Abstract
Background
Currently, patients with type 1 diabetes decide on the amount of insulin to administer based on several factors, including current plasma glucose value, expected meal input, and physical activity (PA). One future therapeutic modality for patients with type 1 diabetes is the artificial endocrine pancreas (AEP). Incorporation of PA could enhance the efficacy of AEP significantly. We compared the main technologies used for PA quantitation.
Subjects and Methods
Data were collected during inpatient studies involving healthy control subjects and type 1 diabetes. We report PA quantified from accelerometers (acceleration units [AU]) and heart rate (HR) monitors during a standardized activity protocol performed after a dinner meal at 7 p.m. from nine control subjects (four were males, 37.4±12.7 years old, body mass index of 24.8±3.8 kg/m2, and fasting plasma glucose of 4.71±0.63 mmol/L) and eight with type 1 diabetes (six were males, 45.2±13.4 years old, body mass index of 25.1±2.9 kg/m2, and fasting plasma glucose of 8.44±2.31 mmol/L).
Results
The patient-to-patient variability was considerably less when examining AU compared with HR monitors. Furthermore, the exercise bouts and rest periods were more evident from the data streams when AUs were used to quantify activity. Unlike the AU, the HR measurements provided little insight for active and rest stages, and HR data required patient-specific standardizations to discern any meaningful pattern in the data.
Conclusions
Our results indicated that AU provides a reliable signal in response to PA, including low-intensity activity. Correlation of this signal with continuous glucose monitoring data would be the next step before exploring inclusion as input for AEP control.
Introduction
Diabetes mellitus continues to increase in prevalence worldwide to pandemic proportions.1 Glucose control in diabetes mellitus continues to be suboptimal.2 Whereas randomized controlled trials achieving tight control in type 2 diabetes have raised safety concerns regarding pharmacotherapeutic approaches, current therapeutic approaches for type 1 diabetes (T1D) continue to be associated with wide glucose variability, frequent hypo- and hyperglycemia, and suboptimal quality of life.3,4 To overcome these limitations, for patients with T1D, closed-loop control systems are being developed. Various control systems are currently being evaluated to achieve closed loop control.5–7 Studies to date indicate that such control systems improve glucose control for short periods in carefully supervised clinical research units. Several factors will need to be addressed to improve glucose control further and extend such control to ambulatory care settings. Important factors influencing glucose variability in this context include meal factors, physical activity (PA), and patient behavior.
Low-intensity PA constitutes about 20% of energy expenditure daily.8 We have recently shown that such low-intensity PA substantially decreases postprandial glucose excursions in healthy control subjects and people with T1D.9 Also, it has been shown that undertaking mild- to moderate-intensity PA decreases cardiovascular risks.10 Yet, detection and quantification of PA in real time remain challenging, and to date this remains an area of limited evaluation in the development of the artificial endocrine pancreas (AEP). Using the microelectromechanical systems technology, it is now possible to use body-worn microsensors such as accelerometers and/or gyroscopes to quantitate motion sensing and associated energy expenditure.8,11
Heart rate (HR) monitors have traditionally been used to quantify exercise periods involving higher energy expenditure than activities of daily living.12,13 Using HR alone as a measure for PA may have several confounders. Increased central sympathetic output may result in elevated HR even when a person is resting, and patients with type 1 diabetes may have cardiac autonomic neuropathy resulting in reduced HR variability.14,15 In in-silico studies for AEP, instantaneous HR monitors as a measure of physical activity have had limited success to quantify the effect of PA on glucose status changes.16,17
To overcome this shortcoming, alternative, and readily quantifiable, measures of PA are needed so that they can be incorporated into an AEP. The data presented here use instantaneous HR and accelerometer data captured concurrently during a supervised inpatient protocol in controlled laboratory conditions. Our primary objective here is to understand the concordance (or agreement) between accelerometers and instantaneous HR monitors during standardized laboratory conditions in healthy controls and patients with T1D.
Methods and Overall Design
In this article we report a secondary data analysis using data collected during an inpatient study to detect patterns in postprandial glucose tolerance in controls and subjects with T1D.9,18 The Mayo Clinic Institutional Review Board approved the research study. Informed consent was obtained from all participants prior to their participation in the study.
