SUMMARY
We solved the crystal structure of human ARGONAUTE1 (hAGO1) bound to endogenous 5′-phosphorylated guide RNAs. To identify changes that evolutionarily rendered hAGO1 inactive, we compared our structure with guide-RNA-containing and cleavage-active hAGO2. Aside from mutation of a catalytic tetrad residue, proline residues at positions 670 and 675 in hAGO1 introduce a kink in the cS7 loop forming a convex surface within the hAGO1 nucleic-acid-binding channel near the inactive catalytic site. We predicted that even upon restoration of the catalytic tetrad, hAGO1-cS7 sterically hinders the placement of a fully paired guide-target RNA duplex into the endonuclease active site. Consistent with this hypothesis, reconstitution of the catalytic tetrad (R805H) led to low-level hAGO1 cleavage activity, whereas combining R805H with cS7 substitutions P670S and P675Q substantially augmented hAGO1 activity. Evolutionary amino acid changes to hAGO1 were readily reversible, suggesting that loading of guide RNA and seed-based miRNA-target RNA pairing constrain its sequence drift.
Keywords: human Argonaute, insertion elements, RNAi, target cleavage
INTRODUCTION
Argonaute (Ago) proteins and microRNAs (miRNAs) form effector ribonucleoprotein complexes resulting in either mRNA cleavage and/or translational repression, thereby post-transcriptionally regulating gene expression in eukaryotes (Bartel, 2004; Hutvagner and Simard, 2008). Early structural studies focused on laterally transferred prokaryotic, readily expressible Ago proteins, and identified the various structural domains and their roles in guide and target RNA binding and cleavage. The PAZ domain binds the 3′-end of the guide strand (Lingel et al., 2004; Ma et al., 2004), while the MID domain binds the 5′-phosphate of the guide strand (Ma et al., 2005; Parker et al., 2005). The PIWI domain is responsible for the endonucleolytic activity and structurally resembles RNase H enzymes (Parker et al., 2004; Song et al., 2004; Yuan et al., 2005). Further structural studies on Thermus thermophilus (Tt) Ago elucidated the molecular mechanisms underlying nucleation and propagation of base-pairing between guide and target strands (Wang et al., 2008a; Wang et al., 2008b), and the conformational transitions leading to target cleavage (Wang et al., 2009). Given that prokaryotic Ago preferentially binds guide DNA in biochemical studies, in contrast to their eukaryotic counterparts that preferably bind guide RNA, structure-function efforts on Ago proteins have recently turned to eukaryotic systems, despite their difficulty in expression and study.
The first crystal structures of eukaryotic Agos were solved and included the budding yeast Kluyveromyces polysporus (Kp) Ago (Nakanishi et al., 2012), as well as human Argonaute2 (hAGO2) from two groups (Elkayam et al., 2012; Schirle and MacRae, 2012), and most recently also hAGO1 (Faehnle et al., 2013), all with either autonomously- or in vitro-loaded guide RNA. These structures of binary eukaryotic Ago complexes bound to 8–10 nucleotide (nt) 5′ phosphorylated guide RNAs defined the intermolecular contacts involving the 2′-OH groups of guide RNA in a eukaryotic system. Furthermore, sequence-independent interactions involving the sugar-phosphate backbone pre-organized the guide RNA seed segment in a near A-form conformation in all three complexes.
A key discovery following analysis of the structure of the binary complex of KpAgo with bound guide RNA was the identification of a hydrogen-bonded network that stabilizes an expanded and repositioned loop, thereby facilitating insertion of an invariant glutamate into the catalytic pocket (Nakanishi et al., 2012). Compared with Agos captured in inactive states, insertion of this glutamate finger was shown to complete a universally conserved catalytic tetrad, in the process activating KpAgo for target RNA cleavage (Nakanishi et al., 2012). The contribution of the glutamate (E) in the catalytic active Ago conformation went unnoticed in the structures of the binary guide-RNA-bound hAGO2 complexes (Elkayam et al., 2012; Schirle and MacRae, 2012), though closer inspection did also validate the formation of the DEDH catalytic tetrad in this system (Nakanishi et al., 2012).
A primary question in the field has been why amongst the four human AGOs, only hAGO2 cleaves target mRNA (Liu et al., 2004; Meister et al., 2004), while the remaining homologs (hAGO1, 3 and 4) facilitate translational repression. hAGO1, 3, and 4 are located next to each other on chromosome 1, while hAGO2 is on chromosome 8. Presumably, an ancestral AGO underwent gene duplication followed by expansion of one of the duplicated genes. While hAGO2 and hAGO3 both contain the DEDH catalytic tetrad, hAGO2 retained target RNA cleavage activity whereas hAGO3 lost activity and only functions in translational repression; hAGO1 and 4 carry active site mutations. Eukaryotic Agos are also larger than their prokaryotic counterparts and contain conserved (designated cS) and variable insertion elements, of as yet unknown biological functions. These cS elements are also different amongst the hAGO proteins.
