Abstract
In view of recent recognition of the existence of two Herpesvirus hominis (HVH) types with antigenic and biological differences, an electron microscopic study was undertaken of pocks produced on the choriallantoic membrane of embryonated eggs after infection with type 1 and type 2 HVH strains. Besides the typical morphological features of herpesvirus infection noted by several investigators, it was observed that type 2 HVH also produced microtubules measuring approximately 19 nm in both nucleus and cytoplasm. Although the nature of these filamentous structures is still unclear, consideration is given in this paper to the possibility that they may represent viral structural subunits, aberrant forms or neoantigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARHELGER R. B., DARLINGTON R. W., RANDALL C. C. An electron microscopic study of equine abortion virus infection in hamster liver. Am J Pathol. 1963 Jun;42:703–713. [PMC free article] [PubMed] [Google Scholar]
- BERNHARD W., BAUER A., HAREL J., OBERLING C. Les formes intracytoplasmiques du virus fibromateux de Shope; études de coupes ultrafines au microscope électronique. Bull Assoc Fr Etud Cancer. 1954;41(4):423–444. [PubMed] [Google Scholar]
- CHANDLER R. L., HAIG D. A., SMITH K. ELECTRON MICROSCOPY OF A PORCINE ADENOVIRUS. J R Microsc Soc. 1965 Jun;84:133–142. doi: 10.1111/j.1365-2818.1965.tb02115.x. [DOI] [PubMed] [Google Scholar]
- CHITWOOD L. A., BRACKEN E. C. REPLICATION OF HERPES SIMPLEX VIRUS IN A METABOLICALLY IMBALANCED SYSTEM. Virology. 1964 Sep;24:116–120. doi: 10.1016/0042-6822(64)90157-6. [DOI] [PubMed] [Google Scholar]
- DALTON A. J., KILHAM L., ZEIGEL R. F. A COMPARISON OF POLYOMA, "K", AND KILHAM RAT VIRUSES WITH THE ELECTRON MICROSCOPE. Virology. 1963 Jul;20:391–398. doi: 10.1016/0042-6822(63)90087-4. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. R., Nahmias A. J., Harwell R. W., Pauls F. P. Association of antigenic type of Herpesvirus hominis with site of viral recovery. J Immunol. 1967 Nov;99(5):974–980. [PubMed] [Google Scholar]
- FAWCETT D. W. Electron microscope observations on intracellular virus-like particles associated with the cells of the Lucké renal adenocarcinoma. J Biophys Biochem Cytol. 1956 Nov 25;2(6):725–741. doi: 10.1083/jcb.2.6.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodheart C. R., Plummer G., Waner J. L. Density difference of DNA of human herpes simplex viruses, types I and II. Virology. 1968 Jul;35(3):473–475. doi: 10.1016/0042-6822(68)90225-0. [DOI] [PubMed] [Google Scholar]
- HOWATSON A. F., ALMEIDA J. D. An electron microscope study of polyoma virus in hamster kidney. J Biophys Biochem Cytol. 1960 Jul;7:753–760. doi: 10.1083/jcb.7.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnins V. I., Stich H. F., Gregory C., Yohn D. S. Localization of tumor antigens in adenovirus-12-induced tumor cells and in adenovirus-12-infected human and hamster cells by ferritin-labeled antibodies. Cancer Res. 1967 Oct;27(10):1874–1877. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunger P. D. Cytoplasmic filaments and associated Lucké viruses in the frog renal adenocarcinoma. J Morphol. 1967 Sep;123(1):63–69. doi: 10.1002/jmor.1051230106. [DOI] [PubMed] [Google Scholar]