Subjects
Table 1 shows demographic characteristics of subjects. Each subject underwent two screening visits as described previously to confirm eligibility.9,18 We used a validated questionnaire to exclude autonomic nervous system dysfunction in both groups of subjects.19
Table 1.
Demographic and Baseline Characteristics for the Nine Healthy Controls and Eight Type 1 Diabetes Patients
| Controls | Type 1 diabetes | |
|---|---|---|
| Number (males) | 9 (4) | 8 (6) |
| Age (years) | 37.4±12.7 | 45.2±13.4 |
| Body mass index (kg/m2) | 24.8±3.8 | 25.1±2.9 |
| Body fat (%)a | 29.3±7.27 | 26.25±5.01 |
| Fasting plasma glucose (mmol/L) | 4.71±0.63 | 8.44±2.31 |
| HbA1c (%) | 5.01±0.22 | 7.25±0.51 |
| Heart rate (beats/min) | ||
| Resting | 69.7±10.3 | 79.8±14.8 |
| Peak | 93.4±9.1 | 96.7±9.7 |
| Creatinine (mg/dL) | 0.86±0.14 | 0.83±0.13 |
Body fat composition was computed using dual X-ray absorptiometry (DPX-IQ scanner with SmartScan version 4.6 software; Hologic, Waltham, MA).
HbA1c, hemoglobin A1c.
PA measurement
Data presented here were collected during inpatient studies at the Mayo Clinic Clinical Research Unit. Throughout the duration of the study, PA data were collected using PAMS tri-axial accelerometer system8 and modular signal recorder (MSR) accelerometers (MSR Electronics GmbH, Seuzach, Switzerland), placed on an elastic belt worn at the waist. The accelerometers weigh about 40 g and were placed lateral to the spine at the center of the waist.
HR measurement
The HR data were captured on IntelliVue patient monitors (Philips Healthcare, Andover, MA) using finger-clip pulse oximeters. The captured HR data were collected using the TrendFace (Ixellence GmbH, Wildau, Germany) system. The TrendFace system is a professional, efficient tool for PC-based data collection and the visualization of vital signs and primary signals from IntelliVue patient monitors. TrendFace collects, visualizes, saves, and exports patient data.
Experimental design
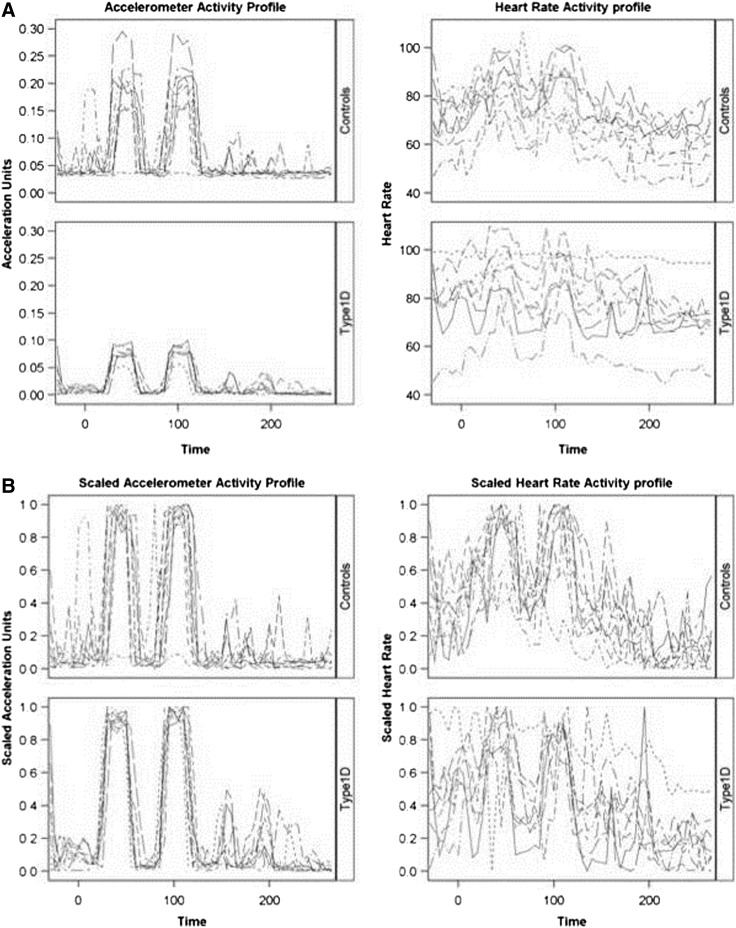
A carefully planned PA routine was planned for the participants, and adherence was captured using MSR accelerometers. PA data were analyzed using the same validated and published method described by Levine et al.8 Although the participants walked about 5.6–6.7 km (3.5–4.2 miles) on a typical study day, the data presented here were collected during walking activity performed after a protocol-standardized dinner meal served at 7 p.m. for the study day that had breakfast containing 1-13C-labeled isotope.9 The post-dinner PA consisted of participants doing two intervals, each 26.5 min in duration (Fig. 1). The walking velocity of 1.9 km/h (kmph) (1.2 m/h) was chosen, consistent with median free-living walking velocity as reported by Levine et al.20 All subjects were asked to retire to bed at 10 p.m. every night, and therefore the dataset presented here represents active as well as resting periods.
FIG. 1.
(A) Unscaled and (B) scaled acceleration and heart rate profiles for patients with type 1 diabetes (Type1D) (n=8) compared with controls (n=9). The scaled values are based on the percentage of patient-specific range observed over time. The sampling frequency is 5 min.