We have solved the crystal structure of binary guide-RNA-bound hAGO1 complex, composed of its wild-type (wt) DEDR incomplete catalytic tetrad, and compared it with the crystal structures of binary guide-RNA-bound hAGO2 (wt DEDH tetrad) complexes (Elkayam et al., 2012; Schirle and MacRae, 2012). We observed conformational differences between binary guide-RNA-bound hAGO1 (this study) and hAGO2 (Elkayam et al., 2012; Schirle and MacRae, 2012) complexes within insertion element cS7 (residues 669–675) proximal to the catalytic tetrad. Single and double amino acid changes within cS7 and a single amino acid change of the catalytic tetrad together partially restored catalytic activity of hAGO1. While our structural studies were underway, domain-swapping experiments between active and inactive hAGO proteins identified segments comprising the N-terminus and the cS7 segment partially restoring target RNA cleavage (Hauptmann et al., 2013; Hur et al., 2013). In addition, a recent structural and biochemical study has also highlighted the role of cS7 in obstructing cleavage by hAGO1 (Faehnle et al., 2013).
RESULTS AND DISCUSSION
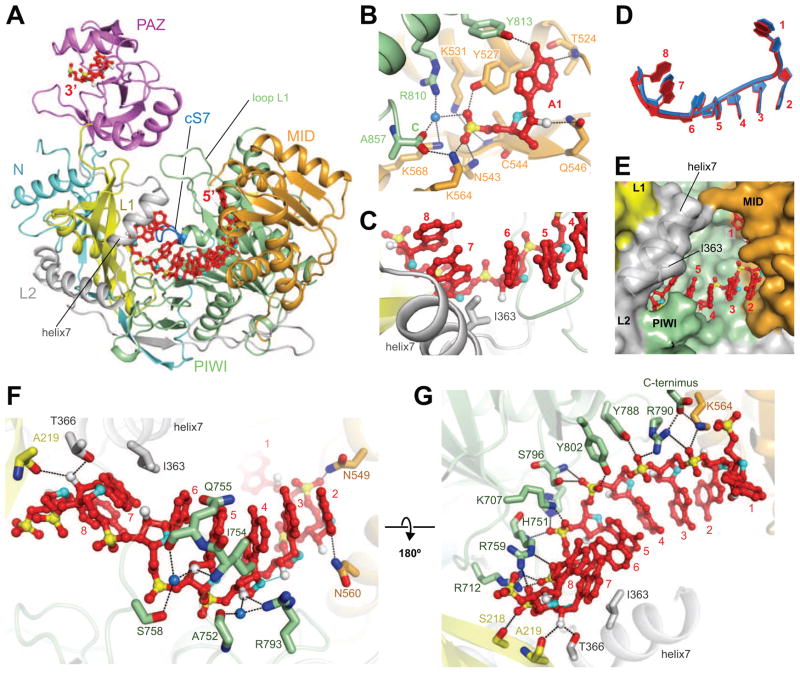
We have solved the 2.3 Å crystal structure of hAGO1 (Figures 1A, S2, and Table S1), expressed from insect cells with stably bound endogenous predominantly 21-nt RNAs (Figure S1). The majority of the hAGO1 protein chain could be traced except for amino acid (aa) segments 1–21, 113–126 and 819–834. hAGO1 adopts a bilobal scaffold with the nucleic acid channel positioned between the N-terminal N and PAZ and the C-terminal MID and PIWI lobes (Figure 1A). We also observed the first eight nucleotide (nt) from the 5′-phosphorylated end and the last two nt from the 3′-end of the bound endogenous RNA, while its middle segment was disordered (Figures 1A and S2A). We generated small RNA cDNA libraries from RNAs naturally loaded into recombinant hAGO1 and hAGO2. The RNAs were of mostly viral origin and predominantly 21-nt in length (Figure S1) with no single sequence read exceeding 0.15% of the total small RNA population (Table S2).
Figure 1.
Crystal Structure of hAGO1.
(A) Structure of hAGO1 bound to guide RNA. hAGO1 with N (cyan), L1 (yellow), PAZ (magenta), L2 (gray), MID (orange), and PIWI (green) domains is drawn in a ribbon representation. Guide RNA positions 1–8 and 20–21 (red) are traceable in the structure of the complex.
(B) 5′-end nucleotide recognition. Residues involving the RNA recognition are shown in a stick representation. A water molecule (blue) is shown as a sphere. The O2′ and O4′ sugar atoms are colored in white and cyan, respectively. Hydrogen bonds are shown as dotted lines. The other color codes are the same as in panel A. Only the first nucleotide is shown for clarity.
(C) A kink is introduced in the bound guide RNA strand by insertion of I363 on helix 7.
(D) Trajectories of hAGO1- and hAGO2-bound guide RNAs. The guide RNAs bound to hAGO1 and to hAGO2 are colored in red and blue, respectively.
