Abstract
The relationship between serotonin (5-HT) and major depressive disorder (MDD) has been extensively studied but certain aspects are still ambiguous. Given the evidence that 5-HT neurotransmission is reduced in depressed subjects, it is possible that one or more of the 5-HT regulators may be altered in the dorsal raphe nucleus (DR) of depressed subjects. Candidates that regulate 5-HT synthesis and neuronal activity of 5-HT neurons include intrinsic regulators such as tryptophan hydroxylase 2 (TPH2), 5-HT autoreceptors, 5-HT transporter (SERT) and transcription factors, as well as afferent regulators such as estrogen and brain-derived neurotrophic factor (BDNF). The present study was designed to quantify mRNA concentrations of the above 5-HT regulators in an isolated population of 5-HT-containing DR neurons of MDD subjects and gender-matched psychiatrically normal control subjects. We found that mRNA concentrations of the 5-HT1D receptor and the transcription factors, NUDR and REST, were significantly increased in DR-captured neurons of female MDD subjects compared to female control subjects. No significant differences were found for the transcripts in male MDD subjects compared to male controls. This study reveals sex-specific alterations in gene expression of the presynaptic 5-HT1D autoreceptors and 5-HT-related transcription factors, NUDR and REST, in DR neurons of women with MDD.
Keywords: Dorsal raphe nucleus, Laser capture microdissection, Major depressive disorder, messenger RNA, Serotonin receptors, Transcription factors
Introduction
Major depressive disorder (MDD) is a common, recurring and debilitating mood disorder. Considerable evidence from clinical in vivo imaging and pharmacological challenge studies as well as human postmortem studies has accumulated to suggest that the serotonin (5-HT) system is disrupted in subjects with MDD. The dorsal raphe nucleus (DR) in the midbrain contains about 50% of the brain’s serotonin neurons and the majority of forebrain 5-HT axons and terminals arise from the midbrain DR (Tork 1990). Several key regulators of 5-HT neurotransmission reside in the DR including the rate-limiting enzyme, tryptophan hydroxylase 2 (TPH2; EC1.14.16.4), the serotonin transporter (SERT) and the 5-HT1A autoreceptor and these targets have been the focus of investigations aimed at elucidating the alterations in serotonin neurotransmission in MDD.
Several studies have examined TPH2 in the DR of depressed or suicide subjects. A number of reports from the same group found that TPH2 mRNA levels are increased and the number of TPH-immunoreactive neurons are increased in the DR in depressed suicide subjects (Underwood et al. 1999; Boldrini et al. 2005; Bach-Mizrachi et al. 2006; Bach-Mizrachi et al. 2008). However, Bonkale and colleagues reported no change in TPH-immunoreactivity in individual subnuclei of the DR between depressed suicide and control subjects, which indicates no alteration in the TPH protein in depressed suicide subjects (Bonkale et al. 2004). Similarly, a study of Nissl-stained sections of the DR found that the number of neurons and volumes in the entire DR were not significantly different between the mood disorder group and control group, and only the ventrolateral subnucleus of the DR showed a 31% reduction in the number of neurons in the mood disorder group relative to controls (Baumann et al. 2002). These studies reveal conflicting results regarding the role of TPH2 in DR neurons of subjects with depression.
The SERT has been another serotonin substrate investigated in depressed subjects. Although a previous report found that the cellular levels of SERT mRNA in the DR was greater in suicide victims (Arango et al. 2001), there are several studies which show opposite results such as a radioligand binding assay of SERT which revealed no changes in SERT distribution and binding sites in the DR between suicide victims with depression and control subjects (Bligh-Glover et al. 2000). Two other studies reported that SERT mRNA expression or the number of SERT binding sites in the DR was not different between those who committed suicide and control subjects (Little et al. 1997; Anisman et al. 2008).
5-HT1A receptors located in the DR represent somatodendritic autoreceptors which regulate the activity of DR neurons; hence a dysfunction of these receptors has been intensely investigated in subjects with MDD. Stockmeier and colleagues (1998) reported that 5-HT1A agonist binding was significantly increased in specific subnuclei of the DR of subjects with MDD (Stockmeier et al. 1998). In contrast, Arango et al. (2001) subsequently reported a decrease in 5-HT1A receptor “binding capacity” in the dorsal raphe of depressed suicide victims relative to controls (Arango et al. 2001). However, a more recent report from these investigators contradicts their earlier finding and reveals an increase in 5-HT1A autoreceptor binding sites in the rostral dorsal raphe of suicide subjects (Boldrini et al., 2008) which is consistent with the original Stockmeier et al. (1998) report. Other findings include an in vivo imaging study that reported decreased [11C]WAY 100635 binding in the brainstem region of the DR in elderly depressed patients which provides further evidence of altered 5-HT1A autoreceptor function in depression (Meltzer et al. 2004).