As a part of the inpatient visit, on each study day the participants performed an abbreviated exercise protocol as follows: 3 min standing still; 3 min each of walking at 1.6, 3.2, and 4.8 kmph; and concluding the 15-min protocol with 3 min of standing. The mean acceleration units (AU) and HR during the middle minute for each of the phases (e.g., at 2, 5, …14 min) were used to test for tracking of the PA metrics with known activity.
Data analysis
The MSR accelerometer captures the PA data along three orthogonal axes x, y, and z, respectively, at 20 Hz with the dynamic range to ±2 g. Instantaneous accelerations along each axis is computed by taking first-order derivative; this removes the influence of the Earth's gravity on the accelerometer and leaves us with actual displacement at each time point. The resultant acceleration represented as AU is then calculated by computing the vector magnitude and doing summation over the desired epoch length (1 min in our case).11
The HR data are measured in beats/min. Thus, although each assessment was captured simultaneously, the relative scales and measurement metrics were not directly comparable. To compare the two datasets, a normalization procedure was applied by rescaling the observations to represent the percentage of the observed range for each participant. For example, if a participant had the lowest and highest HR measurements of 60 and 120 beats/min, a value of 72 beats/min would be rescaled to 0.2 (= [72−60]/[120−60]). As the “baseline” HR data change throughout the day, the minimum HR was selected during the period of evaluation. It was hypothesized that concordance of the accelerometer output and HR measurements would vary during different segments of time (e.g., exercise, sleep), so segment-by-segment analyses were considered.
For the 15-min exercise protocol, we also computed the theoretical maximum HR (i.e., 220 – age) to derive scaled HRs representing the fraction of the theoretical working range for the individual participant (resting to maximum HR). Tests for differences among the five phases of exercise intensity ranging from standing to up to 4.8 kmph were conducted using a random effects model (i.e., a “repeated-measures analysis of variance”). Post-hoc comparisons were conducted. P values reported for these comparisons have not been adjusted for multiple comparisons.
To assess the overall degree of concordance, the intraclass correlation (ICC) was computed for each participant. This calculation used an atypical formulation of the one-way random effects model. In particular, for each participant, the continuously measured accelerations and HR were grouped into 5-min epochs. For each epoch, there were paired measurements (mean HR in the interval, mean accelerations in the interval), and so the ICC was calculated as the percentage of variation within the epoch relative to overall variation for a given participant. To provide an overall estimate of the concordance of the two metrics, the bootstrap was used, and differences in ICC between healthy controls and T1D patients were assessed using the Wilcoxon rank sum test. These quantitative analyses were supplemented with graphical overlays of the scaled values and standard descriptive statistics. Statistical analyses were conducted using SAS system software. Bootstrap estimates and bias-corrected confidence intervals were generated in R using the Bootstrap package.