(E) Solvent-exposed seed nucleotides 2–4 in the binary complex of guide-RNA-bound hAGO1, with the latter shown in a surface representation. The color codes are as in panel A. Helix 7 and I363 are shown as transparent representations.
(F, G) Guide RNA recognition on the 2′ OH groups (panel F) and backbone phosphate oxygens (panel G). The guide RNA is shown as a ball-stick representation. Residues involved in interactions with the guide RNA are depicted in stick representations. The 2′-OH groups are shown as white-colored spheres in panel F, while water molecules are drawn as blue-colored spheres.
See also Figures S1 and S2.
The terminal 5′-monophosphate of the guide RNA is maximally recognized at the interface between the MID and PIWI domains (Figure 1B), with a water molecule involved in the hydrogen-bonding network surrounding the 5′-phosphate. The base moiety of the 5′-most nt, which stacks on Y527, forms two sequence-specific hydrogen bonds to T524 and Y813, while the 2′-hydroxyl (OH) group of the 5′-end nt forms one hydrogen bond to Q546. Both hAGO1 (this study) and hAGO2 (Elkayam et al., 2012; Schirle and MacRae, 2012) utilize common principles for 5′-phosphate recognition.
The seed region of the guide RNA (pos. 2–8) is pre-organized to be in A-form but its continuity is broken between pos. 6 and 7 as a result of insertion of the side chain of I363 on helix 7 (Figure 1C), a feature observed previously for both KpAgo (Nakanishi et al., 2012) and hAGO2 (Elkayam et al., 2012; Schirle and MacRae, 2012). The guide RNA bound to hAGO1 follows the same trajectory as observed for the guide RNA bound to hAGO2, though pos. 5–7 curve a little more outwards due to the slightly differently positioned helix 7 in the two complexes (Figure 1D). Consistent with hAGO1 and 2 recognizing the seed region in the same manner, the residues directly interacting with the seed segment are completely conserved between both proteins. The position of helix 7 limits the solvent access to the seed region, such that only nucleobases 2–4 are accessible for pairing with target (Figure 1E). It therefore appears that the nucleic-acid-binding channel will need to widen to incorporate target mRNAs, potentially by moving helix 7 outwards, probably together with opening loop L1 that covers the channel (Figure 1A).
The 3′-most two nts of the guide strand are bound by the PAZ domain of hAGO1 as seen in the crystal structure of the isolated PAZ domain of hAGO1 bound to a 9-mer siRNA-like duplex (Ma et al., 2004) and in the binary complex of guide strand-bound hAGO2 complex (Elkayam et al., 2012; Schirle and MacRae, 2012). There is extensive intermolecular recognition of 2′-OH and backbone phosphate oxygens of the bound guide RNA in the binary complex with hAGO1 (Figures 1F and G). The 2′-OH groups at positions 4, 5 and 7 form direct and water-mediated hydrogen bonds with side chains from the PIWI and linker L1 (Figure 1F). The phosphate backbone oxygens extending from positions 3 to 8 form a network of direct hydrogen bonds with the side chains of the PIWI domain (Figure 1G).
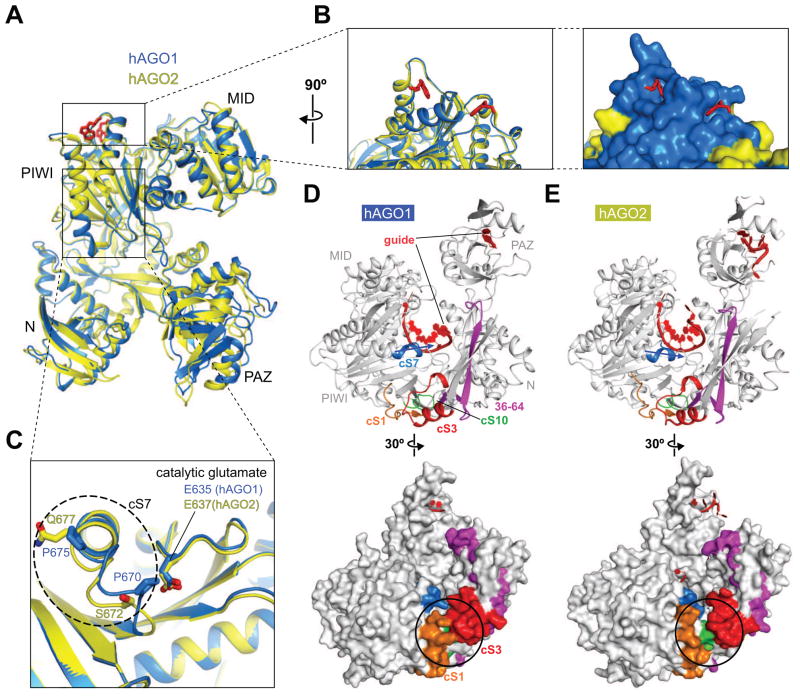
hAGO1 and 2 share more than 82% aa sequence identity and adopt quite similar overall folding topologies (Figures 2A and S3). The residues forming the exterior of the MID-PIWI lobe are well conserved between hAGO1 and 2, as are those that line the nucleic-acid-binding channel. Two tryptophan-binding pockets are located at the surface of the PIWI domain of hAGO2 (Figure 2A) possibly involved in binding to GW repeats of TNRC6 (trinucleotide repeat containing 6) family proteins, which are essential for promoting miRNA-mediated target RNA regulation (Huntzinger and Izaurralde, 2011; Schirle and MacRae, 2012). The corresponding pockets are also found on the surface of the PIWI domain of hAGO1, following superposition of our hAGO1 structure (this study) to the hAGO2 structure (Figure 2B, left panel) (Schirle and MacRae, 2012). The residues around the pockets are well conserved between hAGO1 and hAGO2, suggestive of similar interactions with TNRC6 proteins (Figure 2B, right panel).
