Abstract
Objective
To examine morphologic changes in the somatosensory cortex (SSC) of patients with migraine.
Methods
Cortical thickness of the SSC of patients with migraine was measured in vivo and compared with age- and sex-matched healthy subjects. The cohort was composed of 24 patients with migraine, subdivided into 12 patients who had migraine with aura, 12 patients who had migraine without aura, and 12 controls. Group and individual analyses were performed in the SSC and shown as average maps of significant changes in cortical thickness.
Results
Migraineurs had on average thicker SSCs than the control group. The most significant thickness changes were noticed in the caudal SSC, where the trigeminal area, including head and face, is somatotopically represented.
Conclusions
Our findings indicate the presence of interictal structural changes in the somatosensory cortex (SSC) of migraineurs. The SSC plays a crucial role in the noxious and nonnoxious somatosensory processing. Thickening in the SSC is in line with diffusional abnormalities observed in the subcortical trigeminal somatosensory pathway of the same migraine cohort in a previous study. Repetitive migraine attacks may lead to, or be the result of, neuroplastic changes in cortical and subcortical structures of the trigeminal somatosensory system.
Migraine is a chronic painful disease in which frequent headache attacks affect a great part of the patient’s life, from childhood to late adulthood. The consequences of such persistent suffering on the cortex of migraineurs are not known. Using diffusion tensor imaging (DTI), we have recently shown interictal alterations in trigeminal somatosensory pathway in patients who have migraine with aura (MWA) and migraine without aura (MWoA), confirming the involvement of the somatosensory system in the migraine pathophysiology.1 Based on that evidence, and taking advantage of the large somatotopic representation of the head in the somatosensory cortex (SSC) that receives trigeminal noxious and innocuous sensory inputs, we examined for the presence of cortical thickness changes in the same group of patients.
A number of data suggest that neurogenic inflammation underlies migraine pathophysiology.2 During a migraine attack, initial activation of meningeal nociceptive fibers conveys noxious inputs to the spinal trigeminal nucleus.3,4 Those inputs are mainly directed via the trigeminothalamic tract to the ventroposteromedial nucleus in the thalamus. Finally, noxious inputs are transmitted to the lower portion of the SSC5,6 through the thalamocortical tract.
The thickness of the cortex layer varies across the human cortical mantle, and the SSC is one of the thinnest regions in the brain, with some portions measuring on average 2 mm. Variations of the thickness in specific cortical areas may reflect pathologic or physiologic changes of the intrinsic structure of the cortical laminae. Recently, several studies have shown thickness decreases in neurologic diseases such as multiple sclerosis7 and Alzheimer disease.8 We recently reported changes in the visual cortex of migraineurs,9 with thickening of visual motion related structures that were concurrent with subcortical diffusional changes, and spatially coincidental with functional abnormalities identified previously in such type of patients.10,11 Hence, thickness changes may be either the result or the cause of pathophysiologic mechanisms that take place in the CNS. Even physiologic tasks such as motor training12 and learning13 are known to induce gray matter changes. Based on our previous findings of diffusional changes in the subcortical structures of the sensory system of migraine patients, including second- (MWA) and third-order neurons (MWA and MWoA) of the trigeminal sensory system, we collected data of the same migraine population to investigate whether these white matter changes are accompanied by cortical thickness changes in the SSC.
METHODS
Patients and controls
All the patients were recruited from headache clinics in the Boston area and by advertisements in the hospital. Each migraine volunteer was first screened by phone, and a second, more detailed clinical interview was conducted before MRI acquisition. Pregnant or breast-feeding women, subjects older than 55 years, and subjects having claustrophobia or any MRI incompatibility were not included in our cohort of subjects. In addition, patients with gross anatomic asymmetry or clear pathology on MRI anatomic scans were not included in the study. The Institutional Review Boards (IRBs) of the Massachusetts General Hospital approved this study (IRB no. 2002P-000652).