More recent studies have identified novel regulators of serotonin function. These include a nuclear protein complex, nuclear deformed epidermal autoregulatory factor-1 (Deaf-1) or the human homolog, NUDR, FRE under dual repression binding protein-1 (Freud-1) and RE-1 silencing transcription factor (REST) also known as neuron-restrictive silencing factor (NRSF). These transcription factors bind to the specific repressor sequence in the 5-HT1A promoter and lead to reduction in 5-HT1A receptor transcriptional activity as well as protein expression. A recent study also found that the RE-1 sequence is present within the promoter region of the TPH2 gene (Patel et al. 2007) which can bind REST and repress transcriptional activity of TPH2. In addition, there are several afferent regulators whose terminals innervate the DR and regulate DR neuronal activity. For example, BDNF by its action on tropomyosine-related kinase B receptors (TrkB) and estrogen by its action on estrogen receptors (ERα and ERβ) have been reported to exert profound effects on serotonin DR neurons and serotonin neurotransmission (Joffe and Cohen 1998; Mattson et al. 2004; Shively and Bethea 2004; Lasiuk and Hegadoren 2007; Martinowich and Lu 2008).
The aforementioned regulators all have been shown to regulate various parameters of serotonin function and therefore may be involved in the pathophysiology of major depressive disorder. There have been no previous studies which have measured the cellular expression of all of the above 5-HT-related regulators in isolated DR neurons of both male and female subjects with MDD using a laser-capture microdissection (LCM) approach. The present study was designed to quantify the cellular concentration of mRNA transcripts of several 5-HT-related genes in a pure population of serotonin-containing DR neurons in female and male subjects diagnosed with MDD and in psychiatrically-normal control subjects matched for gender using LCM of immunofluorescently-stained neurons and quantitative real time PCR.
Materials and Methods
Subjects
All procedures in our study were approved by the Institutional Review Board of the University of Mississippi Medical Center and University Hospitals of Cleveland. Human brain specimens were obtained in the course of routine autopsies conducted at the Cuyahoga County Coroner’s Office, Cleveland, OH, after obtaining written consent from the legally defined next-of-kin. Blood and urine samples from all subjects were examined by the coroner’s office for psychotropic medications and substances of abuse. Subjects included 6 female and 6 male subjects diagnosed with MDD. The 6 female and 6 male control subjects never met criteria for an Axis I illness and had no history of a neurological disorder. Each MDD subject was matched with a control subject for gender and as closely as possible for age and post-mortem interval (PMI). Some of the pairs were also matched for race. The demographics for each subject are summarized in Table 1.
Table 1.
Demographic characteristic of human subjects
| Pair | Control Subjects | MDD subjects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex/Race/Age (Yr) | PMI | pH | Cause of death | Age/Race/Age (Yr) | PMI | pH | Cause of death | Age of onset | Suicide | Episodes single/multiple | |
| Females | |||||||||||
| 1 | F/B/51 | 22 | 6.3 | Heart | F/W/56 | 17 | 6.7 | Atherosclerotic aneurism | 37 | No | M |
| 2 | F/W/83 | 25 | 6.7 | Heart | F/W/87 | 24 | 6.6 | Aortic aneurism | 46 | No | M |
| 3 | F/W/49 | 29 | 6.6 | Heart disease | F/W/48 | 24 | 6.1 | CO poisoning | 34 | Suicide | M |
| 4 | F/B/80 | 21 | 6.8 | HCD | F/W/72 | 19 | 6.6 | Drowning | 33 | Suicide | S |
| 5 | F/W/46 | 24 | 6.3 | Homicide | F/W/50 | 28 | 6.5 | Heart disease | 30 | No | M |
| 6 | F/W/67 | 16 | 6.4 | Heart | F/W/67 | 24 | 5.6 | Acute bronchopneumonia/diabetic mellitus | 40 | No | S |
| Males | |||||||||||