Results
In total, 24 subjects (12 healthy controls and 12 with T1D) were studied as a part of the primary protocol; however, not all participants yielded sufficient data for this secondary analysis. One control participant was missing data from both sources (accelerometer and HR monitors) for the entire study duration (i.e., 60 epochs of time). One participant with T1D was missing a single epoch of data for the accelerometer starting at min 205, and there were 12 participants (four controls and eight T1D patients) who were missing only HR data ranging from a single epoch to as many as 12 epochs over the study period. Because the overarching goal of this study was to assess the concordance of the two measuring devices, only participants with three or fewer epochs of time with missing data (i.e., 95% data available or better) were included in the analysis. The final study group consisted of nine healthy participants and eight participants with T1D. The demographics of these participants are presented in Table 1.
Figure 1 illustrates the unscaled (Fig. 1A) and scaled (Fig. 1B) activity measurements based on AU and HR for control subjects and participants with T1D. It is apparent that the patterns of activity differed by measurement technique. The patient-to-patient variability was considerably less when examining the activity by the accelerometer (i.e., longitudinal profiles on the AU scales overlapped by participants more heavily than on the HR scale). Furthermore, when AU values were used to quantify activity, the exercise intervals and rest periods are far more evident from the data streams. Unlike the AU values, the HR measurements provided little insight into the underlying protocol for active and rest stages. Although scaling did improve the consistency of HR measurements across patients, the degree to which the AU values aligned with the HR data was the subject of further investigation.
Concordance, as measured by the ICC ranged from very poor concordance (negative ICC estimates) to very high (estimates approaching 1.0). Table 2 presents the ICC for the scaled measurements for all participants, along with the 95% bootstrap confidence intervals for the median and mean ICCs for healthy controls and T1D patients. There were no differences in ICC observed between patient classifications (P=0.89).
Table 2.
Intraclass Correlation Coefficients for Healthy Controls and Type 1 Diabetes Patients Using the Paired Assessments Within Patient and Epoch of Time
| Group, subject | Estimate | 95% CI |
|---|---|---|
| Healthy controls (n=9) | ||
| 101 | 0.81 | (0.57, 0.92) |
| 102 | 0.60 | (0.22, 0.82) |
| 103 | 0.55 | (0.16, 0.80) |
| 105 | 0.47 | (0.04, 0.75) |
| 106 | 0.49 | (0.06, 0.76) |
| 109 | 0.70 | (0.38, 0.87) |
| 110 | 0.79 | (0.54, 0.91) |
| 111 | 0.60 | (0.22, 0.82) |
| 112 | −0.16 | (−0.56, 0.3) |
| Mean | 0.54 | (0.28, 0.67) |
| Median | 0.60 | (0.47, 0.60) |
| Type 1 diabetes (n=8) | ||
| 203 | 0.80 | (0.56, 0.92) |
| 204 | −0.39 | (−0.71, 0.06) |
| 205 | 0.93 | (0.84, 0.97) |
| 206 | 0.42 | (−0.01, 0.73) |
| 208 | 0.51 | (0.09, 0.78) |
| 209 | 0.78 | (0.51, 0.91) |
| 210 | 0.38 | (−0.06, 0.7) |
| 211 | 0.87 | (0.7, 0.95) |
| Mean | 0.54 | (0.12, 0.73) |
| Median | 0.64 | (0.38, 0.83) |
The Wilcoxon rank sum test for differences in concordance between the controls and type 1 diabetes patients was not significant (P=0.89). The 95% confidence intervals (CI) for the mean and median summary statistics are bootstrap estimates. Subjects 104, 107, 108, 201, 202, 207, and 212 did not have sufficient data for estimation (see Results).
