Abstract
Purpose
Habitual running has been associated with reduced risk of cataract development in one prospective study. The purpose of the current analyses is to provide further evidence of this potentially important benefit of vigorous exercise, and to test whether moderate exercise (e.g., walking) provides a significant and equivalent reduction in cataract risk as vigorous exercise (e.g. running).
Methods
Cox proportional hazard analyses of self-reported, physician-diagnosed incident cataracts vs. baseline energy expenditure (metabolic equivalents or METs) in 32,610 runners and 14,917 walkers during 6.2-year follow-up. Results are reported as hazard ratios (HR), percent risk reductions (100*(HR-1)), and 95% confidence intervals (95%CI).
Results
Runners and walkers reported 733 and 1,074 incident cataracts during follow-up, respectively. When adjusted for sex, race, age, education, smoking, and intakes of meat, fruit and alcohol, lower cataract risk was significantly associated with both running (HR=0.960 per METh/d, 95%CI 0.935 to 0.986) and walking (HR=0.918 per METh/d, 95%CI: 0.881 to 0.956,), with no significant difference in the per METh/d risk reduction between running and walking, or between men and women. Compared to running or walking at or below guideline levels (≤1.8 METh/d), incident cataract risk was significantly lower for running or walking 1.8 to 3.6 (16.4% lower, 95%CI: 6.4% to 25.3%), 3.6 to 5.4 (19.0% lower, 95%CI: 5.6% to 30.4%), 5.4 to 7.2 (26.2% lower, 95%CI: 11.2% to 38.7%), 7.2 to 9.0 (34.1% lower, 95%CI: 10.0% to 51.2%), and ≥9 METh/d (41.6% lower, 95%CI: 19.8% to 57.4%).
Conclusion
Moderate (walking) and vigorous (running) exercise were both significantly associated with lower cataract risk, and their effects similar. Cataract risk appears to decrease linearly with increasing exercise energy expenditure through 9 METh/d.
Keywords: Vision, prevention, epidemiology, exercise
Many health benefits are ascribed to physical activity. These include lower rates of all-cause mortality, coronary heart disease, high blood pressure, stroke, type 2 diabetes, metabolic syndrome, colon cancer, and breast cancer, improved cardiorespiratory and muscular fitness, and healthier body mass and composition [21]. Most are conditions associated with aging. Curiously absent from the list are vision diseases. Cataracts affect over one-half of all Americans over the age of 65 and globally are the leading cause of blindness [2]. In addition to trauma and age, the risk for cataract may increase with exposure to sunlight, diabetes, smoking, alcohol, obesity, and dyslipoproteinemia [2,10].
We previously reported that cataract risk decreased significantly with greater distance run and greater cardiorespiratory fitness in 29,025 male runners who participated in the 7.6-year follow-up of the original National Runners’ Health Study [34]. Men who ran ≥64 km/wk had 35% lower risk of developing cataracts than those who ran <16 km/wk. Greater cardiorespiratory fitness was associated with lower cataract risk in men as well. The lack of significance in women may have been due to insufficient statistical power given their smaller sample size (N=11,967) and younger age. Physical activity was also reported to affect cataract risk in a small case-control study of 110 cataract surgery patients and 50 age-matched controls [20]. The odds for cataract were 7-fold greater in the right eye, and 4.4-fold greater in the left eye in the less active patient.
There is a manifest need to replicate these important associations in a second independent sample in order for physical activity and cardiorespiratory fitness to become recognized as potentially modifiable cataract risk factors. In addition, it remains to be determined whether the inverse association between running and cataract risk applies to women, whether other vigorous physical activities provide the same benefit as running, whether walking also lowers cataract risk, and whether the decrease in risk is equivalent for equivalent doses of moderate (e.g., walking) and vigorous exercise (e.g., running). To this end, we have analyzed the incidence of physician-diagnosed cataracts in a new, independent sample of nearly 50,000 runners and walkers who were resurveyed approximately 6.2 years after baseline, in order to confirm our initial findings in a independent sample, to extend the results to women, and to broaden the association with respect to the types of physical activity that may confer cataract risk reduction.
Methods
The National Runners’ Health Study II and the National Walkers’ Health Study were initially recruited primarily between 1998 and 2001 through solicitation of subscribers of activity-targeted publications and participants at footrace events. The 2006 partial re-survey of the National Runners’ Health Study II and the National Walkers’ Health Study [36,37] were obtained to identify and qualify approximately 50,000 runners and walkers for a proposed clinical trial, rather than a prospective follow-up study per se. These represented approximately a third of the original walkers (33.2%), and one-half of the original runners surveyed (51.7%). The difference in recruitment rates was due to the greater effort made to recruit runners (two mailings) than walkers (one mailing). Compared to non-responders, those that responded were slightly more likely to be female, younger, slightly less educated, weighed slightly more, were less likely to report taking medications for blood pressure, hypertension, or diabetes, but reported approximately the same number of km/day run if a runner or walked if a walker as reported on their baseline questionnaire [35].