| 7 | M/B/53 | 23 | 6.8 | Electrocution | M/W/65 | 30 | 6.3 | SIGSW to chest | 65 | Suicide | S |
| 8 | M/W/78 | 22 | 6.8 | Severe ACD w/MI and cardiomegaly. | M/W/82 | 12 | 6.5 | CO | 73 | Suicide | M |
| 9 | M/W/74 | 21 | 6.6 | Abdominal aortic aneurysm | M/W/64 | 26 | 6.9 | SIGSW head | 63 | No | S |
| 10 | M/H/43 | 22 | 6.6 | Crushing impacts to head, trunk and extremities | M/W/47 | 11 | 6.8 | SIGSW head | 20 | Suicide | M |
| 11 | M/W/37 | 17 | 6.5 | acute hemorrhagic pancreatitis due to choledocholithiasis | M/W/36 | 11 | 6.9 | Undetermined | 35 | No | M |
| 12 | M/W/77 | 24 | 6.6 | Heart | M/W/81 | 33 | 6.8 | Drowning | 81 | Suicide | S |
MDD, Major depressive disorder; F, female; M, male; W, white; B, black; H, Hispanic; PMI, post-mortem interval in hours; Episodes (S, single; M, multiple); HCD, hypertensive cardiac disease; ACD, atherosclerotic cardiovascular disease; CO, carbon monoxide; MI, Myocardial Infarctions; SIGSW, self-inflicted gunshot wound.
Retrospective, informant-based psychiatric assessments were performed for all depressed and control subjects as previously described (Stockmeier et al. 2004). About 3 months after the death of the subjects, a trained interviewer administered the Schedule for Affective Disorders and Schizophrenia: Lifetime Version (SADS-L;(Endicott and Spitzer 1978)) to knowledgeable next-of-kin of the depressed subjects, as previously described (Stockmeier et al. 1997). Axis I psychopathology was independently assessed by a clinical psychologist and psychiatrist and consensus diagnosis was reached in conference using information from the interview, previous hospitalizations, doctors’ records, and the coroner’s office. Responses from the subjects evaluated with the SADS-L were also recorded in the Structured Clinical Interview for DSM-IV Psychiatric Disorders (SCID;(First MB 1996)), and regardless of the structured diagnostic interview used, all subjects met criteria for MDD according to the Diagnostic and Statistical Manual of Mental Disorders IV (American Psychiatric Association, 1994). All 6 female depressed subjects met DSM-IV criteria for an Axis I diagnosis of MDD, all 6 met this criteria for MDD in the last month of life. Four of the female MDD subjects had multiple depressive episodes during their life and two female subjects had a single episode. Six male depressed subjects met DSM-IV criteria for an Axis I diagnosis of MDD, and all 6 met this criteria for MDD in the last month of life. Three male subjects had multiple depressive episodes during their life and three male subjects had a single episode. Five females and two male MDD subjects had an antidepressant prescription at the time of death, but toxicology screening of these subjects were negative for any antidepressant and no other psychotropic drug was detected in any of the subjects. The mean age of onset of depression for females was 36.67±2.33 yrs and for males was 56.17±9.63 yrs whereas the average duration of age of illness for females was 26.67±4.79 yrs and males was 6.33±4.36 yrs.
Tissue sampling
The study was carried out on frozen tissue blocks of midbrain containing the DR which were immediately frozen after autopsy and stored at −80°C. Before and after sectioning each block of tissue - the blade, blade holder, anti-rolling blade and area inside the cryostat were wiped with 95% ethanol and RNase wipes to avoid cross-contamination and RNase contamination. In order to ensure that sections were collected consistently from DR, reference slides (20μm) were collected at regular intervals of 240μm which were used for Nissl staining. Midbrain sections (10μm) were collected on Silane-Prep glass slides (Sigma-Aldrich, USA) at −20 °C in a Leica cryostat CM3050S (Leica Microsystem, Nussloch, Germany). During sectioning, the tissue mounted slides were immediately placed in a microslide box on dry ice and then transferred to −80 °C freezer for storage.