The same three equal groups of volunteers from our previous DTI study were selected following the International Headache Society classification14: MWA, MWoA, and healthy controls (HCs). Each group was composed of 12 sexand age-matched subjects. Patients’ ages ranged from 21 to 52 years (MWA: 33.8 ± 9.2 [mean ± SD], MWoA: 36.0 ± 7.7, HC: 31.0 ± 8.0; p ± 0.35), and patients had experienced episodic migraine attacks for the past 20 years on average (MWA: 20.3 ± 11.2, MWoA: 19.5 ± 8.5), with age at onset mostly during adolescence (MWA: 13.6 ± 4.6, MWoA: 16.5 ± 7.1). The average pain intensity of the attacks was rated approximately 6 out of 10 on the numeric analog scale, with a frequency of approximately four migraine attacks per month (MWA: 4.1 ± 3.6, MWoA: 4.0 ± 3.6). In the MWA subgroup, the pain predominantly started on the left side in 4 patients, on the right side in 2, and bilaterally in 6. In the MWoA subgroup, the pain onset was equally distributed in the 12 patients, 4 patients in each category. In the MWA subgroup, visual aura occurred predominantly on the same side of the headache in 4 patients, on alternating sides in another 4 patients, and on the contralateral side in 2 patients. Two patients could not define the predominant side of their aura. Additionally, 4 patients also described occasional sensory (e.g., numbness, tingling) but not motor and language disturbances. Six of the MWA patients and 4 of the MWoA were under prophylactic treatment. None of the patients had a previous history of either other trigeminal chronic pain or current acute pain or any other major health disorder that could confound the results.
Scanning protocol
Brain images were obtained and three-dimensionally reconstructed by two high-resolution magnetization-prepared rapid acquisitions with gradient echoes (MP-RAGE) on a 3.0-T Siemens Allegra Scanner (Erlangen, Germany). Both MP-RAGE sequences (1 × 1 × 1.3 mm, 128 slices, 256×256 matrix, echo time = 3.25 msec; repetition time = 2,530 msec; flip = 7°) were motion corrected and averaged to create one image volume. Using Free-surfer (http://surfer.nmr.mgh.harvard.edu), the MP-RAGE images were later segmented, reconstructed, inflated, and flattened as described below.
Cortical thickness analysis
For the surface reconstruction and cortical thickness estimation, brain surfaces were reconstructed and inflated as described by Dale and Fischl.15–18 Cortical thickness measurements were obtained by reconstructing the gray–white matter boundary and the cortical surface. The distance between these two surfaces was then calculated at each point across the cortical mantle. The maps of cortical thickness were created using spatial intensity gradients across tissue classes and were not restricted to individual voxel intensities, allowing subvoxel resolution and submillimetric difference detection between the migraine subtype groups and HCs. The thickness measures were mapped onto the inflated surfaces of each subject’s brain, and aligned according to cortical folding and thickness measurements into a common spherical system. Data were then smoothed on the surface tessellation using an iterative nearest-neighbor procedure. Smoothing was restricted to the cortical surface, thus avoiding averaging of data across sulci or outside the gray matter. This method has the advantage of matching morphologically homologous cortical areas based on the main gyri/sulci patterns with minimal metric distortion. A mean measure of the cortical thickness at each point of the cortical surface was then computed. Using an automatically parcellating technique, neuroanatomic regions (sulci and gyri) associated with somatosensory processing were labeled including the central sulcus, the post-central gyrus, and the postcentral sulcus, as well as the precentral gyrus that is part of the motor cortex. In addition, a control region of interest (ROI) was placed in the precentral sulcus, a region not involved in somatosensory processing.
Statistical comparison of surface maps was computed using a random effect model for each subject to generate a t test for each cortical location over the SSC between migraine subtype groups and HCs. ROIs were defined as clusters of p < 0.05 in the SSC. These ROIs, created on a standard brain, were then mapped back to each individual subject using spherical morphing17 to find homologous regions across subjects. A mean thickness score over each location was calculated for each subject, and the mean measure of SSC was computed.
Clinical correlations
Clinical correlations were computed between the mean thickness average of each location (significant clusters) found in SSC and migraine duration, age at onset, frequency of the headache attacks, and disease time span in each patient.