The participants completed a four-page survey on walking history (average weekly mileage over the preceding 5 years, minutes required to walk or run one mile, frequency of walks per week >10 min, longest usual walk), height, weight and body circumferences, diet (vegetarianism and the current weekly intakes of alcohol, red meat, fish, fruit), current and past cigarette use, and history of diseases. Intakes of meat and fruit were based on the questions “During an average week, how many servings of beef, lamb, or pork do you eat”, and “...pieces of fruit do you eat”. Alcoholic beverage consumption was ascertained from the corresponding questions for 4-oz (112 mL) glasses of wine, 12-oz (336 mL) bottles of beer, and mixed drinks and liqueurs, and alcohol intake estimated from 10.8 g/4-oz glass of wine, 13.2 g/12-oz bottle of beer, and 15.1 g/mixed drink. Education was solicited by requesting the participant provide “years of education (examples: HS=12; BS or BA = 16; MS or MA = 18; PhD or MD = 20).” The study protocol was approved by the University of California Berkeley committee for the protection of human subjects, and all subjects provided a signed statement of informed consent.
Walking (in walkers) and running (in runners) were reported in miles per week, while running (in walkers), walking (in runners), swimming, cycling, and other exercise were reported as hours spent per week. Usual pace (minutes per mile) was reported for walking in walkers, and for running in runners. Non-running exercise energy expenditure in the runners, and non-walking exercise energy expenditures in the walkers, was calculated using the metabolic equivalent (MET) tables published by Ainsworth et al. [1], where one MET is the energy expended sitting at rest (3.5 ml O2•kg-1•min-1). Physical activities that expended between 3 to 6 METs were classified as moderate-intensity physical activities, those that expended more than 6 METs were defined as vigorous, and those that expended less than 3 METs as light. In walkers, METh/d walked was calculated by converting reported distance into duration (i.e., distance/mph) and then taking the product of the average hours walked per day and the MET value for the reported pace [1]. The 8.7% of men and 8% of women who did not provided their usual walking pace had their values estimated by stochastic imputation using a sex-specific function of age. Specifically, normally distributed random error having the same variance as the residual error was added to the predicted values from sex-specific least squares regression analyses of age and age2. Simply excluding these subjects produced little change in the regression coefficient, suggesting that the imputation of missing values had a negligible impact on the analyses. The analyses were also repeated assigning the same walking pace (18.3 min/mi) for all subjects, again with negligible impact on the analyses. Running MET values were calculated as 1.02 MET•hours per km [37]. Previously, we reported strong correlations between repeated questionnaires for self-reported running distance (r=0.89) [31].
For this report, baseline cardiorespiratory fitness in the runners was defined as speed in meters per second (m/s) of the runners’ best 10 km race during the previous five years. Published data support the use of running 10-km performance to estimate maximal oxygen consumption (VO2max) [3,5,7].
Participants reported whether they had been diagnosed by a physician for cataract, and the year of the diagnosis. Specifically, the participants responded to the question “If you were told by a physician that you had any of the following conditions between 1991 and 2006, please give the year of onset:” with “cataract ___” included as one of the conditions. Cox proportional hazard analyses were used to estimate the hazard ratios (HR) per METh/d of running, walking, and other vigorous, moderate, and light intensity exercise, using the statistical package Stata (StataCorp LP, College Station TX, version 11). Survival was the length of time between baseline and diagnosis of cataract or receipt of the follow-up questionnaire completion date. Linear combinations of estimators were used to compare regression slopes for running, walking, and other exercise. Results are reported as hazard ratios (HR), percent reductions in risk (100*(HR-1)), with 95% confidence intervals (95%CI). Covariates included baseline age, age2, sex, race, years of education, smoking (baseline smoker vs. nonsmoker), and intakes of meat, fruit, and alcohol. The analyses were repeated using quadratic functions of education and dietary intake, and quintiles of age, education, and dietary variables to insure that the effects of exercise were robust to the functional form of the covariates. As these data are observational, they cannot prove causality. The use of the terminology “increasing METh/d” in reference to the independent variable and “decreasing cataract risk” in reference to the dependent variables pertain only to their mathematical functional relationship and is not intend to imply a causal one.
Results
Sample characteristics
There were 15,945 walkers (3,349 males, 12,596 females), and 33,060 runners (16,983 males, 16,077 females) who were eligible for the analyses. Among these, 1028 walkers (330 males, 698 females) and 450 runners (321 males, 129 females) were excluded for pre-existing cataracts. Heights and weights were provided by 98.2% of the walkers and 99% of the runners. There were 23,733 runners (72.8%) who provided their best 10-km race performance time during the previous 5 years. For these reasons, the number of subjects varies slightly for analyses involving BMI and 10-km race performance. All but 28 of the 1807 reported cataracts had onset at age 45 years or older.
Table 1 shows that energy expended by walking in the walkers was less than half that reported by running in the runners. In addition, the walkers were older than the runners, consumed less alcohol and more fruit, and were heavier. Because baseline recruitment began later in walkers than runners, their follow-up duration was 9.1 months shorter. The runners completed their 10-km races at an average pace (±SD) of 3.81±0.56 m/s for men and 3.36±0.55 m/s for women.
Table 1.