Immunofluorescence staining tissue sections for LCM
A quick staining protocol was standardized to locate and identify the serotonin-containing neurons and to preserve the integrity of RNA. All solutions used were cold (0 °C) during the staining protocol except the dehydration solutions. The solutions are also treated with ProtectRNA™ RNase Inhibitor (Sigma-Aldrich, USA). Slide-mounted tissue sections were removed from the −80°C freezer and immediately immersed into 4% parafomaldehyde solution for 2 min followed by 10 sec wash with 1X PBS. The slides were then placed on a cooling block (Molecular Devices, CA, USA) and a liquid repellent boundary was marked around the tissue section using liquid repellent slide marker (Super pap pen, Tokyo, Japan). The cold slides were then incubated in blocking buffer for 3 min. followed by incubation with primary monoclonal anti-tryptophan hydroxylase-2 antibody (WH3; IgG3, 1:50; Sigma-Aldrich, USA,) for 2 min. This was followed by 10 second 1X PBS wash and again the slides were placed back on a cooling block before being stained with Cye 5 fluorescently tag secondary anti-mouse antibody (anti-mouse; against IgG3; 1:100; Jackson Laboratories) for 2 minute and then washed for 10 sec with 1X PBS. The tissue sections were immediately dehydrated using increased concentration of alcohol (75% and 95%) for 30 seconds each and two immersions in 100% alcohol for 1 min each, followed by xylene for 5 minute. The sections were allowed to air dry for 10 minutes in a fume hood and immediately transferred to a vacuum sealed desiccator. Slides were stored in the desiccator until processed with the LCM to capture DR neurons from the tissue sections.
Laser Capture Microdissection
The TPH2 immunofluorescent-stained neurons were visualized using a fluorescence filter and captured using the infrared laser beam of the Veritas laser microdissection system (Molecular Devices, CA, USA) which does not affect the RNA integrity of captured cells. This method also allows us to capture a pure population of 5-HT-containing neurons without contamination from non-serotonergic cells and surrounding neuropil in the DR. Laser capture parameters were 30–45mW power, with a 2500μsec pulse, and a spot diameter of 10–15μm. Approximately 1500 TPH2-positive cells were captured from three tissue sections of the DR nucleus for each subject. The anatomical level of the sampled tissue sections corresponded to the mid-rostral level of the DR where all DR subnuclei are visible and the trochlear nucleus is present. Each control and MDD matched-paired tissue section and captured cells were processed in parallel to avoid any variance due to reagents and handling.
RNA isolation
Total RNA from captured cells was isolated using the PicoPure RNA isolation kit following the manufacturer’s protocol (Molecular Devices, CA, USA). To eliminate genomic DNA contamination, RNA samples were treated with RNase-free DNase I as described by the manufacturer (Qiagen, Valencia, CA, USA). RNA was eluted in two steps with 14μl of elution buffer, bringing the final volume up to 26 μl after elution. A 1 μl aliquot of each sample was processed for RNA integrity analysis on an Agilent Bioanalyzer 2100 with Agilent Lab-on-a-Chip Picochip RNA kit (Agilent technologies, Foster City, CA, USA). RNA integrity number (RIN) was used to assess the RNA quality of LCM samples as previously described (Schoor et al. 2003; Fleige and Pfaffl 2006; Schroeder et al. 2006). The mean RIN value for the LCM samples was 6.3±0.7. There was no effect of immunofluorescent-staining, PMI, pH, age, sex or race on RIN number. Similarly, there was no difference in the RIN value of depressed and their matched control subjects.
cDNA synthesis
After isolation of RNA, all samples were immediately processed for reverse transcription (RT) reaction to avoid potential RNA degradation. cDNA was synthesized from 50ng total RNA isolated from each LCM sample using Sensiscript RT kit manufacturer specification (Qiagen, Valencia, CA, USA). To avoid the bias toward the 3′ amplification, a mixture of random and oligo dT primer was used for RT reaction. The RT reaction cDNA was stored at −20°C until further use.
Primer design
All sequences for genes of interest were downloaded from the NCBI nucleotides data base. Vector NTI (Invitrogen Corporation, Carlsbad, CA) and IDT web-based primer design software (Integrated DNA Technologies, Coralville, IA) were used to design primers. The sequences of primers, location of each primer and gene bank accessory number is shown in Table 2. The specificity of each primer was confirmed by running the PCR product on an agarose gel and followed by cloning the amplified sequence using pCR 2.1 TOPO vector from each of these primer pairs and confirming the cloned sequence by DNA sequencing (data not shown).
Table 2.