RESULTS
Cortical thickness findings common to both subgroups of migraineurs
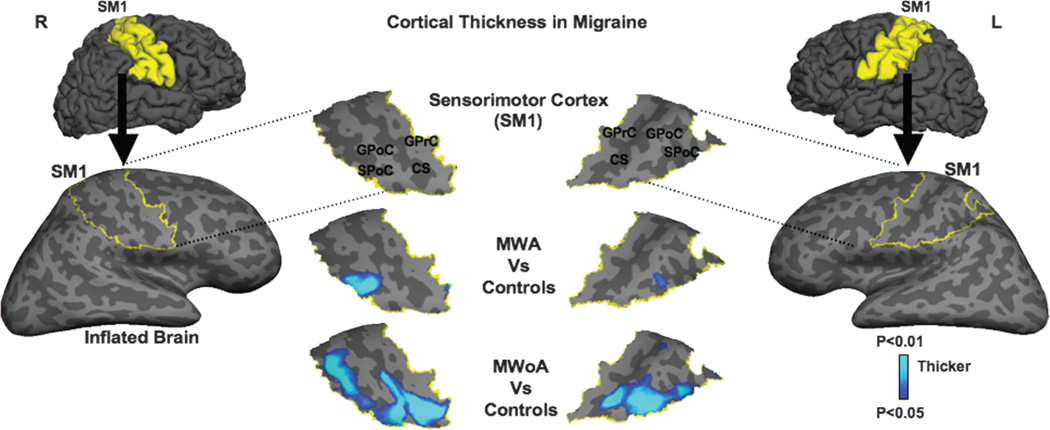
Migraineurs had on average thicker SSCs than the control group. The thickness changes were noticed in the caudal portion of the SSC, where the trigeminal area, including head and face, is somatotopically represented (figure).
Figure. Mean cortical thickness maps in migraine.
Lateral views of the folded and inflated brain hemispheres exposing sulci (dark gray) and gyri (light gray) with the right and left sensorimotor cortices (SMCs) delineated in yellow (lateral columns). When the migraine subgroups were compared with healthy controls, significant cortical thickening was found in the caudal SMCs of migraineurs, mostly in the somatosensory cortex, where the head is somatotopically represented (center columns). The light–dark blue shading code represents p values for cortical thickness changes. The lighter blue shading indicates thicker cortex. CS = central sulcus; GPoC = gyrus postcentralis; GprC = gyrus precentralis; MWA = migraine with aura; MWoA = migraine without aura; SPoC = sulcus postcentralis.
Cortical thickness findings in migraine subgroups
MWA patients showed thickening clusters in the lower postcentral sulcus bilaterally when compared with controls (right: p ≤ 0.005, left: p ≤ 0.003). The area of thickening bordered the posterior flank of the postcentral gyrus (putative border of Brodmann areas 1 and 2). In patients with MWoA, clusters were not only located in the postcentral sulcus of both hemispheres, but also in the central sulcus and postcentral gyrus (putative areas 3a, 3b, and 1), extending from the caudal area to more rostral regions, especially in the central and postcentral sulci (right: p ≤ 0.004, left: p ≤ 0.003). In MWoA patients, the thickening of the right SSC extended to the posterior flank of the precentral gyrus (putative area 4), which is part of the motor cortex. The most significant area of thickening on MWoA was localized in the caudal region of the central sulcus fundus, which corresponds approximately to the Brodmann area 3. There was no significant difference in cortical thickness of the precentral sulcus between groups (MWA + MWoA vs HC—right: p ≤ 0.238, left: p ≤ 0.107; MWA vs HC—right: p ≤ 0.38, left: p ≤ 0.164; MWoA vs HC—right: p ≤ 0.301, left: p ≤ 0.207).
Clinical correlations
We did not find any clear correlation between the cortical thickness changes and the clinical data (duration, age at onset, frequency, and disease time span).