Sample characteristics
| Male | Female | |||
|---|---|---|---|---|
| Runners | Walkers | Runners | Walkers | |
| Sample (N) | 16,662 | 3,019 | 15948 | 11898 |
| Age (years)* | 48.01±10.82 | 60.73±10.88 | 40.74±10.52 | 52.25±11.68 |
| Follow-up (years)* | 6.30±0.91 | 5.62±1.16 | 6.55±0.94 | 5.70±1.27 |
| Education (years)* | 16.79±2.46 | 16.33±2.70 | 16.35±2.31 | 15.28±2.54 |
| Baseline smokers (%) | 1.21 | 3.64 | 1.67 | 3.71 |
| Meat (servings/d)* | 0.44±0.40 | 0.46±0.41 | 0.27±0.30 | 0.37±0.34 |
| Fruit (pieces/d)* | 1.53±1.18 | 1.60±1.21 | 1.53±1.06 | 1.68±1.13 |
| Alcohol (g/d)* | 9.83±13.44 | 9.08±13.32 | 5.88±8.21 | 4.93±9.09 |
| BMI (kg/m2)* | 24.09±2.58 | 26.71±4.10 | 21.61±2.50 | 25.46±5.20 |
| Waist circumference (cm)* | 84.83±6.19 | 93.48±10.01 | 70.03±6.72 | 78.51±12.11 |
| Energy expenditure (METh/d)* | ||||
| Running | 5.30±3.12 | 4.75±3.02 | ||
| Walking | 2.21±1.67 | 2.16±1.63 | ||
| Other vigorous exercise | 1.70±3.21 | 1.75±3.43 | 2.06±3.33 | 1.47±2.95 |
| Other exercise, moderate | 0.76±1.63 | 0.43±1.50 | 0.83±1.73 | 0.36±1.26 |
| Other exercise, light | 0.02±0.30 | 0.04±0.59 | 0.03±0.36 | 0.03±0.25 |
| Other exercise, strength | 0.52±1.26 | 0.21±0.89 | 0.54±1.26 | 0.21±0.76 |
mean±SD
Walking
When simultaneously adjusted for baseline age, sex, race, group (walking vs. running), smoking (smoker vs. nonsmoker), diet (meat and fruit intake), and alcohol intake, baseline walking was associated with 8.2% lower risk for incident cataracts per METh/d walked (Table 2, P=3×10-5). The inclusion of a quadratic term for METh/d walked did not significantly improve the model's fit (P=0.14). The results were consistent in both women (8.9% lower risk per METh/d, 95%CI: 4.3% to 13.4% lower, P=0.0002) and men (6.2% lower risk per METh/d, 95%CI: 0.7% higher to 12.7% lower, P=0.08, results not displayed), albeit not as significant in men. For sexes combined, the reduction in risk was independent of both BMI (7.7% lower adjusted risk per METh/d, P=0.0001) and walking intensity (7.7% lower adjusted risk per METh/d, 95%CI: 3.8% to 11.4% lower P=0.0001), since cataract risk in walkers was associated with neither BMI (HR=1.001 per kg/m2, 95%CI: 0.987 to 1.016, P=0.85) nor walking intensity (HR=0.865 per m/s, 95%CI: 0.700 to 1.069, P=0.18). The reduction in cataract risk was also significant when walkers were analyzed separately from the runners (7.6% lower per METh/d walked, 95%CI: 3.7% to 11.3% lower, P=0.0001).
Table 2.
Hazard ratios (95% confidence intervals) for self-reported physician-diagnosed incident cataracts vs. exercise from Cox proportional hazard analyses.
| BMI not included | BMI included | |
|---|---|---|
| Sample size (N) | 47,527 | 46,940 |
| Incident events | 1807 | 1772 |
| Runners (0,1) | 0.72‡ (0.60, 0.86) | 0.71† (0.59, 0.85) |
| Energy expenditure (per METh/d) | ||
| Running | 0.96† (0.94, 0.99) | 0.96† (0.94, 0.99) |
| Walking | 0.92§ (0.88, 0.96) | 0.92§ (0.89, 0.96) |
| Other vigorous | 0.98* (0.97, 1.00) | 0.98* (0.97, 1.00) |
| Other moderate | 1.02 (1.00, 1.05) | 1.02 (1.00, 1.05) |
| Other light | 1.02 (0.92, 1.10) | 1.02 (0.92, 1.10) |
| BMI (per kg/m2) | 1.01 (0.99, 1.02) | |
| Comparison between coefficients Ratio of hazard ratio | ||
| Running vs. walking | 1.05 (1.00, 1.10) | 1.05 (1.00, 1.10) |
| Running vs. other vigorous | 0.98 (0.95, 1.01) | 0.98 (0.95, 1.01) |
| Walking vs. other moderate | 0.90 (0.85, 0.94) | 0.90 (0.86, 0.95) |
The model included all of the variables listed above, along with baseline age (age, age2), sex, race (self identified African-American, Hispanic, Asian, Native American), education, smoking (current smoker vs. non-smoker), and intakes of meat, fruit, and alcohol. Significance levels for individual coefficients are coded
P<0.05
P<0.01
P<0.001
P<0.0001.
Running
When adjusted for other covariates, the runners as a group were at significantly lower risk than the walkers (Table 2), which was not explained by the exercise dose (28.4% lower, P=0.0003) or BMI (29.4% lower, still P=0.0003). The runner-walker difference was consistent across sex, i.e. 26.2% lower in males (95%CI: 3.4% to 43.6% lower, P=0.03) and 25.6% lower in females (95%CI: 3.5% to 42.7% lower, P=0.02). Running longer distances was associated with lower cataract risk for the sexes combined (Table 2, P=0.002) and for males (3.6% lower per METh/d, 95%CI: 0.3% to 6.7% lower, P=0.03) and females separately (4.9% lower per METh/d, 95%CI: 0.4% to 9.2% lower, P=0.03). The inclusion of a quadratic term for METh/d walked did not significantly improve the model's fit (P=0.43, sexes combined). The reduction in risk per METh/d run persisted when adjusted for BMI (Table 2, P=0.008). As in the walkers, cataract risk in runners was not significantly related to either BMI (HR=1.005 per kg/m2, 95%CI: 0.975 to 1.035, P=0.75) or running intensity (HR=0.854 per m/s, 95%CI: 0.712 to 1.025, P=0.09). The reduction in cataract risk was also significant when runners were analyzed separately from the walkers (3.8% lower per METh/d run, 95%CI: 1.2% to 6.4% lower, P=0.005).