Figure 2.
Structural Comparison between hAGO1 and hAGO2.
(A) Structural resemblance between hAGO1 and hAGO2 following superposition of their structures. The current hAGO1 (blue) and the tryptophan-bound hAGO2 (yellow) (PDB ID: 4EI3) structures are superposed on their MID-PIWI lobes. The two tryptophans (red) observed in tryptophan-bound hAGO2 are depicted in stick representations.
(B) The plausible tryptophan-binding pockets on the PIWI domain of hAGO1. The local structures around the tryptophan-binding pockets are quite similar between hAGO2 and hAGO1, as shown for hAGO2 containing a pair of bound tryptophans (left panel). Different amino acids residues between hAGO1 and hAGO2 are colored in yellow on the surface representation of hAGO1 (blue) (right panel).
(C) Structural difference within cS7 between hAGO1 and hAGO2. The cS7 element is shown in a dashed ellipse with hAGO1 in blue and hAGO2 in yellow.
(D, E) Different local structures of the cS cluster. The cS1 (orange), cS3 (red), cS7 (blue), and cS10 (green) form a cluster at the edge of the nucleic-acid-binding channel of hAGO1 (panel D) and hAGO2 (panel E). The N-terminal amino acids 36–64 are colored in magenta. The directionality of cS7 for hAGO1 (panel D, top view) and hAGO2 (panel E, top view) are highlighted with arrows. The different local structures composed of the cS1, cS3 and cS10 for hAGO1 (panel D, bottom view) and hAGO2 (panel E, bottom view) are highlighted with black circles on their surface representations.
Eukaryotic Agos contain multiple insertions into specific positions within the overall protein scaffold. Inserted regions that are conserved throughout all eukaryotic Agos are referred to as conserved segments (cS) (Nakanishi et al., 2012). The crystal structures of KpAgo identified four insertions, labeled cS1, cS3, cS7 and cS10, that cluster at one of the two edges of the nucleic-acid-binding channel and bridge the gap among the N domain, L1 linker and PIWI domain, thereby generating a longer channel than its prokaryotic counterpart.
The cluster of cS regions in hAGO1 (cS1, 20–35: cS3, 139–156: cS7, 669–675; cS10, 735–742) (Figures 2D, top view, and S3) and hAGO2 (cS1, 22–37: cS3, 141–158: cS7, 671–677; cS10, 737–744) (Figures 2E, top view, and S3) exhibits the greatest variability with 25 out of 49 aa residues altered (Figure S3). These differences result in the formation of a hydrophobic core between cS1 and cS3 in hAGO1, thereby positioning the N and PIWI domains in close proximity (black circled segment in Figure 2D, bottom view). In contrast, the corresponding cS1 and cS3 segments in hAGO2 are gapped (black circled segment in Figure 2E, bottom view), thereby separating the N and PIWI domains (Elkayam et al., 2012; Schirle and MacRae, 2012). In other words, the narrow gap between cS1 and cS3 leading upwards into the nucleic acid-binding channel in hAGO2 (Figure 2E, bottom view) is plugged through hydrophobic core formation in hAGO1 (Figure 2D, bottom view), possibly leading to distinct RNA-protein interaction network when accommodating extensively paired target RNAs.