Primer sequences
| Gene | Transcript access number. | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | PCR Product length (bp) |
|---|---|---|---|---|
| 5-HT1A | NM_000524 | CCGTCATTTACGCATACTTC | CAC TGG CGG CAG AAC TTA CAC | 79 |
| 5-HT1B | NM_000863 | TTGTGTGTTG GCTACCCTTC TTC | AAC GTA TCA GTT TAT GGA ATG CTT G | 186 |
| 5-HT1D | NM_000864 | AGGAAGGAGCCAAATGTGTG | GTGGCATTCAGGGATCTGTT | 118 |
| SERT/5HTT/SLC6A4 | NM_001045 | T GGGTACTCAG CAGTTCCAAG | TCC ACA GCA TAG CCA ATC ACT | 159 |
| NUDR/Deaf-1 | NM_021008 | GTTGGTCA ATGGGCTGGA G | CCT GGT TTG TGG CAG CTT CTC | 152 |
| Freud-1/CC2D1A | NM_017721 | GACA ATCAGCGGAC AATCGG | TGT GCA AAC AGG ACT CTC CAG | 156 |
| REST | NM_005612 | TGTCCTTACTCAAGTTCTCAGAAGA | GAGGCCACATAACTGCACTG | 101 |
| TPH2 | NM_173353 | AAATACTGGGCACGGAGAGGGT | ACAACTGCTGTCTTGCCACTTTCG | 170 |
| TPH1 | NM_004179 | CCAAAGAAGATTTCTGACCTGGACC | ATACTCTCTGCAAGCATGGGTTGG | 254 |
| TPH2-T | AK094614 | T AGACTCATTT ACGAAAATTC ATGTGTGCAA | AGTCATCTGACATACGTGGCTTGACG | 153 |
| ERα | NM_000125 | TTTGACCCTCCATGATCAGGTCCA | ACACAAACTCCTCTCCCTGCAGAT | 233 |
| ERβ | NM_001437 | GACATGCTCCTGGCAACTACTTCA | GGCAATCACCCAAACCAAAGCATC | 195 |
| TrkB | NM_006180 | ACCAATCACACGGAGTACCAC | GTGTCCCCGATGTCATTCGC | 220 |
| TREK-1 | NM_001017424 | TGGCTGTGTACTCTTTGTGGCTCT | ATCCAGAACCACACGACAGGCTTA | 183 |
| GAPDH | NM_002046 | AAGGTCGGAGTCAACGGATTTGGT | TGATGACAAGCTTCCCGTTCTCAG | 196 |
| RP-II | X74870 | GCACTTGCCACAGACAGACAACAA | AGCCATCAAAGGAGATGACGTGGT | 245 |
Real time PCR
The 1ul cDNA was subjected to Real-Time/Quantitative Polymerase Chain Reaction (Q-PCR) analysis for the quantification of mRNA transcripts for 5-HT1A, 5-HT1B, 5-HT1D, Freud-1, NUDR, REST, TPH1, TPH2, TPH2-T, SERT, ERα, ERβ, TrkB and TREK-1 using gene specific primers (Table 2). Q-PCR was carried out in a 96 well format using MyiQ Single color real time PCR cycler (BioRad, Hercules, CA, USA), and iQ SYBR Green PCR Master Mix at annealing temperature of 55 °C (BioRad, Hercules, CA, USA). The Q-PCR products were checked by performing a melting curve analysis and running Q-PCR products on 2% agarose gel containing ethidium bromide. A concentration curve with known concentrations of plasmid DNA containing the specific primer pair amplified sequence was used to calculate the primer pair’s efficiency for each of the primer pairs including internal controls. The primer efficiency for each primer pair in Table 2 was between 95 and 105%. The final relative concentration of each transcript was calculated using both glyderaldehyde 3-phosphate dehydrogenase (GAPDH) and RNA polymerase II (RP-II) as internal control genes for normalization with ΔΔCt method. Conversion of ΔCt value to fold changes (2−ΔΔCt) were calculated for each transcript (Livak and Schmittgen, 2001).
Several reports have shown that specific reference genes may be regulated under certain conditions and may therefore be unsuitable for normalization purposes (Dheda et al. 2004). Also some reports show that use of a single internal standard gene as a reference gene or ribosomal RNA gene may lead to faulty results (Tricarico et al. 2002). Therefore, a combination of genes were used as internal standards, in which one was the general reference gene, GAPDH and other was the ribosomal gene RP-II since RP-II is minimally influenced by chemical stimulation and has resistance to cellular activation (Radonic et al. 2004). We used the geometric mean as the normalization factor (Vandesompele et al. 2002).