Age was the strongest predictor of cataract risk in the analyses. The number of incident cataract by quintiles of age was 10 (age<37.2), 46 (37.2≤age<44.0), 119 (44.0≤age<50.0), 329 (50.0≤age<57.6), and 1303 (57.6≤age). The hazard ratios for METhr/d run and walked were 0.961 (95%CI: 0.94, 0.99) and 0.916 (95%CI: 0.88, 0.96) adjusted for age as a cubic function, respectively, and 0.950 (95%CI: 0.92, 0.98) and 0.893 (95%CI: 0.86, 0.93) when adjusted for quintiles of age. Moreover, the effects of running and walking on cataract risk persisted when the analyses were restricted to the highest quintile of age (N=9510), i.e. 4.7% lower per METh/d run (95%CI: 1.2% to 8.1% lower, P=0.007) and 6.6% lower per METh/d walked (95%CI: 2.2% to 10.7% lower, P=0.003). In addition, quadratic terms were found to be nonsignificant for education, and intakes of meat, fruit, and alcohol, and the inclusion of the quadratic terms produced negligible effect on the coefficients for running, walking, and other physical activities. The analyses were also repeated using indicator functions to adjust for quartiles of education, intakes of meat, fruit, and alcohol, again with negligible effect on the hazard ratios for running, walking, and other physical activities,
Walking vs. Running
There was no significant difference in the risk reduction between METh/d run and per METh/d walked (Table 2, P=0.07). Figures 1 and 2 display the reductions in cataract risk by quintiles and categories of METh/d walked or run, adjusted for the runner-walker group difference and other covariates. Figure 2 suggests that the decline in risk was linear with increasing exercise energy expenditure through at least 9 METh/d. For the sexes combined, the risk decreased 5.3% per METh/d run or walked (95%CI: 3.1% to 7.4% decrease, P=1.7×10-6) with little evidence for nonlinearity (P=0.45 for quadratic term, (METh/d)2, when included in the model). The generally linear decline in risk was observed for both males (4.0% per METh/d, 95%CI: 1.9% to 5.4%, P=0.007) and females (6.8% per METh/d, 95%CI: 4.6% to 10.0%, P=3.9×10-5), with both sexes exhibiting no obvious departure from linearity (P=0.70 and P=0.30, respectively) as represented by the significance of (METh/d)2. The reduction in cataract risk per METh/d run or walked was not significantly different between males and females (HRmale/HRfemale= 1.028, 95%CI: 0.987 to 1.070, P=0.19).
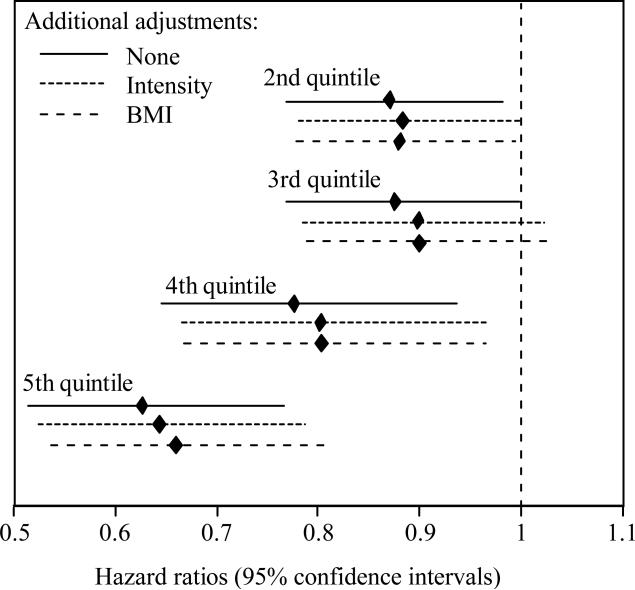
Figure 1.
Hazard ratios (95% confidence intervals) of incident cataracts by quintiles of METh/d energy expended by walking or running, adjusted for age (age, age2), sex, race, education, smoking status, and, intakes of meat, fruit, and alcohol. Compared to the lowest quintile (≤1.57 METh/d), incident cataract risk was significantly lower in the 2nd (P=0.03), 3rd (P=0.05), 4th (P=0.007), and 5th quintiles (P=2.5×10-6), where 40th, 60th, and 80th percentiles were 2.81, 4.69, and 6.57 METh/d, respectively.