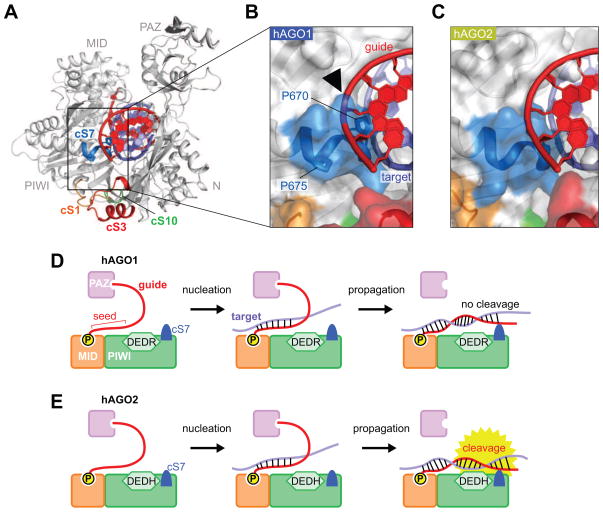
An additional important difference between hAGO1 and 2 is that the loop of cS7 of hAGO1 is tilted at P670 and P675, while no such tilt is observed in hAGO2 whose corresponding residues are S672 and Q677, respectively (Figure 2C). As a result, the cS7 loop sticks out into the space associated with the nucleic-acid-binding channel, thereby likely preventing access of the base-paired target RNA strand to the catalytic E635. To estimate whether cS7 intercepts accommodation of guide-target duplex within the nucleic-acid-binding channel, the crystal structure of the ternary complex of guide-target-duplex-bound TtAgo (PDB ID: 3HK2) was superposed on the MID-PIWI lobe of either hAGO1 or 2 (Figures 3A–C). The docked guide RNA strand on hAGO2 adopts a complementary shape to the channel wall comprised of the PIWI domain (Figure 3C), whereas the docked RNA guide strand on hAGO1 clashes with the cS7 loop (see black arrow, Figure 3B). This suggests that the cS7 loop of hAGO1 obstructs to some extent the nucleic-acid-binding channel and interferes with placement of the fully paired guide-target RNA duplex, thereby potentially preventing the RNA target from reaching the DEDR tetrad in the hAGO1 complex (see model in Figure 3D) in contrast to hAGO2 (see model in Figure 3E).
Figure 3.
cS7 Loop of hAGO1 Serves to Sterically Hinder Guide-Target Accommodation in the Nucleic-Acid-Binding Channel.
(A) hAGO1 modeled with guide-target RNA duplex. The model results from superposition of the current guide RNA-hAGO1 binary complex and the TtAgo guide-target ternary complex as superpositioned on their MID-PIWI domains. hAGO1 (white) is shown as a ribbon representation. The modeled guide and target are colored in red and slate, respectively.
(B, C) The cS7 insertion element of hAGO1 potentially clashes with the bound guide RNA strand. The expanded boxed segments highlight the surface around the cS7 of hAGO1 (panel B) and hAGO2 (panel C). The surface representations are shown as transparent. The black arrowhead points to the potential clash between hAGO1-cS7 and the bound guide RNA strand in panel B. The color code for cS insertion elements is the same as in panel A.
(D, E) Proposed model for RNA target cleavage for hAGO1 (panel D) and hAGO2 (panel E). In hAGO1, the cS7 element protrudes towards guide-target duplex, thereby impacting on cleavage efficacy following release of the target strand from the PAZ domain during the propagation step (panel D). In hAGO2, the cS7 element is recessed, and does not impact on target cleavage. The DEDR and DEDH catalytic tetrads are shown as hexagons. Only the PAZ, MID and PIWI domains are depicted in panels D and E for clarity.
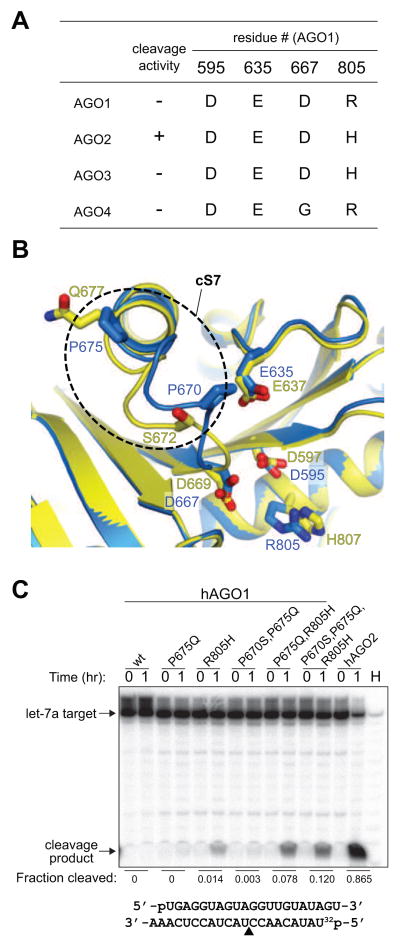
We tested the impact of replacing amino acids in the cS7 insertion element within the context of substituting R805H (Figures 4A, B) to potentiate hAGO1 cleavage activity. Reconstitution of the DEDH catalytic tetrad resulted in the onset of AGO1 guide-dependent RNA cleavage activity (Figure 4C). Replacement of prolines at position 670 and 675 with serine and glutamine, respectively, without an accompanying R805H replacement did not lead to any substantial activity (Figure 4C). However, the P675Q substitution in the presence of the DEDH reconstituted catalytic tetrad, enhanced cleavage activity. Additional replacement of proline to serine (P670S) increased the activity further (7.8% to 12%, Figure 4C). These results support our hypothesis that in hAGO1, the cS7 loop is kinked by prolines (Figure 4B), and replacement of at least one cS7 residue is sufficient for further promoting hAGO1 cleavage activity.