DISCUSSION
Our current findings show interictal thickening of the cortical mantle in the SSC of patients with migraine when compared with ageand sex-matched controls. Previous studies with chronic pain disorders, including migraine19 and back pain,20 have described abnormal variations in the frontal and temporal cortices using voxel-based morphometry, but not in the SSC. In one of their studies, Apkarian et al.20 measured cortical volume in chronic back pain patients and noticed thinning of the dorsolateral prefrontal cortex. However, chronic back pain is a disorder that initiates much later in life than migraine, which may influence the response of neuronal systems (adapta-tion/breakdown) to overstimulation caused by chronic pain. In addition, chronic back pain has relatively spread somatotopic distribution (pain occurs in multiple dermatomes) compared with migraine (trigeminal nerve), a fact that may explain their lack of reported changes in the SSC. The technique we applied is extremely reliable and sensitive to measure cortical thickness differences21 even in submillimeters with improved spatial localization, and uses of more stable structural parameters.22
Functional and structural plasticity in the so-matosensory cortex have been noticed in rats23 after altered sensory experience. In humans, a comparable thickening of the SSC at the border between the postcentral gyrus and sulcus has recently been reported in chronic stroke patients when compared with HCs.24 The area of thickening spatially coincided with blood oxygen level– dependent activation after tactile stimulation of the hemiparetic hand of those patients, which indicates coupled structural and functional cortical changes in the SSC. This observation is in line with colocalized functional and structural changes reported in the visual cortex of patients with migraine.9 The maximum degree of thickening observed in the SSC of stroke patients reached 13%, which is also similar to the degree of gray matter changes in the motor cortex,25,26 whereas in our migraine cohort the average group thickening magnitude in the SSC reached 21% (MWA vs HCs: right side), and in the control ROI thickening was not statistically significant. This higher degree of structural changes in the SSC of patients with migraine can be explained by the long-term overstimulation of sensory fields in the cortex induced by the frequent headache attacks, with most of our patients experiencing migraine since childhood (age at onset for all patients: 14.6 ± 5.9 years). This is consistent with increases in the sensorimotor cortex thickness or volume after extensive learning and training.12,13
We observed thickening of the lower segment of the posterior flank SSC, where the face and the head are somatotopically represented in both migraine groups.27 The area of cortical thickening was larger in the MWoA. Although each area of the SSC carries a parallel somatotopic map, with the craniofacial region represented caudally, its function differs in the anteroposterior extension.28 For example, in the central sulcus there are primarily cutaneous responses in minor trigeminal receptor fields. These receptor fields increase in size and complexity toward more posterior regions of the SSC, responding simultaneously to peripheral cutaneous and deep receptors, as well as integrating high-order corticocortical inputs.28 This suggests the cortical integration in the SSC of neuronal inputs from other cortical regions during the trigeminal noxious experience from the headache attacks in both migraine subgroups.
However, the structural changes in the SSC of both subgroups described here seem to be mostly related to the trigeminal somatosensory system. Thickening of the SSC was accompanied in the same migraine cohort by reduced fractional anisotropy (FA) in the subcortical trigeminal somatosensory pathway leading to the SSC.1 The tracts affected were the trigeminothalamic tract in the MWA group (second-order neurons) and the thalamocortical tract in both migraine subgroups (third-order neurons), MWA and MWoA. FA is a DTI parameter that reflects the diffusional property of molecules in the neuronal structures29 and may indicate for instance changes in the axonal diameter or integrity.30–32 These concomitant cortical and subcortical abnormalities may represent either an innate trigeminal hyperexcitability in migraineurs, or the result of sustained stimulation of the trigeminal somatosensory system provoked by migraine attacks.
It remains unexplained why the thickening in the MWoA group extended to anterosuperior regions in the SSC compared with MWA, which would have theoretically some implications to sensory receptors from other somatotopic regions of the body. The same MWoA cohort presented lower FA values in the ventrolateral periaqueductal gray compared with the MWA and control groups.1 The latter finding suggested a possible dysfunction of the descending modulatory system in our MWoAcohort, which could result in a lowering of the threshold for headache attacks and general pain through a lack of inhibition of the sensory inputs from the trigeminal and nontrigeminal areas of the body. Hence, the cortical changes in the sensory cortex of this MWoA group would not be restricted to the craniofacial somatotopic regions. Further studies should be conducted with a higher number of patient to determine whether these gray and white matter changes are specific to the patient cohort studied or the pathophysiologic trait of migraine in general.