Statistical analysis
Data for real time PCR was analyzed using a matched-pairs design. For real time samples, three different individual runs were performed with each triplicate sample. Statistical tests compared MDD to matched control separately for female and male groups and as well as for the combined gender groups. Similar analyses were conducted for all genes. A maximum-likelihood mixed-models test was used to estimate parameters of the models, assuming pairs and subjects within pairs were random components (SAS; Version 9.1). As a first step, unadjusted models were fit to compare depressives vs. controls without adjusting for potential confounders. Gender-specific adjusted models included the main effect for comparing depressives vs. controls and covariates for age, PMI, pH and cause of case death (suicide vs. non-suicide). Interactions between the main effect, depressives vs. controls, and each of the potentially confounding covariates were investigated and dropped from the model. The covariate adjusted analyses produced similar results; the results for the unadjusted model are reported for simplicity. Results for the depressed and control groups are reported as mean ± SEM based on the mixed model. Results for main effects are considered significant if p<0.05.
Results
TPH2 immunofluorescent staining of serotonin DR neurons
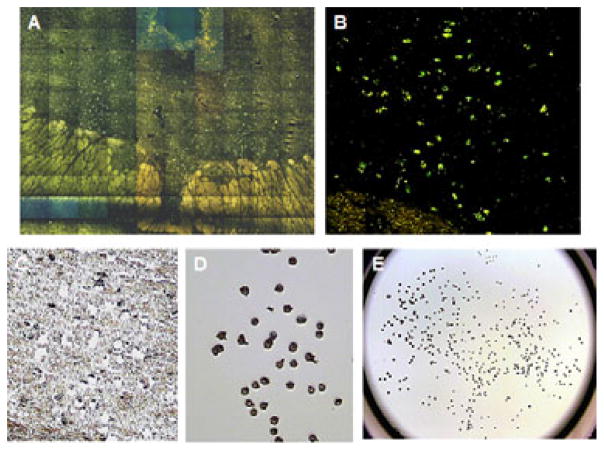
Using a rapid staining protocol, serotonin neurons in the DR exhibited robust immunofluorescence with the primary monoclonal anti-tryptophan hydroxylase 2 antibody resulting in a crisp contrast image that allowed easy identification and sampling of stained neurons from other background neurons and neuropil (Figs. 1A and B). This sampling method greatly reduced potential cross-contamination from the area surrounding the serotonin neurons. Individual TPH2-stained cells were selected from the DR nucleus and the laser fired on those selected serotonin cells. An area of remaining intact tissue can be seen after the selected serotonin cells were captured from a DR tissue section (Fig 1C). The selected serotonin neurons adhered to the base of polymer cap after the capture laser fired on selected serotonin cells (Fig. 1D). This process is performed on the entire DR nucleus within a section allowing the collection of the maximum number of serotonin neurons at the mid-rostral anatomical level (Fig. 1E).
Figure 1.
Representative photomicrographs of dorsal raphe nucleus, immunofluoroscent-stained cells and captured neurons obtained from the laser-capture microdissection instrument. A. High magnification (20x) composite image of entire DR illustrating TPH2-immunofluoroscent stained neurons. B. Photomicrograph of TPH2-immunofluoroscent labeled DR neurons (20x). C. Brightfield photomicrograph of tissue section after the TPH2-stained neurons were captured (20x). D. Photomicrograph of laser-captured serotonin DR neurons adhered to the cap after LCM processing of tissue section in C (20x). E. Photomicrograph illustrating laser-captured serotonin DR neurons adhered to the entire cap after LCM processing of the tissue section.
Tryptophan hydroxylase isoforms
There was no significant difference in mean mRNA concentrations of TPH1, TPH2 or truncated TPH2 (TPH2-T) isoforms in laser-captured DR neurons of female or male MDD subjects compared to gender-matched control subjects (Table 3 and Table 4). Similarly, no difference in expression of the above transcripts was found when gender groups were combined and analyzed between MDD and control subjects (Table 5). The truncated form of TPH2-T was expressed in the serotonin neurons but in a very low concentration as compared to TPH1 and TPH2 mRNAs when the expression is normalized to internal control gene GAPDH and RP-II.
Table 3.