Figure 4.
Cleavage Activity of hAGO1 Mutants.
(A) Alignment of four catalytic residues in hAGO1, hAGO2, hAGO3 and hAGO4.
(B) Structural difference in insertion element cS7 between hAGO1 (blue) and hAGO2 (yellow) drawn in ribbon representation. The cS7 of hAGO1 is highlighted in dotted circle. Important residues on cS7 and the catalytic tetrad are depicted in stick representation.
(C) Cleavage activity of hAGO1 mutants. Baculovirus-expressed and purified recombinant hAGO proteins were loaded with 5′ phosphorylated guide RNAs representing mature hsa-let-7a sequence. Radiolabeled 21-nt RNA, complementary to the let-7a guide was used as cleavage substrate. The cleavage site is located across of the 10th and 11th nucleotide from the 5′ end of the guide RNA, yielding a 9-nt product. Guide-RNA load-normalized quantitation, by fraction cleaved, is shown. Abbreviation: H, alkaline hydrolysis ladder of 5′ labeled target RNA.
Although the substitution of P670S and P675Q in the presence of the DEDH partially converted hAGO1 into a catalytic protein, the cleavage activity was lower than that observed for hAGO2 (12% versus 87%, Figure 4C). This difference could be explained by the recent report that the N-terminal aa 1–64 of hAGO2 also contribute to cleavage activity (Hauptmann et al., 2013). Furthermore, in the same report the authors converted hAGO3 into a catalytically active protein by swapping the domains composed of aa 1–64 (comprising cS1) and 137–160 (cS3), with the corresponding segments of hAGO2 (Hauptmann et al., 2013). Since the hAGO3 cS3 is much longer than the cS3 of any other AGO, its larger size presumably provides an obstacle at the edge of the channel. In summary, variations within eukaryote-specific inserts appear to contribute specialized roles within the nucleic-acid-binding channel for interactions with guide-passenger duplex and/or guide-target duplex. This feature also relates to the proposed involvement of the N domain of hAGO2 in the initiation of unwinding of guide-passenger duplexes (Kwak and Tomari, 2012). Given that the N-terminal 1–21 aa segment is disordered in our crystal structure, we are unable to comment on the specific function of the N-terminus in target-RNA accommodation and cleavage.
Based on the sequence analysis of hAGO proteins, their nucleic-acid-binding channel are conserved and predicted to retain the same shape at the seed segment-binding side, whereas there is more variability proximal to the catalytic site and the exit of the channel. We speculate that this may result in variable placement of extensively base-paired target mRNAs among hAGOs. Considering that only 2 out of the 4 catalytic tetrad residues vary among hAGOs, the first two invariant residues may play additional non-catalytic structural roles involved in target RNA accommodation (Figure 4A).
In summary, we focused on a structural and biochemical comparison between the binary guide-RNA-bound complexes of hAGO1 (this study) and hAGO2 (Elkayam et al., 2012; Schirle and MacRae, 2012). Our work expands beyond the domain swap experiments (Hauptmann et al., 2013), defining a specific subset of single amino acid substitutions within insertion segment cS7 of hAGO1, and also relates to single amino acid substitutions conceived from independently solving hAGO1 crystal structures (Faehnle et al., 2013). Interestingly, the substitution of leucine at pos. 674 of hAGO1 to phenylalanine in cS7 also led to a similar enhancement of target RNA cleavage activity (Faehnle et al., 2013). This position was identified by a systematic mutagenesis screen of residues non-conserved within the PIWI domain of hAGO1 and 2 (Faehnle et al., 2013). F674 forms a strong hydrophobic interaction with the PIWI domain in hAGO2 preventing the cS7 from pointing into the nucleic-acid-binding channel. Restoration of the hydrophobic interaction between cS7 and the PIWI domain is predicted to act similar to P670S and P675Q substitutions in hAGO1. Together, these studies emphasize the critical role of non-catalytic residues within the insertion segment cS7 of hAGO2 for target RNA cleavage.