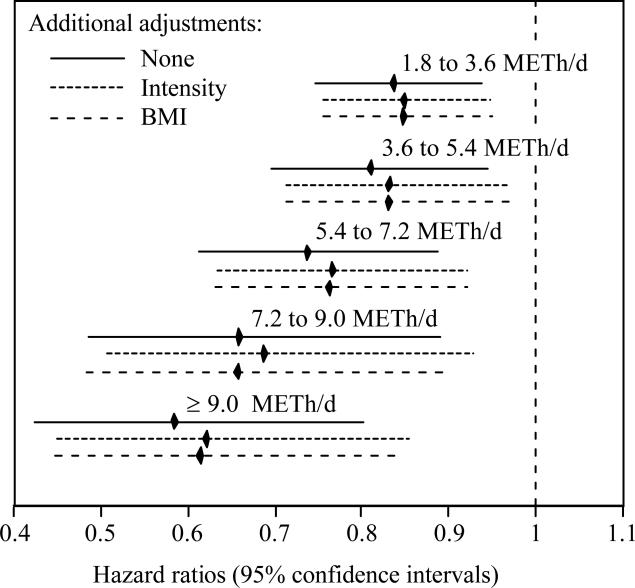
Figure 2.
Hazard ratios (95% confidence intervals) of incident cataracts by categories of METh/d walked or run, adjusted for age (age, age2), sex, race, education, smoking status, and, intakes of meat, fruit, and alcohol. Compared to running or walking at or below guideline levels (≤1.8 METh/d), incident cataract risk was significantly reduced by running or walking 1.8 to 3.6 (P=0.002), 3.6 to 5.4 (P=0.006), 5.4 to 7.2 (P=0.001), 7.2 to 9.0 (P=0.004), and ≥9 METh/d (P=0.0004).
Cardiorespiratory fitness
There were 517 incident cataracts in the 23,733 runners who provided 10-km performance times. Their risk for cataracts decreased significantly in association with increasing cardiorespiratory fitness as measured by running speed during a 10-km race (22.8% lower per m/s, 95%CI: 7.8% to 35.3% lower, P=0.004) which was not explained by usual METh/d run and other exercise (17.9% lower adjusted risk per m/s, 95%CI: 0.7% to 32.1% lower, P=0.04), BMI (23.8% lower adjusted risk per m/s, 95%CI: 8.0% to 36.9% lower, P=0.005), or even usual running intensity (21.9% lower adjusted risk per m/s, 95%CI: 3.4% to 36.8% lower, P=0.02). Figure 3 shows that relative to the lowest quintile of cardiorespiratory fitness, the risk for cataracts declined in the 2nd (10% reduction, P=0.41), 3rd (20%, P=0.10), 4th (37.5%, P=0.0008), and 5th quintiles of fitness (33.4% reduction, P=0.002), which was scarcely affected by adjustments for BMI or usual running intensity.
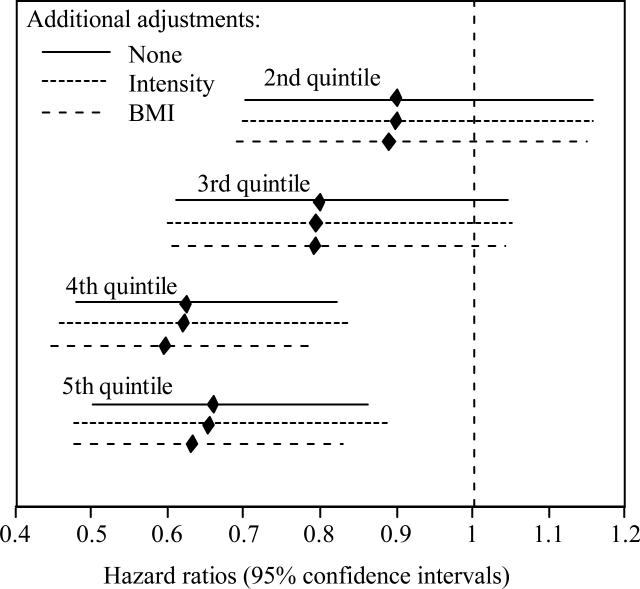
Figure 3.
Hazard ratios (95% confidence intervals) of incident cataracts in runners by quintiles of cardiorespiratory fitness (10-km footrance performance times) adjusted for age (age, age2), sex, race, education, smoking status, and, intakes of meat, fruit, and alcohol. Compared to the lowest quintile, incident cataract risk was lower in the 2nd (P=0.41), 3rd (P=0.10), 4th (P=0.0008), and 5th quintiles of cardiorespiratory fitness (P=0.002).
Other exercise
There was also a marginally significant (P=0.05) effect of increasing levels of other (non-running) vigorous exercise on cataract risk (Table 2), which persisted with adjustment for baseline BMI. Other moderate intensity exercise was not significant (P= 0.07). The decline in cataract risk was not significantly greater for running than non-running vigorous exercise (Table 2, P=0.12), but was significantly greater for walking than non-walking moderate exercise (P<10-5).
