27 the different injection procedures and injection sites among researchers might be another reason for the different half-lives of vitreous bevacizumab. The vitreous half-lives of drugs or molecules with different molecular weights are listed in Table 2.
Table 2.
Vitreous Half-Lives of Drugs or Molecules After Intravitreal Injections
| Drugs or molecules | Molecular weight | Vitreous half-life | Species | Surgical intervention | Vitreous half-life in vitrectomized eyes |
|---|---|---|---|---|---|
| Bevacizumab | 149 kDa | 4.32 days (Bakri et al.9) | Rabbit | None | |
| 5.95 days (Nomoto et al.7) | Rabbit | None | |||
| 6.61 days (Sinapis et al.8) | Rabbit | None | |||
| 7.06 days (our data) | Rabbit | Vitrectomy | 6.99 days | ||
| 6.7 days (Zhu et al.30) | Human | None | |||
| 9.82 days (Krohne et al.31) | Human | None | |||
| 7.85 days in 1.5 mg and 11.67 days in 3.0 mg injections (Meyer et al.32) | Human | None | |||
| Rituximab | 145 kDa | 4.7 days (Kim et al.33) | Rabbit | None | |
| Ranibizumab | 48 kDa | 2.63 days (Gaudreault et al.26) | Monkey | None | |
| 2.88 days (Bakriet al.18) | Rabbit | None | |||
| 2.9 days (Gaudreault et al.34) | Rabbit | None | |||
| 7.19 days (Krohne et al.35) | Human | None | |||
| VEGF (hVEGF165) | 42 kDa (dimer) | 2.46 h (Lee et al.11) | Rabbit | Vitrectomy | 12.5 min |
| Vancomycin | 1449.3 Da | 2.6 days (Coco et al.19) | Rabbit | None | |
| Amphotericin B | 924.09 Da | 9.1 days (Doft et al.10) | Rabbit | Vitrectomy and lensectomy | 1.4 days |
| Ceftazidime | 636.6 Da | 20.0 h (Barza et al.36) | Rabbit | None | |
| Ceftriaxone | 576.55 Da | 9.1 h (Barza et al.36) | Rabbit | None | |
| Cefepime | 571.49 Da | 14.3 h (Barza et al.36) | Rabbit | None | |
| Methotrexate | 454.45 Da | 5.9 h (Ozkan et al.37) | Rabbit | None | |
| Triamcinolone acetonide | 434.5 Da | 2.89 days (Chin et al.12) | Rabbit | Vitrectomy | 1.57 days |
| Ceftizoxime | 405.38 Da | 5.7 h (Barza et al.36) | Rabbit | None | |
| Ketorolac tromethamine | 376.41 Da | 3.09 h (Wang et al.21) | Rabbit | None | |
| Voriconazole | 349.311 Da | 2.5 h (Shen et al.38) | Rabbit | None | |
| Ciprofloxacin | 331.3 Da | 2.2 h (Pearson et al.39) | Rabbit | Vitrectomy and lensectomy | 1 h |
| Trifluorothymidine | 296.20 Da | 3.15 h (Pang et al.40) | Rabbit | None |
In terms of clinical application, we reach a conclusion that the clearance and vitreous concentration of intravitreally injected bevacizumab in vitrectomized eyes are comparable to those of nonvitrectomized eyes. Accordingly, we can expect similar efficacy and a similar dosing schedule for bevacizumab in vitrectomized eye as in nonvitrectomized eyes. However, although we showed comparable vitreous concentrations of bevacizumab in vitrectomized eyes, it does not guarantee similar drug delivery to targeted ocular tissues or equivalent therapeutic efficacy. The absence of the vitreous gel may influence the pathway of the drug movement and alter the drug concentration within the retina and choroid thereby reducing drug efficacy. Further research investigating the intraretinal drug concentration and retina histopathology may provide some answers regarding this issue. Most importantly, to determine the clinical relevance, a prospective clinical trial comparing the efficacy of IVB between vitrectomized and nonvitrectomized eyes is necessary. However, the anatomic status of the retina in vitrectomized eyes and individual systemic factors are usually different from those of nonvitrectomized eyes, thus, a direct comparison between the 2 groups may not be feasible.
Another important point of clinical implication is the rapid rise of bevacizumab concentrations in the AH, which could be utilized for pathologic conditions, where anterior chamber bevacizumab delivery is required. In cases of neovascular glaucoma, higher levels of IVB may reach the iris and trabecular meshwork in vitrectomized eyes compared to that in nonvitrectomized eyes immediately after injection, which is more effective for neovascularization regression. There have been previous reports of effective anterior segment neovascularization regression after IVB injection in vitrectomized eyes.28,29
There are some limitations to our study. First, the PK properties of human eyes are known to be different from that in rabbit eyes. Compared to human eyes, the vitreous occupies a smaller proportion within rabbit eyes, which results in higher IVB concentrations. In addition, there is a possibility of different drug clearance mechanisms for different species. Second, although we removed as much vitreous as possible (more than 80% of vitreous volume subjectively), there would have been remnant vitreous behind the lens and on the retinal surface. The remnant vitreous may have affected IVB clearance and attenuated the effect of vitrectomy. Despite these limitations, this study is the first study to directly compare IVB PK parameters, using traditional immunoassay methods, between vitrectomized and nonvitrectomized rabbit eyes without lensectomy, the results of which have important implications for clinical practice.