Δ Ct value and Fold change for female subjects.
| Genes | Δ Ct Control (Mean±SEM) | Δ Ct MDD (Mean±SEM) | Fold Change (Mean±SEM) | p-value |
|---|---|---|---|---|
| TPH1 | −0.08±0.40 | −0.70±0.56 | 1.53±0.48 | 0.3928 |
| TPH2 | −2.21±0.17 | −1.77±0.22 | 0.74±0.20 | 0.1518 |
| TPH2-T | 17.27±0.62 | 15.85±0.77 | 2.68±0.70 | 0.1794 |
| 5-HT1A | 13.49±0.68 | 11.59±0.59 | 3.73±0.64 | 0.0619 |
| 5-HT1B | 13.36±0.50 | 11.70±0.66 | 3.16±0.59 | 0.0742 |
| 5-HT1D | 16.16±0.43 | 14.48±0.32 | 3.20±0.38* | 0.0110 |
| NUDR | 15.09±0.44 | 13.32±0.32 | 3.43±0.39* | 0.0088 |
| FREUD-1 | 18.43±0.78 | 16.72±0.71 | 3.26±0.75 | 0.1384 |
| REST | 15.57±0.24 | 13.91±0.69 | 3.15±0.51* | 0.0458 |
| SERT | 12.74±0.93 | 11.08±0.80 | 3.16±0.87 | 0.2067 |
| TREK-1 | 2.11±0.40 | 2.19±0.15 | 0.95±0.30 | 0.8599 |
| TrkB | 4.46±0.37 | 4.38±0.33 | 1.06±0.35 | 0.8708 |
| ERα | 4.64±0.16 | 3.95±0.46 | 1.61±0.34 | 0.1862 |
| ERβ | 7.88±0.20 | 7.54±0.21 | 1.26±0.20 | 0.2640 |
p≤0.05
Table 4.
Δ Ct value and Fold change for male subjects.
| Genes | Δ Ct Control (Mean±SEM) | ΔCt MDD (Mean±SEM) | Fold Change (Mean±SEM) | p-value |
|---|---|---|---|---|
| TPH1 | 0.15±0.37 | −0.58±0.38 | 1.66±0.37 | 0.1940 |
| TPH2 | −2.66±0.16 | −2.77±0.06 | 1.08±0.12 | 0.5616 |
| TPH2-T | 14.56±0.71 | 16.17±0.94 | 0.33±0.84 | 0.2035 |
| 5-HT1A | 11.30±0.42 | 11.54±0.86 | 0.84±0.67 | 0.7997 |
| 5-HT1B | 12.57±0.74 | 13.02±0.76 | 0.73±0.75 | 0.6784 |
| 5-HT1D | 15.13±0.76 | 15.54±0.98 | 0.75±0.87 | 0.7454 |
| NUDR | 15.20±0.65 | 14.76±0.75 | 1.35±0.70 | 0.6679 |
| FREUD-1 | 18.27±0.71 | 18.37±0.99 | 0.94±0.86 | 0.9397 |
| REST | 14.89±1.06 | 15.05±0.64 | 0.90±0.87 | 0.9017 |
| SERT | 10.89±0.42 | 10.94±1.11 | 0.97±0.84 | 0.9689 |
| TREK-1 | 2.76±0.26 | 2.41±0.26 | 1.28±0.26 | 0.3554 |
| TrkB | 3.75±0.13 | 3.88±0.12 | 0.91±0.13 | 0.4900 |
| ERα | 4.37±0.65 | 3.53±0.32 | 1.79±0.52 | 0.2754 |
| ERβ | 8.36±0.43 | 7.81±0.19 | 1.46±0.33 | 0.2692 |
Table 5.
Δ Ct value and Fold change value after combining both groups.