Different recruitment rates between the runners (51.7%) and walkers (33.2%) did not affect the analyses. Repeating the analyses using only the first 33.2% of the runners recruited (to match the 33.2% recruitment rate in the walkers) produced results consistent entirely consistent with the complete sample, namely: 1) the decline in cataract risk was significant for the sexes combined (5.0% lower per METh/d run, 95%CI: 1.9% to 8.0% lower, P=0.001) and for males (3.8% lower, 95%CI: 0% to 7.5% lower, P=0.04) and females (7.3% lower, 95%CI: 1.9% to 12.5% lower, P=0.008) separately; 2) adjustment for BMI did not eliminate the decline in cataract risk (4.5% lower risk per METh/d run, 95%CI: 2.3% to 7.5% lower, P=0.04); 3) the decline in cataract risk per METh/d was not significantly different between running and walking (P=0.19), 4) cataract risk declined significantly with cardiorespiratory fitness (HR: 24.8% decline per m/s, 95%CI: 7.1% to 39.1% decrease, P=0.008); and 5) relative to the lowest quintile of cardiorespiratory fitness, the risk for cataracts was lower for the 2nd (9.5% lower risk, P=0.39), 3rd (17.5%, P=0.08), 4th (37.1%, P=0.005), and 5th quintiles of fitness (25.4% lower risk, P=0.009). The different recruitment rates for runners and walkers was intentional, the result of there being two follow-up mailings sent to the runners, but only one follow-up mailing to walkers.
Discussion
These results provide independent support that baseline running and cardiovascular fitness were associated with risks of cataract in men during follow-up. The declines in risk reported here (i.e., 41.6% lower risk for ≥9 METh/d) corresponds well with that of the highest running levels reported previously (i.e., 35% lower risk for ≥9.3 METh/d [34]). A 28% decline in risk corresponding to a two standard deviation difference in METhr/d run or walked represents a moderate reduction in cataract risk (i.e., for every 10 people with cataracts in the reference group, small, moderate and large reductions in risk would correspond to 9, 7 and 5 people with cataracts in the exercise comparison groups; the corresponding hazard-ratio thresholds are 0.9, 0.7 and 0.5, and these thresholds can also be interpreted as 10%, 30% and 50% reductions in risk [9]).
The risk reduction for the highest vs. lowest quintile of 10-km race performance reported here is not inconsistent with the risk reductions for the fittest performance category previously reported [34]. Both our current and previous report showed a reduction in cataract risk with cardiorespiratory fitness that was not explained by usual distance run.
In addition to confirming the initial report, these results also extend the original finding in several important respects: to women, to other vigorous exercise, and to walking. Women are at greater risk for developing cataracts than men [29]. Only 1.1% of the 5.1% of US adults who report any vigorous exercise are runners, with use of cardiovascular equipment being twice as common [27]. Extending the results to walking is particularly important given its rank as the most commonly performed exercise in the United States [27]. Whether one chooses walking or running for exercise does not appear to affect the reduction in cataract risk for the same exercise dose. As with our original findings, the current data suggest that the dose-response relationship between cataract risk and METh/d run or walked is linear throughout the range of reported activity, with no indication of diminishing return (Figure 2). This would suggest an advantage to running given that the average energy expenditure for running was more than twice as great as that for walking (Table 1). More walkers walked than runners ran at or below the guideline levels (48.1% vs. 12.2%), and fewer walkers than runners exceeded the guideline levels by walking or running by ≥2-fold (15.4% vs. 61.1%), ≥3-fold (4.5% vs. 40.1%), or ≥4-fold (1.1% vs. 17.9%).
The statistical power provided by our cohort's large sample size and broad activity range and the quality of the exposure variable probably explain why the inverse association between cataracts and exercise was repeatedly observed in the National Runners’ and Walkers’ Health Studies but apparently not in other cohorts, given the lack of reports. The National Runners’ and Walkers’ Studies are the only large prospective cohorts recruited specifically to study the health benefits of exercise, and therefore include substantially greater numbers of physically active subjects who are exercising at substantially higher activity levels than other cohorts that are geographically, gender, or institutionally based. For example, only about 20% of the women in the Women's Health Initiative [18] and the Nurses’ Health Study [19] walked ≥8 mi/wk (approximately 1.43 METh/d [1]), whereas nearly half of the National Walkers’ Health Study did. The primarily sedentary populations studied by others may also be more inclined to overestimate exercise, e.g., only 0.2% of women and 0.4% of men met current guidelines as objectively measured by accelerometry in the National Health and Nutrition Examination Survey [17] compared to 42% by time-based questionnaire estimates [24]. In addition, we calculated running and walking energy expenditure from distance run and walked, which appear to be superior metrics for epidemiological analyses of physical activity. Specifically, energy expenditure estimated from running and walking distance show associations with body weight that are over twice as great as those obtained from reported time and intensity [36,37]. Presumably, errors and biases affecting the time-based running and walking energy expenditures also affect time-based calculations of energy expenditure for other physical activities.
Several mechanisms could contribute to the cataract-protective effects of exercise and cardiorespiratory fitness. Oxidation and systemic inflammation are thought to be important in the etiology of age-related nuclear cataracts [23,25]. High-density lipoproteins (HDL) have both anti-oxidative and anti-inflammatory activities [28], predispose men to run longer distances [40], exhibit a pronounced, concordant, dose-response relationship with running distance [30], and are inversely related to plasma triglyceride concentrations [28]. Low HDL-cholesterol and high triglycerides have been associated with cataracts, albeit the results are variable across studies and cataract type [8,9]. C-reactive protein levels predict incident cataract prospectively [23] and are inversely correlated with cardiorespiratory fitness (i.e., VO2max) [13]. Type 2 diabetes, glucose intolerance, insulin resistance, gout, elevated serum uric acid levels, hypertension, and total and abdominal obesity have all be associated with cataract risk, and are reduced by physical activity (discussed in [34]). The lack of association between BMI and cataract risk in this study could be because these cohorts tend to be considerably leaner and includes fewer obese subjects than other cohorts and therefore many have included too few subjects at risk for their obesity [30-33].