In conclusion, the clearance of IVB is not substantially increased after vitrectomy in rabbit eyes. Our experimental data suggest that vitreous concentrations of bevacizumab in vitrectomized patients may be comparable to those without a vitrectomy history, thus, modification of the standard dosing regimen may not be necessary.
Acknowledgments
Grant No. 11-2011-016 of the Seoul National University Bundang Hospital Research fund, grant from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Grant No. A111161), and grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) (No. 2012R1A2A2A02012821).
Author Disclosure Statement
No competing financial interests exist for any author.
References
- 1.Aiello L.P. Avery R.L. Arrigg P.G., et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 2.Spilsbury K. Garrett K.L. Shen W.Y., et al. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am. J. Pathol. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenfeld P.J. Brown D.M. Heier J.S., et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen Q.D. Brown D.M. Marcus D.M., et al. Ranibizumab for Diabetic Macular Edema: Results from 2 Phase III Randomized Trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro P.A. Heier J.S. Feiner L., et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102–1112 e1. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Martin D.F. Maguire M.G. Ying G.S., et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nomoto H. Shiraga F. Kuno N., et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest. Ophthalmol. Vis. Sci. 2009;50:4807–4813. doi: 10.1167/iovs.08-3148. [DOI] [PubMed] [Google Scholar]
- 8.Sinapis C.I. Routsias J.G. Sinapis A.I., et al. Pharmacokinetics of intravitreal bevacizumab (Avastin(R)) in rabbits. Clin. Ophthalmol. 2011;5:697–704. doi: 10.2147/OPTH.S19555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakri S.J. Snyder M.R. Reid J.M., et al. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114:855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Doft B.H. Weiskopf J. Nilsson-Ehle I. Wingard L.B., Jr. Amphotericin clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology. 1985;92:1601–1605. doi: 10.1016/s0161-6420(85)33838-1. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.S. Ghosn C. Yu Z., et al. Vitreous VEGF clearance is increased after vitrectomy. Invest. Ophthalmol. Vis. Sci. 2010;51:2135–2138. doi: 10.1167/iovs.09-3582. [DOI] [PubMed] [Google Scholar]
- 12.Chin H.S. Park T.S. Moon Y.S. Oh J.H. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25:556–560. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Jarus G. Blumenkranz M. Hernandez E. Sossi N. Clearance of intravitreal fluorouracil. Normal and aphakic vitrectomized eyes. Ophthalmology. 1985;92:91–96. doi: 10.1016/s0161-6420(85)34063-0. [DOI] [PubMed] [Google Scholar]
- 14.Yanyali A. Aytug B. Horozoglu F. Nohutcu A.F. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am. J. Ophthalmol. 2007;144:124–126. doi: 10.1016/j.ajo.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 15.Kakinoki M. Sawada O. Sawada T., et al. Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest. Ophthalmol. Vis. Sci. 2012;53:5877–5880. doi: 10.1167/iovs.12-10164. [DOI] [PubMed] [Google Scholar]
- 16.Christoforidis J.B. Williams M.M. Wang J., et al. Anatomic and pharmacokinetic properties of intravitreal bevacizumab and ranibizumab after vitrectomy and lensectomy. Retina. 2013;33:946–952. doi: 10.1097/IAE.0b013e3182753b12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakri S.J. Snyder M.R. Pulido J.S., et al. Six-month stability of bevacizumab (Avastin) binding to vascular endothelial growth factor after withdrawal into a syringe and refrigeration or freezing. Retina. 2006;26:519–522. doi: 10.1097/01.iae.0000225354.92444.7a. [DOI] [PubMed] [Google Scholar]
- 18.Bakri S.J. Snyder M.R. Reid J.M., et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Coco R.M. Lopez M.I. Pastor J.C. Nozal M.J. Pharmacokinetics of intravitreal vancomycin in normal and infected rabbit eyes. J. Ocul. Pharmacol. Ther. 1998;14:555–563. doi: 10.1089/jop.1998.14.555. [DOI] [PubMed] [Google Scholar]
- 20.Schindler R.H. Chandler D. Thresher R. Machemer R. The clearance of intravitreal triamcinolone acetonide. Am. J. Ophthalmol. 1982;93:415–417. doi: 10.1016/0002-9394(82)90130-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang M. Liu W. Lu Q., et al. Pharmacokinetic comparison of ketorolac after intracameral, intravitreal, and suprachoroidal administration in rabbits. Neurosurgery. 2012 doi: 10.1097/IAE.0b013e3182576d1d. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Knudsen L.L. Dissing T. Hansen M.N. Nielsen-Kudsk F. Ocular fluorescein kinetics before and after vitrectomy on swine. Graefes. Arch. Clin. Exp. Ophthalmol. 2001;239:832–839. doi: 10.1007/s004170100355. [DOI] [PubMed] [Google Scholar]