EXPERIMENTAL PROCEDURES
Purification of hAGO1
The gene of hAGO1 was cloned into pFastBac HTB (Invitrogen) and expressed as His-TEV tag fused protein in High Five cells. The cells were harvested 3 days after infection and suspended with buffer A (10 mM sodium phosphate buffer, pH 7.3, 1.5 M NaCl, 25 mM imidazole, 10 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride). The cell extract was obtained by French Press, followed by ultracentrifugation for 1 h. The supernatant was loaded onto a HisTrap column (GE healthcare) equilibrated with buffer A and was eluted with a linear gradient of 25 mM to 1.5 M imidazole. After dialysis against buffer B (10 mM sodium phosphate buffer, pH 7.3, 0.5 M NaCl, 25 mM imidazole, 10 mM β-mercaptoethanol) in the presence of TEV, the sample was loaded again on a HisTrap column to remove the His-TEV tag. The flow-through fraction was dialyzed against buffer C (20 mM Tris-HCl, pH 8.0, 50 mM KCl and 10 mM β-mercaptoethanol), and then was loaded onto a MonoQ column (GE healthcare) to further remove co-purifying nucleic acids. The eluted sample was loaded onto Superdex 200 (GE Healthcare) equilibrated with buffer D (20 mM Tris-HCl, pH 8.0, 200 mM NaCl, and 5 mM dithiothreitol (DTT)). After concentration, the purified hAGO1 was flash frozen by liquid nitrogen and was stored at −80 °C until crystallization.
Mutations were generated by PCR within the expression vector obtained above. The mutant proteins were prepared as above omitting the MonoQ column step, followed by dialysis against buffer E (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 10% glycerol) for 3 h and then against buffer F (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 5 mM MgCl2, 1 mM DTT, and 50% glycerol) overnight. Cloning and expression of hAGO2 were carried out similarly. All the hAGO1 mutants and hAGO2 were stored at −80 °C until used for the in vitro cleavage assay.
Crystallization Conditions
The purified hAGO1 (9 mg/ml) was mixed with the same volume of the crystallization buffer (96 mM Tris-HCl, pH 7.5, 126 mM lithium sulfate, 29% PEG3350, 16.5% glycerol, 50 mM sodium fluoride, 40 mM sodium citrate tribasic dihydrate, and 100 mM cesium chloride), followed by sitting-drop vapor diffusion at 20 °C. Crystals were directly flash-frozen in liquid nitrogen with the crystallization buffer serving as cryoprotectant.
Structure Determination
We collected diffraction data at NE-CAT 24ID-E and processed it by HKL2000 (Otwinowski, 1997). The phase was obtained by molecular replacement via Phaser-MR (McCoy et al., 2007) using the published hAGO2 structure (PDB ID: 4EI1) as a search model. The initial map showed a continuous electron density positioned within the nucleic-acid-binding channel. Iterative model building and refinement for the protein part using Coot (Jones et al., 1991) and Phenix (Adams et al., 2002), respectively, resulted in the ambiguous electron densities for the 5′-end eight nucleotides within the channel and 3′-end two nucleotide in the PAZ domain. The RNA was modeled as poly A and U chains for the 5′-end eight nucleotides and for the 3′-end two nucleotides, respectively. Simulated-annealing Fo-Fc omit map was calculated by CNS (Brunger, 2007). The Ramachandran plot analysis for hAGO1 shows N737 to be in a disallowed region (Figure S2B). The corresponding residue, K739, of hAGO2 is also in a disallowed region in all the crystal structure (Figure S2C; PDB IDs: 4EI1, 4EI3 and 4F3T).
Isolation of Small RNAs from T. ni Cells and Purified hAGO1 and hAGO2 Proteins
Total RNA was isolated from approx. 1 million High Five (T. ni) uninfected or infected (with AGO1 baculovirus) cells using Trizol (Invitrogen) per manufacturer’s instructions. Additionally, 10 μg of purified hAGO proteins were subjected to Trizol RNA extraction. Barcoded small RNA cDNA library preparation and sequencing was performed essentially as described (Hafner et al., 2011). RNA inputs of 2 μg (from T. ni cells) or 20 ng (AGO isolated RNAs) were used per sample. Alignment was performed using Bowtie2 (Langmead and Salzberg, 2012). Annotation was accomplished using custom Perl scripts with the following reference sequence sources: ACMPNV genome (Ayres et al., 1994), D. melanogaster genome (FB2013_03, except for rRNA which was added from ENTREZ NCBI), and an internally-curated human tRNA database, based originally on (Lowe and Eddy, 1997). The sequencing data in this publication have been deposited in the NCBI’s Gene Expression Omnibus (GEO).
hAGO Cleavage Assay
21-nt hsa-let-7a guide, 5′-pUGAGGUAGUAGGUUGUAUAGU, and target oligoribonucleotides, 5′-UAUACAACCUACUACCUCAAA, were used for hAGO cleavage assays. hAGO loading was performed by incubating 100 nM of 5′-phosphorylated let-7a guide RNA with 550 nM of AGO at 37°C for 1 h in 25 mM HEPES-KOH, pH 7.5, 5 mM MgCl2, 50 mM KCl, 5 mM DTT, 0.2 mM EDTA. Following loading, 50 nM of 5′ 32P-labeled let-7a target RNA and 0.5 μg/μL yeast tRNA were added, and the reaction mixture incubated at 37 °C for up to 1 h. Reactions were terminated by addition of 8 M urea containing gel-loading dye and cleavage products were resolved on an 18% denaturing polyacrylamide sequencing gel, visualized by phosphorimager (Typhoon FLA-9500, GE Healthcare), and quantified using ImageJ (v1.47i). Alkaline hydrolysis of radiolabelled let-7a target was performed using 0.1 M Na2CO3, pH 10.7 at 95°C for 30 s in the presence of 1.2 μg/μL yeast tRNA. Hydrolysis products with 2′,3′-cyclic phosphate and 2′ or 3′ monophosphate termini resolve as doublets whereas RNA cleavage products containing 3′ hydroxyl ends run as single bands.