Caveats
These results are derived in a sample of convenience; however, we believe that the biological processes that relate exercise to cataract risk do not differ dramatically between the current sample and a more formally specified population. Moreover, we have already established the association between cataract risk and running in a well-designed prospective study with 80% follow-up [34]. Incident cataracts were identified by self-reporting of physician diagnoses, and there is no identification of the type of cataract. We do not know the proportion of subjects who received dilated eye or ophthalmic slit lamp examinations to properly assess cataract, or the proportion of those with clinically insignificant cataracts who may not have been informed of their condition, or whether those proportions might vary systematically by exercise or cardiorespiratory fitness. Is it also not known whether there were less frequent clinical visits among more active walkers and runners that might account for the associations reported here. However, higher mileage runners and walkers are leaner than those less active [32,33,38,39], and leaner men have been reported to have more frequent eye examinations (i.e., opportunity for cataract diagnoses) than heavier men [22]. Others report that vigorously active males had more routine medical check-ups than less active men in the Health Professionals Follow-up Study [15], and that there was no difference in routine medical check-up by activity level in the Nurses Health Study [16]. It is also possible that visual ability, or the need to wear glasses, could affect running and walking behavior. Finally, it is worth noting that the majority of older people have some degree of cataract. Of greater clinical significance is the effects of running and walking on visually-significant cataract (affecting quality of life, thereby indicating surgery), which was not assessed in the study.
Age was the strongest predictor of cataract risk, and was also inversely related to physical activity. Adjustment for age as a quadratic function, cubic function (not displayed), or as age quintiles (not displayed) did not eliminate the significant inverse association between METh/d run or walked and cataract risk, however, noise in the relationship between calendar and biological age could result in the incomplete adjustment for age, and thus age could still contribute in part to the cataract-exercise association.
In conclusion, the public health significance of delaying the onset of age-related cataracts is potentially great. Cataracts are the most common cause of low vision in the United States, and account for almost one-half of all blindness worldwide [4]. One estimate suggests that a 10-year delay in the development of cataracts would produce a 45% reduction in the need for cataract surgeries [14]. To date, not smoking [12] and limiting sunlight exposure [26] are the only modifiable risk factor for cataracts. The cataract-protective effects of exercise were observed even though higher mileage runners and walkers may have received greater sunlight exposure as a consequence of their activity. These results suggest that cataract risk reduction should be listed as a potential benefit of exercise.
Acknowledgement
The author had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This research was supported by grant HL094717 from the National Heart, Lung, and Blood Institute and was conducted at the Ernest Orlando Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California). The author wishes to thank Ms. Kathryn Hoffman for her help in collecting the data. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure
The author has no financial conflict of interest to disclose. The results of the present study do not constitute endorsement by ACSM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 2.Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365:599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 3.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. US Armed Forces Med J. 1959;10:875–88. [PubMed] [Google Scholar]
- 4.Congdon N, O'Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 5.Cooper KH. A means of assessing maximal oxygen intake: correlation between field and treadmill testing. JAMA. 1968;203:201–4. [PubMed] [Google Scholar]
- 6.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 7.Hellerstein HK. Limitations of marathon running in the rehabilitation of coronary patients: anatomic and physiologic determinants. Ann NY Acad Sci. 1977;301:484–94. doi: 10.1111/j.1749-6632.1977.tb38224.x. [DOI] [PubMed] [Google Scholar]
- 8.Hiller R, Sperduto RD, Reed GF, D'Agostino RB, Wilson PW. Serum lipids and age-related lens opacities: a longitudinal investigation: the Framingham Studies. Ophthalmology. 2003;110:578–83. doi: 10.1016/S0161-6420(02)01762-1. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins WG, Marshall SW, Batterham AM, Hanin J. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41:3–13. doi: 10.1249/MSS.0b013e31818cb278. [DOI] [PubMed] [Google Scholar]
- 10.Jacques PF, Moeller SM, Hankinson SE, Chylack LT, Jr, Rogers G, Tung W, Wolfe JK, Willett WC, Taylor A. Weight status, abdominal adiposity, diabetes, and early age-related lens opacities. Am J Clin Nutr. 2003;78:400–5. doi: 10.1093/ajcn/78.3.400. [DOI] [PubMed] [Google Scholar]
- 11.Klein BE, Klein R, Lee KE. Cardiovascular disease, selected cardiovascular disease risk factors, and age-related cataracts: the Beaver Dam Eye Study. Am J Ophthalmol. 1997;123:338–46. doi: 10.1016/s0002-9394(14)70129-1. [DOI] [PubMed] [Google Scholar]
- 12.Klein BE, Klein R, Linton KL, Franke T. Cigarette smoking and lens opacities: the Beaver Dam Eye Study. Am J Prev Med. 1993;9:27–30. [PubMed] [Google Scholar]