- 23.Maurice D.M. Injection of drugs into the vitreous body. In: Leopold I.H., editor; Burns R.P., editor. Symposium on Ocular Therapy. New York: John Wiley & Sons, Inc.; 1976. pp. 59–72. [Google Scholar]
- 24.Laude A. Tan L.E. Wilson C.G., et al. Intravitreal therapy for neovascular age-related macular degeneration and inter-individual variations in vitreous pharmacokinetics. Prog. Retin. Eye. Res. 2010;29:466–475. doi: 10.1016/j.preteyeres.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Heiduschka P. Fietz H. Hofmeister S., et al. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest. Ophthalmol. Vis. Sci. 2007;48:2814–2823. doi: 10.1167/iovs.06-1171. [DOI] [PubMed] [Google Scholar]
- 26.Gaudreault J. Fei D. Rusit J., et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest. Ophthalmol. Vis. Sci. 2005;46:726–733. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 27.Miura Y. Uematsu M. Teshima M., et al. Injection site and pharmacokinetics after intravitreal injection of immunoglobulin G. J. Ocul. Pharmacol. Ther. 2011;27:35–41. doi: 10.1089/jop.2010.0112. [DOI] [PubMed] [Google Scholar]
- 28.Batman C. Ozdamar Y. The effect of bevacizumab for anterior segment neovascularization after silicone oil removal in eyes with previous vitreoretinal surgery. Eye (Lond) 2010;24:1243–1246. doi: 10.1038/eye.2009.304. [DOI] [PubMed] [Google Scholar]
- 29.Miki A. Oshima Y. Otori Y., et al. One-year results of intravitreal bevacizumab as an adjunct to trabeculectomy for neovascular glaucoma in eyes with previous vitrectomy. Eye (Lond) 2011;25:658–659. doi: 10.1038/eye.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu Q. Ziemssen F. Henke-Fahle S. Tatar O. Szurman P. Aisenbrey S. Schneiderhan-Marra N. Xu X. Grisanti S. Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750–1755, 1755 e1751. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 31.Krohne T.U. Eter N. Holz F.G. Meyer C.H. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am. J. Ophthalmol. 2008;146:508–512. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 32.Meyer C.H. Krohne T.U. Holz F.G. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina. 2011;31:1877–1884. doi: 10.1097/IAE.0b013e318217373c. [DOI] [PubMed] [Google Scholar]
- 33.Kim H. Csaky K.G. Chan C.C. Bungay P.M. Lutz R.J. Dedrick R.L. Yuan P. Rosenberg J. Grillo-Lopez A.J. Wilson W.H. Robinson M.R. The pharmacokinetics of rituximab following an intravitreal injection. Exp. Eye Res. 2006;82:760–766. doi: 10.1016/j.exer.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Gaudreault J. Fei D. Beyer J.C. Ryan A. Rangell L. Shiu V. Damico L.A. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina. 2007;27:1260–1266. doi: 10.1097/IAE.0b013e318134eecd. [DOI] [PubMed] [Google Scholar]
- 35.Krohne T.U. Liu Z. Holz F.G. Meyer C.H. Intraocular pharmacokinetics of ranibizumab following a single intravitreal injection in humans. Am. J. Ophthalmol. 2012;154:682–686. doi: 10.1016/j.ajo.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 36.Barza M. Lynch E. Baum J.L. Pharmacokinetics of newer cephalosporins after subconjunctival and intravitreal injection in rabbits. Arch. Ophthalmol. 1993;111:121–125. doi: 10.1001/archopht.1993.01090010125038. [DOI] [PubMed] [Google Scholar]
- 37.Ozkan E.B. Ozcan A.A. Alparslan N. Intravitreal injection of methotrexate in an experimental rabbit model: determination of pharmacokinetics. Indian J. Ophthalmol. 2011;59:197–200. doi: 10.4103/0301-4738.81026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Y.C. Wang M.Y. Wang C.Y. Tsai T.C. Tsai H.Y. Lee Y.F. Wei L.C. Clearance of intravitreal voriconazole. Invest. Ophthalmol. Vis. Sci. 2007;48:2238–2241. doi: 10.1167/iovs.06-1362. [DOI] [PubMed] [Google Scholar]
- 39.Pearson P.A. Hainsworth D.P. Ashton P. Clearance and distribution of ciprofloxacin after intravitreal injection. Retina. 1993;13:326–330. doi: 10.1097/00006982-199313040-00010. [DOI] [PubMed] [Google Scholar]
- 40.Pang M.P. Branchflower R.V. Chang A.T. Peyman G.A. Blatt H. Minatoya H.K. Half-life and vitreous clearance of trifluorothymidine after intravitreal injection in the rabbit eye. Can. J. Ophthalmol. 1992;27:6–9. [PubMed] [Google Scholar]