- MATSUMOTO S. Electron microscopy of nerve cells infected with street rabies virus. Virology. 1962 May;17:198–202. doi: 10.1016/0042-6822(62)90099-5. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses as observed in the electron microscope. I. Herpes simplex virus. J Exp Med. 1954 Aug 1;100(2):195–202. doi: 10.1084/jem.100.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., MOORE D. H. An evaluation of host cell changes accompanying viral multiplication as observed in the electron microscope. Ann N Y Acad Sci. 1957 Oct 21;68(2):302–323. doi: 10.1111/j.1749-6632.1957.tb56087.x. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Intranuclear formation of filaments in herpesvirus hominis infection of mice. Arch Gesamte Virusforsch. 1967;21(3):463–468. doi: 10.1007/BF01241746. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Dowdle W. R., Naib Z. M., Highsmith A., Harwell R. W., Josey W. E. Relation of pock size on chorioallantoic membrane to antigenic type of herpesvirus hominis. Proc Soc Exp Biol Med. 1968 Apr;127(4):1022–1028. doi: 10.3181/00379727-127-32861. [DOI] [PubMed] [Google Scholar]
- RECZKO E. [Electron microscopic research on the virus of vesicular stomatitis]. Arch Gesamte Virusforsch. 1961;10:588–605. [PubMed] [Google Scholar]
- RECZKO E. [Electron microscopic study of the abdominal skin of the young pig infected with original swine pox]. Arch Gesamte Virusforsch. 1959;9:193–213. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls W. E., Laurel D., Melnick J. L., Glicksman J. M., Kaufman R. H. A search for viruses in smegma, premalignant and early malignant cervical tissues. The isolation of Herpesviruses with distinct antigenic properties. Am J Epidemiol. 1968 May;87(3):647–655. doi: 10.1093/oxfordjournals.aje.a120855. [DOI] [PubMed] [Google Scholar]
- Reczko E., Böhm H. O., Straub O. C. Zur Feinstruktur des Rhinopneumonitisvirus der Pferde. Arch Gesamte Virusforsch. 1965;17(2):231–250. [PubMed] [Google Scholar]
- Ruebner B. H., Hirano T., Slusser R., Osborn J., Medearis D. N., Jr Cytomegalovirus infection. Viral ultrastructure with particular reference to the relationship of lysosomes to cytoplasmic inclusions. Am J Pathol. 1966 Jun;48(6):971–989. [PMC free article] [PubMed] [Google Scholar]
- SIEGEL B. V. Filamentous-structures in ectromelia virusinfected cells. Nature. 1960 Jun 4;186:820–821. doi: 10.1038/186820a0. [DOI] [PubMed] [Google Scholar]
- Schneweis K. E. Die Typen 1 und 2 des Herpes-simplex-Virus bei verschiedenen Krankheitsbildern. Dtsch Med Wochenschr. 1967 Dec 15;92(50):2313–2314. doi: 10.1055/s-0028-1106138. [DOI] [PubMed] [Google Scholar]
- Siegert R., Falke D. Elektronenmikroskopische Untersuchungen über die Entwicklung des Herpesvirus hominis in Kulturzellen. Arch Gesamte Virusforsch. 1966;19(2):230–249. [PubMed] [Google Scholar]
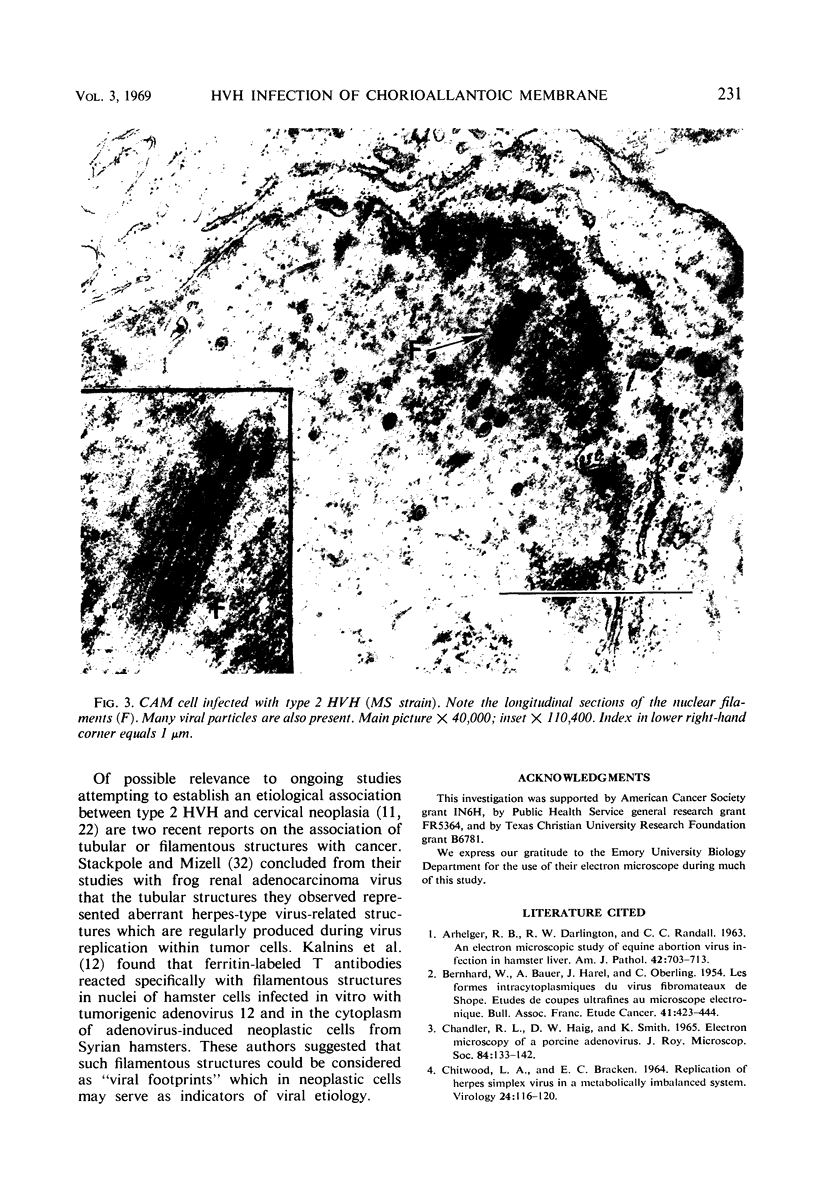
- Stackpole C. W., Mizell M. Electron microscopic observations on herpes-type virus-related structures in the frog renal adenocarcinoma. Virology. 1968 Sep;36(1):63–72. doi: 10.1016/0042-6822(68)90117-7. [DOI] [PubMed] [Google Scholar]
- WATRACH A. M. Intranuclear filaments associated with infectious laryngotracheitis virus. Virology. 1962 Oct;18:324–327. doi: 10.1016/0042-6822(62)90020-x. [DOI] [PubMed] [Google Scholar]
- Zambernard J., Vatter A. E., McKinnell R. G. The fine structure of nuclear and cytoplasmic inclusions in primary renal tumors of mutant leopard frogs. Cancer Res. 1966 Aug;26(8):1688–1700. [PubMed] [Google Scholar]