Supplementary Material
Highlights.
Overall structure of human AGO1 is extremely similar to that of human AGO2.
Eukaryote-insertion segments form different local structures at the channel exit.
Alteration of catalytic tetrad and of cS7 sequence restores RNase activity.
Acknowledgments
We thank the staff of the NE-CAT beamline at the Advanced Photon Source for assistance. This work was supported by National Institute of Health grants to D.J.P and T.T.
Footnotes
ACCESSION NUMBERS
The coordinates and structure factors for the guide-RNA-bound hAGO1 have been deposited in the Protein Data Bank with accession codes 4KXT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- Ayres MD, Howard SC, Kuzio J, Lopez-Ferber M, Possee RD. The complete DNA sequence of Autographa californica nuclear polyhedrosis virus. Virology. 1994;202:586–605. doi: 10.1006/viro.1994.1380. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Brunger AT. Version 1.2 of the Crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- Elkayam E, Kuhn CD, Tocilj A, Haase AD, Greene EM, Hannon GJ, Joshua-Tor L. The structure of human argonaute-2 in complex with miR-20a. Cell. 2012;150:100–110. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faehnle CR, Elkayam E, Haase AD, Hannon GJ, Joshua-Tor L. The Making of a Slicer: Activation of Human Argonaute-1. Cell Reports published online on June. 2013;27:2013. doi: 10.1016/j.celrep.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Renwick N, Brown M, Mihailovic A, Holoch D, Lin C, Pena JT, Nusbaum JD, Morozov P, Ludwig J, et al. RNA-ligase-dependent biases in miRNA representation in deep-sequenced small RNA cDNA libraries. RNA. 2011;17:1697–1712. doi: 10.1261/rna.2799511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann J, Dueck A, Harlander S, Pfaff J, Merkl R, Meister G. Turning catalytically inactive human Argonaute proteins into active slicer enzymes. Nat Struct Mol Biol. 2013 doi: 10.1038/nsmb.2577. [DOI] [PubMed] [Google Scholar]
- Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12:99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- Hur JK, Zinchenko MK, Djuranovic S, Green R. Regulation of Argonaute slicer activity by guide RNA 3′ end interactions with the N-terminal lobe. J Biol Chem. 2013;288:7829–7840. doi: 10.1074/jbc.M112.441030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kwak PB, Tomari Y. The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat Struct Mol Biol. 2012;19:145–151. doi: 10.1038/nsmb.2232. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingel A, Simon B, Izaurralde E, Sattler M. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nat Struct Mol Biol. 2004;11:576–577. doi: 10.1038/nsmb777. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JB, Ye K, Patel DJ. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature. 2004;429:318–322. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JB, Yuan YR, Meister G, Pei Y, Tuschl T, Patel DJ. Structural basis for 5′-end-specific recognition of guide RNA by the A. fulgidus Piwi protein. Nature. 2005;434:666–670. doi: 10.1038/nature03514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Weinberg DE, Bartel DP, Patel DJ. Structure of yeast Argonaute with guide RNA. Nature. 2012;486:368–374. doi: 10.1038/nature11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski ZMW. Processing of X-ray diffraction data collected in oscillation mode. Meth Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Parker JS, Roe SM, Barford D. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 2004;23:4727–4737. doi: 10.1038/sj.emboj.7600488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JS, Roe SM, Barford D. Structural insights into mRNA recognition from a PIWI domain-siRNA guide complex. Nature. 2005;434:663–666. doi: 10.1038/nature03462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–1040. doi: 10.1126/science.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- Wang Y, Juranek S, Li H, Sheng G, Tuschl T, Patel DJ. Structure of an argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature. 2008a;456:921–926. doi: 10.1038/nature07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Juranek S, Li H, Sheng G, Wardle GS, Tuschl T, Patel DJ. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature. 2009;461:754–761. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sheng G, Juranek S, Tuschl T, Patel DJ. Structure of the guide-strand-containing argonaute silencing complex. Nature. 2008b;456:209–213. doi: 10.1038/nature07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan YR, Pei Y, Ma JB, Kuryavyi V, Zhadina M, Meister G, Chen HY, Dauter Z, Tuschl T, Patel DJ. Crystal structure of A. aeolicus argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol Cell. 2005;19:405–419. doi: 10.1016/j.molcel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.