In the T1D subjects, the 15-min exercise protocol was conducted to yield more insight in the agreement of AU and HR data over a more broad range of activity levels (stationary to 4.8 kmph; Fig. 2). Both AU and scaled HR demonstrated changes with relationship to changes in intensity of exercise (P<0.001 for both measures), but using HR, neither 1.6 or 3.2 kmph differed statistically from the initial standing measurements (P=0.22 and 0.23, respectively). AUs were statistically different for all five periods (P<0.001 for all 10 comparisons). These results suggest that AU data were more sensitive to low-intensity activity than HR. In fact, HR only became statistically different from the initial standing period once the 4.8 kmph speed had been reached (P<0.001).
FIG. 2.
Longitudinal profiles of (A) acceleration units (AU) and (B) heart rate (HR) (scaled for the working range of theoretical maximum [220 – age] and resting HR) by subject. With AU, each walking speed was statistically different from one another in addition to being different from the initial standing period (P<0.001 for all pairwise comparisons). In contrast, when HR was used, only 4.8 km/h (kmph) was statistically different (P<0.001) from the initial standing HR, and 3.2 kmph and 4.8 kmph were also statistically different (P=0.004).
Discussion
PA is a known contributor to insulin sensitivity and blood glucose levels and lowers glucose concentrations by increasing energy expenditure.9,18 Several modalities and devices could be used to measure energy expenditure. In the current study, we used two different devices: PA sensors and HR capture. We found that the PA sensors we used were more accurate than HR capture at detecting and quantitating PA during low-intensity PA that mimics activities of daily living. Our results demonstrate that accelerometers rather than HR sensors are better markers of both periods of activity and activity intensity.
Accelerometers have been compared with HR monitors in studies involving normal people. Although HR as a surrogate for PAs has been used extensively, it appears to be a good metric only at moderate to vigorous activities. This makes individual calibration necessary to derive energy expenditure by performing exercise at submaximal levels,21,22 which is not only impractical but also expensive for everyday use. Furthermore, HR is far more sensitive to subtle ambient changes such as anxiety, stress, emotions, and change surrounding (for example, temperature, altitude, weather, etc.).23,24 This further validates our findings that accelerometers rather than HR sensors are better markers of both periods and intensity of PA of daily living. PA associated with activities of daily living likely constitutes about 20% of daily energy expenditure. We have recently shown that in both control subjects and those with T1D on insulin pumps, low-intensity postprandial PA significantly decreases postprandial glucose excursions measured using real-time continuous glucose monitors.9
Studies to date in people with diabetes mellitus have tried using HR data as a surrogate measure for PA with limited success.16,17 In in silico studies the initial simulations were run to fit mathematical models that incorporated varying duration (15 min and 30 min) of exercise of varying intensity (e.g., 50% of maximum O2 uptake in the first study and 50% and 100% increase in HR above baseline levels). Although these simulations give a good insight in developing such models, very limited data are available from human studies to date comparing PA measured by accelerometers and HR in people with T1D. One such study by van Bon et al.25 showed limited agreement between PA measured by accelerometers and HR monitors.
In a recent study Wen et al.10 have shown that even a small amount of daily PA resulted in a 14% reduced risk in mortality, and this further reduced by 4% for every additional 15 min of PA. This was further validated by Nigam and Juneau.26 Increased PA without a doubt has beneficial health effects. In the area of PA quantification, efforts so far have mainly focused on development of technologies to measure PA in daily living and to understand the mechanism (i.e., to understand the effect) of PA on weight gain and glycemic control.8,9,11,20
Efforts to use this knowledge for therapeutic purposes are limited.27 For therapeutic use, particularly in the case of T1D, it is important to have a device that is accurate, reliable, convenient, and least disruptive to the existing set of sensor–pump that they use. Accelerometers are low-cost, small, and accurate PA sensors that can be easily incorporated into a continuous glucose monitor and/or a continuous subcutaneous insulin infusion pump. This will greatly reduce the burden of carrying or wearing multiple sensors.