Regarding the laterality of the migraine attacks, some of our MWA patients described that their headache attacks were ipsilateral to the visual aura. Although migraine is usually described as unilateral, its occurrence may alternate between sides, which is true for both headache and aura attacks. Although patients were almost always certain about the predominant laterality of the headache initiation (in percentage), the retrospective description of the aura side (visual field affected) regarding the headache potentially lead to some confusion for the patients. In the beacon prospective study by Russell et al.,33 it was stated that the unilateral aura/headache symptoms with contralateral occurrence was 90%, but 10% of the patients had headaches ipsilateral to the aura. Perhaps the ipsilateral occurrence was in fact a clinical characteristic in some of our MWA patients. Another factor is that headache attacks may initiate on both sides after the aura event (bilateral); in this case, both answers (ipsilateral and contralateral) are right. Nonetheless, is possible that the side predominance of the headache attacks may influence the cortical thickness pattern in both migraine subgroups. In the MWoA group, where there is an equal distribution of headache attacks regarding predominant side initiation (4 left/4 right/4 bilateral), the area of thickening in the SSC is relatively equal in both hemispheres. In the migraine with aura group though, where there is bias distribution of migraine attack on the left side (4 left/2 right/6 bilateral), the area of thickening in the SSC is larger in the contralateral right hemisphere. However, additional studies with more patients should be conducted in the future using fMRI and prospective data to investigate in details the laterality issue.
In conclusion, the current findings of gray matter thickening of the SSC in addition to the previous discovery of white matter diffusional changes in the trigeminal somatosensory pathway of the same migraine cohort indicate that somatosensory mechanisms are important components in the migraine pathophysiology. These cortical and subcortical changes may be either the result or cause of repetitive migraine attacks, which may also affect other systems.1,9 This may explain the high comorbidity of migraine with other pain disorders, including back pain,34 temporomandibular disorders,35 and fibro-myalgia36 besides sensory disturbances such as allodynia.37
ACKNOWLEDGMENT
The authors thank Drs. M. Moskowitz, E. Loder, E. Spierings, and M. Vincent for their support.
Supported by NIH grant 5P01 NS 35611-09 (N.H., A.F.M.D., J.S.), the Swiss Heart Foundation (C.G.), and the Dean’s award of Harvard School of Dental Medicine (A.F.M.D.).
GLOSSARY
- CS
central sulcus
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- GPoC
gyrus postcentralis
- GprC
gyrus precentralis
- HC
healthy control
- IRB
institutional review board
- MP-RAGE
magnetization-prepared rapid acquisitions with gradient echoes
- MWA
migraine with aura
- MWoA
migraine without aura
- ROI
region of interest
- SMC
sensorimotor cortex
- SPoC
sulcus postcentralis
- SSC
somatosensory cortex
Footnotes
Disclosure: The authors report no conflicts of interest.
REFERENCES
- 1.DaSilva AF, Granziera C, Tuch DS, Snyder J, Hadjikhani N. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 2007;18:301–305. doi: 10.1097/WNR.0b013e32801776bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64:S9–S15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 3.Mitsikostas DD, Sanchez del Rio M. Receptor systems mediating c-fos expression within trigeminal nucleus caudalis in animal models of migraine. Brain Res Brain Res Rev. 2001;35:20–35. doi: 10.1016/s0165-0173(00)00048-5. [DOI] [PubMed] [Google Scholar]
- 4.Sessle BJ. Acute and chronic craniofacial pain: brain-stem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 5.Price DD, Verne GN. Does the spinothalamic tract to ventroposterior lateral thalamus and somatosensory cortex have roles in both pain sensation and pain-related emotions? J Pain. 2002;3:105–108. doi: 10.1054/jpai.2002.122950. [DOI] [PubMed] [Google Scholar]
- 6.Price DD. Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv. 2002;2:392–403. doi: 10.1124/mi.2.6.392. [DOI] [PubMed] [Google Scholar]
- 7.Sailer M, Fischl B, Salat D, Tempelmann C, Schonfeld MA, Busa E. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–1744. doi: 10.1093/brain/awg175. [DOI] [PubMed] [Google Scholar]