- 13.Kullo IJ, Khaleghi M, Hensrud DD. Markers of inflammation are inversely associated with VO2 max in asymptomatic men. J Appl Physiol. 2007;102:1374–9. doi: 10.1152/japplphysiol.01028.2006. [DOI] [PubMed] [Google Scholar]
- 14.Kupfer C. On presentation of the Friedenwald Award of the Association for Research in Vision and Ophthalmology to Dr Joram Piatigorsky. Invest Ophthalmol Vis Sci. 1987;28:2–8. [PubMed] [Google Scholar]
- 15.Leitzmann MF, Giovannucci EL, Rimm EB, et al. The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med. 1998;128:417–25. doi: 10.7326/0003-4819-128-6-199803150-00001. [DOI] [PubMed] [Google Scholar]
- 16.Leitzmann MF, Rimm EB, Willett WC, et al. Recreational physical activity and the risk for cholecystectomy in women. N Engl J Med. 1999;341:777–84. doi: 10.1056/NEJM199909093411101. [DOI] [PubMed] [Google Scholar]
- 17.Luke A, Dugas LR, Durazo-Arvizu RA, Cao G, Cooper RS. Assessing Physical Activity and its Relationship to Cardiovascular Risk Factors: NHANES 2003-2006. BMC Public Health. 2011;11:387. doi: 10.1186/1471-2458-11-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manson JE, Greenland P, LaCroix AZ, et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–25. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–8. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 20.Paunksnis A, Kusleika S, Kusleikaite M. The relationship of the intensity of lens opacity with physical activity. Medicina (Kaunas) 2006;42:738–43. [PubMed] [Google Scholar]
- 21.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report, 2008. U.S. Department of Health and Human Services; Washington, DC: 2008. pp. A1–H14. [Google Scholar]
- 22.Schaumberg DA, Glynn RJ, Christen WG, Hankinson SE, Hennekens CH. Relations of body fat distribution and height with cataract in men. Am J Clin Nutr. 2000;72:1495–1502. doi: 10.1093/ajcn/72.6.1495. [DOI] [PubMed] [Google Scholar]
- 23.Schaumberg DA, Ridker PM, Glynn RJ, Christen WG, Dana MR, Hennekens CH. High levels of plasma C-reactive protein and future risk for age-related cataract. Ann Epidemiol. 1999;9:166–71. doi: 10.1016/s1047-2797(98)00049-0. [DOI] [PubMed] [Google Scholar]
- 24.Sisson SB, Camhi SM, Church TS, et al. Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metab Syndr Relat Disord. 2009;7:529–36. doi: 10.1089/met.2009.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor A. Cataract: relationship between nutrition and oxidation. J Am Coll Nutr. 1993;12:138–46. doi: 10.1080/07315724.1993.10718294. [DOI] [PubMed] [Google Scholar]
- 26.Taylor HR, West SK, Rosenthal FS, et al. Effect of ultraviolet radiation on cataract formation. N Engl J Med. 1988;319:1429–33. doi: 10.1056/NEJM198812013192201. [DOI] [PubMed] [Google Scholar]
- 27.Tudor-Locke C, Johnson WD, Katzmarzyk PT. Frequently reported activities by intensity for U.S. adults: the American Time Use Survey. Am J Prev Med. 2010;39:e13–20. doi: 10.1016/j.amepre.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 28.von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Waudby CJ, Berg RL, Linneman JG, et al. Cataract research using electronic health records. BMC Ophthalmol. 2011;11:32. doi: 10.1186/1471-2415-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams PT. Relationship of distance run per week to coronary heart disease risk factors in 8283 male runners. The National Runners’ Health Study. Arch Intern Med. 1997;157:191–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obes Relat Metab Disord. 2004;28:120–8. doi: 10.1038/sj.ijo.0802480. [DOI] [PubMed] [Google Scholar]
- 32.Williams PT. Nonlinear relationships between weekly walking distance and adiposity in 27,596 women. Med Sci Sports Exerc. 2005;37:1893–901. doi: 10.1249/01.mss.0000175860.51204.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams PT. Association between walking distance and percentiles of body mass index in older and younger men. Br J Sports Med. 2008;42:352–6. doi: 10.1136/bjsm.2007.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PT. Prospective epidemiological cohort study of reduced risk for incident cataract with vigorous physical activity and cardiorespiratory fitness during a 7-year follow-up. Invest Ophthalmol Vis Sci. 2009;50:95–100. doi: 10.1167/iovs.08-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams PT. Distance walked and run as improved metrics over time-based energy estimation in epidemiological studies and prevention; evidence from medication use. PLoS One. 2012;7:e41906. doi: 10.1371/journal.pone.0041906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams PT. Advantage of distance- versus time-based estimates of walking in predicting adiposity. Med Sci Sports Exerc. 2012;44:1728–37. doi: 10.1249/MSS.0b013e318258af3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams PT. Non-exchangeability of running vs. other exercise in their association with adiposity, and its implications for public health recommendations. PLoSOne. 2012;7:e36360. doi: 10.1371/journal.pone.0036360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williams PT, Pate RR. Cross-sectional relationships of exercise and age to adiposity in 60,617 male runners. Med Sci Sports Exerc. 2005;37:1329–37. doi: 10.1249/01.mss.0000174894.05236.45. [DOI] [PubMed] [Google Scholar]
- 39.Williams PT, Satariano WA. Relationships of age and weekly running distance to BMI and circumferences in 41,582 physically active women. Obes Res. 2005;13:1370–80. doi: 10.1038/oby.2005.166. [DOI] [PubMed] [Google Scholar]
- 40.Williams PT, Wood PD, Haskell WL, Vranizan K. The effects of running mileage and duration on plasma lipoprotein levels. JAMA. 1982;24:2674–9. [PubMed] [Google Scholar]