Concurrently we are investigating the chronologic relationship between the energy expenditure measured with our approaches and the change in glucose measured by continuous glucose monitoring. Such work may lead to the development of computational models that incorporate the accelerometer data to account for the change in glucose level after PA. The PA module upon validation and testing could be added to the clinically useful modular AEP system as proposed by Patek et al.7 to enable clinical testing of closed control with and without PA input.
We used pulse oximeters to capture HR in our study. We acknowledge that this is an impractical way of capturing HR data during free living. The alternative way to capture HR data is by recording the electrocardiogram, which although very accurate is not practical for everyday use. Also, it is important to note that although the AU value was better at tracking epochs and intensity of PA compared with HR, it is true for locomotion activity only (such as walking, running, etc.) and would not be sensitive to non-locomotion activities such as upper body movements. A simple solution to this scenario would be to wear an additional accelerometer to capture upper body movement and an embedded accelerometer in an abdominally worn AEP unit to capture complete body movement.
In conclusion, we have compared accelerometers with HR monitors as devices to capture energy expenditure in healthy controls and for the first time in T1D. Accelerometers are significantly better at low-intensity PA, which constitutes 20% of daily energy expenditure, and input from accelerometers could be used in closed-loop control after appropriate further development as discussed earlier in this article.
Acknowledgments
This study was supported by grant DK 085516 (to A.B. and Y.C.K.) from the National Institute of Diabetes and Digestive and Kidney Diseases and by National Institutes of Health/National Center for Research Resources Clinical and Translational Science Award grant UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. We thank the staff at our Clinical and Translational Science Award and the research volunteers and their families.
Author Disclosure Statement
No competing financial interests exist. C.M., D.O.K., A.B., Y.C.K., L.H., and R.C. actively contributed with writing of this manuscript. S.K.M. and H.L. actively participated in recruitment and conducting the research study. C.M., D.O.K., R.C., and H.L. were primarily responsible for data collection, integrity, and analysis. Y.C.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Genuth S. Alberti KG. Bennett P. Buse J. Defronzo R. Kahn R. Kitzmiller J. Knowler WC. Lebovitz H. Lernmark A. Nathan D. Palmer J. Rizza R. Saudek C. Shaw J. Steffes M. Stern M. Tuomilehto J. Zimmet P. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 2.Ismail-Beigi F. Clinical practice. Glycemic management of type 2 diabetes mellitus. N Engl J Med. 2012;366:1319–1327. doi: 10.1056/NEJMcp1013127. [DOI] [PubMed] [Google Scholar]
- 3.Kovatchev BP. Otto E. Cox D. Gonder-Frederick L. Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 4.Penckofer S. Quinn L. Byrn M. Ferrans C. Miller M. Strange P. Does glycemic variability impact mood and quality of life? Diabetes Technol Ther. 2012;14:303–3010. doi: 10.1089/dia.2011.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobelli C. Renard E. Kovatchev B. Artificial pancreas: past, present, future. Diabetes. 2011;60:2672–2682. doi: 10.2337/db11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumareswaran K. Evans ML. Hovorka R. Closed-loop insulin delivery: towards improved diabetes care. Discov Med. 2012;13:159–170. [PubMed] [Google Scholar]
- 7.Patek S. Magni L. Dassau E. Karvetski C. Toffanin C. De Nicolao G. Del Favero S. Breton M. Man CD. Renard E. Zisser H. Doyle FJ., 3rd Cobelli C. Kovatchev BP. International Artificial Pancreas (iAP) Study Group: Modular closed-loop control of diabetes. IEEE Trans Biomed Eng. 2012;59:2986–2999. doi: 10.1109/TBME.2012.2192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine JA. Lanningham-Foster LM. McCrady SK. Krizan AC. Olson LR. Kane PH. Jensen MD. Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–586. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]