- 8.Thompson PM, Hayashi KM, Sowell ER, Gogtay N, Giedd JN, Rapoport JL. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23:S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 9.Granziera C, DaSilva AF, Snyder J, Tuch DS, Hadjikhani N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med. 2006;3:e402. doi: 10.1371/journal.pmed.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aurora SK, Welch KM, Al-Sayed F. The threshold for phosphenes is lower in migraine. Cephalalgia. 2003;23:258–263. doi: 10.1046/j.1468-2982.2003.00471.x. [DOI] [PubMed] [Google Scholar]
- 11.Hadjikhani N, Sanchez Del Rio M, Wu O, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA. 2001;98:4687–4692. doi: 10.1073/pnas.071582498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 13.Draganski B, Gaser C, Kempermann G, et al. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2nd ed) 2004;44:1–160. [Google Scholar]
- 15.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 16.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 19.Rocca MA, Ceccarelli A, Falini A, et al. Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke. 2006;37:1765–1770. doi: 10.1161/01.STR.0000226589.00599.4d. [DOI] [PubMed] [Google Scholar]
- 20.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X, Jovicich J, Salat D, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 22.Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer’s disease. Brain. 2006;129:2885–2893. doi: 10.1093/brain/awl256. [DOI] [PubMed] [Google Scholar]
- 23.Hickmott PW, Steen PA. Large-scale changes in dendritic structure during reorganization of adult somatosensory cortex. Nat Neurosci. 2005;8:140–142. doi: 10.1038/nn1384. [DOI] [PubMed] [Google Scholar]
- 24.Schaechter JD, Moore CI, Connell BD, Rosen BR, Dijkhuizen RM. Structural and functional plasticity in the somatosensory cortex of chronic stroke patients. Brain. 2006;129:2722–2733. doi: 10.1093/brain/awl214. [DOI] [PubMed] [Google Scholar]
- 25.Anderson BJ, Eckburg PB, Relucio KI. Alterations in the thickness of motor cortical subregions after motor-skill learning and exercise. Learn Mem. 2002;9:1–9. doi: 10.1101/lm.43402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleim JA, Barbay S, Cooper NR, et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem. 2002;77:63–77. doi: 10.1006/nlme.2000.4004. [DOI] [PubMed] [Google Scholar]
- 27.DaSilva AF, Becerra L, Makris N, et al. Somatotopic activation in the human trigeminal pain pathway. J Neurosci. 2002;22:8183–8192. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaas JH. Somatosensory system. In: Mai GPJK, editor. The human nervous system. London: Elsevier; 2003. pp. 1061–1086. [Google Scholar]
- 29.DaSilva AF, Tuch DS, Wiegell MR, Hadjikhani N. Diffusion tensor imaging: a primer on diffusion tensor imaging of anatomical substructure. Neurosurgical Focus. 2003;15:1–4. doi: 10.3171/foc.2003.15.1.4. [DOI] [PubMed] [Google Scholar]
- 30.Schmithorst VJ, Wilke M. Differences in white matter architecture between musicians and non-musicians: a diffusion tensor imaging study. Neurosci Lett. 2002;321:57–60. doi: 10.1016/s0304-3940(02)00054-x. [DOI] [PubMed] [Google Scholar]
- 31.Bengtsson SL, Nagy Z, Skare S, Forsman L, Forssberg H, Ullen F. Extensive piano practicing has regionally specific effects on white matter development. Nat Neurosci. 2005;8:1148–1150. doi: 10.1038/nn1516. [DOI] [PubMed] [Google Scholar]
- 32.Hagihara H, Hara M, Tsunekawa K, Nakagawa Y, Sawada M, Nakano K. Tonic-clonic seizures induce division of neuronal progenitor cells with concomitant changes in expression of neurotrophic factors in the brain of pilocarpine-treated mice. Brain Res Mol Brain Res. 2005;139:258–266. doi: 10.1016/j.molbrainres.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Russell MB, Iversen HK, Olesen J. Improved description of the migraine aura by a diagnostic aura diary. Cephalalgia. 1994;14:107–117. doi: 10.1046/j.1468-2982.1994.1402107.x. [DOI] [PubMed] [Google Scholar]
- 34.Hagen EM, Svensen E, Eriksen HR, Ihlebaek CM, Ursin H. Comorbid subjective health complaints in low back pain. Spine. 2006;31:1491–1495. doi: 10.1097/01.brs.0000219947.71168.08. [DOI] [PubMed] [Google Scholar]
- 35.Liljestrom MR, Le Bell Y, Anttila P, et al. Headache children with temporomandibular disorders have several types of pain and other symptoms. Cephalalgia. 2005;25:1054–1060. doi: 10.1111/j.1468-2982.2005.00957.x. [DOI] [PubMed] [Google Scholar]
- 36.Ifergane G, Buskila D, Simiseshvely N, Zeev K, Cohen H. Prevalence of fibromyalgia syndrome in migraine patients. Cephalalgia. 2006;26:451–456. doi: 10.1111/j.1468-2982.2005.01060.x. [DOI] [PubMed] [Google Scholar]
- 37.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]