- 9.Manohar C. Levine JA. Nandy DK. Saad A. Dalla Man C. McCrady-Spitzer SK. Basu R. Cobelli C. Carter RE. Basu A. Kudva YC. The effect of walking on postprandial glycemic excursion in patients with type 1 diabetes mellitus and healthy people. Diabetes Care. 2012;35:2493–2499. doi: 10.2337/dc11-2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen CP. Wai JP. Tsai MK. Yang YC. Cheng TY. Lee MC. Chan HT. Tsao CK. Tsai SP. Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 11.Manohar CU. Koepp GA. McCrady-Spitzer SK. Levine JA. A stand-alone accelerometer system for free-living individuals to measure and promote physical activity. ICAN Infant Child Adolesc Nutr. 2012;4:222–229. [Google Scholar]
- 12.Fletcher GF. Balady G. Frowlicher VF. Hartley LH. Haskell WL. Pollock ML. Exercise standards. A statement for healthcare professionals from the American Heart Association. Writing Group. Circulation. 1995;91:580–615. doi: 10.1161/01.cir.91.2.580. [DOI] [PubMed] [Google Scholar]
- 13.Roy A. Parker RS. Dynamic modeling of exercise effects on plasma glucose and insulin levels. J Diabetes Sci Technol. 2007;1:338–347. doi: 10.1177/193229680700100305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maser RE. Lenhard MJ. Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab. 2005;90:5896–5903. doi: 10.1210/jc.2005-0754. [DOI] [PubMed] [Google Scholar]
- 15.Magder SA. The ups and downs of heart rate. Crit Care Med. 2012;40:239–245. doi: 10.1097/CCM.0b013e318232e50c. [DOI] [PubMed] [Google Scholar]
- 16.Breton MD. Physical activity-the major unaccounted impediment to closed loop control. J Diabetes Sci Technol. 2008;2:169–174. doi: 10.1177/193229680800200127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Man CD. Breton MD. Cobelli C. Physical activity into the meal glucose-insulin model of type 1 diabetes: in silico studies. J Diabetes Sci Technol. 2009;3:56–67. doi: 10.1177/193229680900300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saad A. Man CD. Nandy DK. Levine JA. Bharucha AE. Rizza RA. Basu R. Carter RE. Cobelli C. Kudva YC. Basu A. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes. 2012;61:2691–2700. doi: 10.2337/db11-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suarez GA. Opfer-Gehrking TL. Offord KP. Atkinson EJ. O'Brien PC. Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52:523–528. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- 20.Levine JA. McCrady SK. Lanningham-Foster LM. Kane PH. Foster RC. Manohar CU. The role of free-living daily walking in human weight gain and obesity. Diabetes. 2008;57:548–545. doi: 10.2337/db07-0815. [DOI] [PubMed] [Google Scholar]
- 21.Melanson EL. Freedson PS. Physical activity assessment: a review of methods. Crit Rev Food Sci Nutr. 1996;36:385–396. doi: 10.1080/10408399609527732. [DOI] [PubMed] [Google Scholar]
- 22.Welk GL, editor. Physical Activity Assessments for Health-Research. Champaign, IL: Human Kinetics; 2002. [Google Scholar]
- 23.Montoye H. Kemper H. Saris W. Washburn R. Measuring Physical Activity and Energy Expenditure. Champaign, IL: Human Kinetics; 1996. [Google Scholar]
- 24.Crouter SE. Albright C. Bassett DR. Accuracy of Polar S410 heart rate monitor to estimate energy cost of exercise. Med Sci Sports Exerc. 2004;36:1433–1439. doi: 10.1249/01.mss.0000135794.01507.48. [DOI] [PubMed] [Google Scholar]
- 25.van Bon AC. Verbitskiy E. von Basum G. Hoekstra JB. DeVries JH. Exercise in closed-loop control: a major hurdle. J Diabetes Sci Technol. 2011;5:1337–1341. doi: 10.1177/193229681100500604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nigam A. Juneau M. Survival benefit associated with low-level physical activity. Lancet. 2011;378:1202–1203. doi: 10.1016/S0140-6736(11)61029-5. [DOI] [PubMed] [Google Scholar]
- 27.Koepp GA. Manohar CU. McCrady-Spitzer SK. Ben-Ner A. Flint-Paulson D. Runge CF. Levine JA. Treadmill desks: a one-year prospective trial. Obesity (Silver Spring) 2012 Nov 6; doi: 10.1002/oby.20121. [DOI] [PubMed] [Google Scholar]