| Genes | Δ Ct Control (Mean±SEM) | Δ Ct MDD (Mean±SEM) | Fold Change (Mean±SEM) | p-value |
|---|---|---|---|---|
| TPH1 | 0.03±0.26 | −0.64±0.32 | 1.59±0.29 | 0.1182 |
| TPH2 | −2.44±0.13 | −2.27±0.19 | 0.89±0.16 | 0.4732 |
| TPH2-T | 15.92±0.61 | 16.01±0.58 | 0.94±0.59 | 0.9132 |
| 5-HT1A | 12.39±0.50 | 11.57±0.50 | 1.77±0.50 | 0.2562 |
| 5-HT1B | 12.96±0.44 | 12.36±0.52 | 1.52±0.48 | 0.3877 |
| 5-HT1D | 15.65±0.44 | 15.01±0.52 | 1.55±0.48 | 0.3622 |
| NUDR | 15.15±0.37 | 14.04±0.45 | 2.15±0.41 | 0.0700 |
| FREUD-1 | 18.35±0.51 | 17.55±0.63 | 1.75±0.57 | 0.3312 |
| REST | 15.23±0.53 | 14.48±0.48 | 1.68±0.50 | 0.3035 |
| SERT | 11.81±0.56 | 11.01±0.65 | 1.75±0.61 | 0.3601 |
| TREK-1 | 2.44±0.25 | 2.30±0.15 | 1.10±0.20 | 0.6325 |
| TrkB | 4.10±0.21 | 4.13±0.18 | 0.98±0.20 | 0.9350 |
| ERα | 4.51±0.32 | 3.74±0.27 | 1.70±0.30 | 0.0853 |
| ERβ | 8.12±0.24 | 7.68±0.14 | 1.36±0.19 | 0.1207 |
5-HT, TrkB and estrogen receptors
The mean mRNA level of 5-HT1A (3.73 fold, p=0.0619) and 5-HT1B (3.16 fold, p=0.0742) receptors show a borderline elevation in the female MDD subjects relative to controls while in male MDD subjects there was no change in mRNA levels (Table 3 and Table 4). Levels of mRNA for either 5-HT1A or 5-HT1B did not differ between depressed and control subjects when combining both gender groups (Table 5).
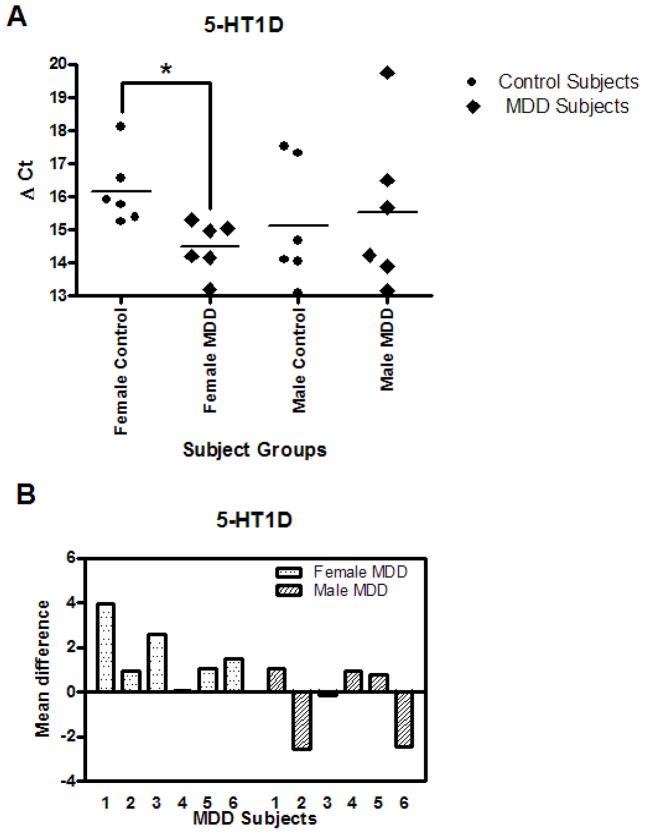
The mean mRNA level of 5-HT1D receptors showed significantly higher expression (3.20 fold, p=0.0110) in the captured DR neurons in the female MDD subjects as compared to their matched female control subjects (Table 3). The results for mRNA expression for 5-HT1D receptor are summarized in Fig. 2; the delta Ct value for each subject is plotted on scattered plot (Fig. 2A). All 6 depressed women subjects showed an increase in mean difference of the 5-HT1D receptor delta Ct relative to their matched control (Fig. 2B). 5-HT1D receptor mRNA was not significantly different in male MDD subjects compared to matched controls nor was it different in the pooled gender groups (Table 4 and 5).
Figure 2.
A. 5-HT1D mRNA levels in DR neurons of 6 female and 6 male control and MDD subjects expressed as mean Δ Ct values. B. 5-HT1D mRNA levels of female and male MDD subjects expressed as Δ Ct mean difference values from paired controls.
The TrkB receptor mRNA expression was not significantly different in the DR neurons of depressed subjects as compared gender-matched control subjects or across genders (Table 3, 4 and 5). Estrogen receptor ERα mRNA was elevated in depressed subject group relative to the control group but the data did not reach statistical significance (p=0.0853) (Table 5). Similarly, there was no change in the expression of ERβ mRNA among the depressed subject groups within or across genders (Table 3, 4 and 5).
Transcription factors
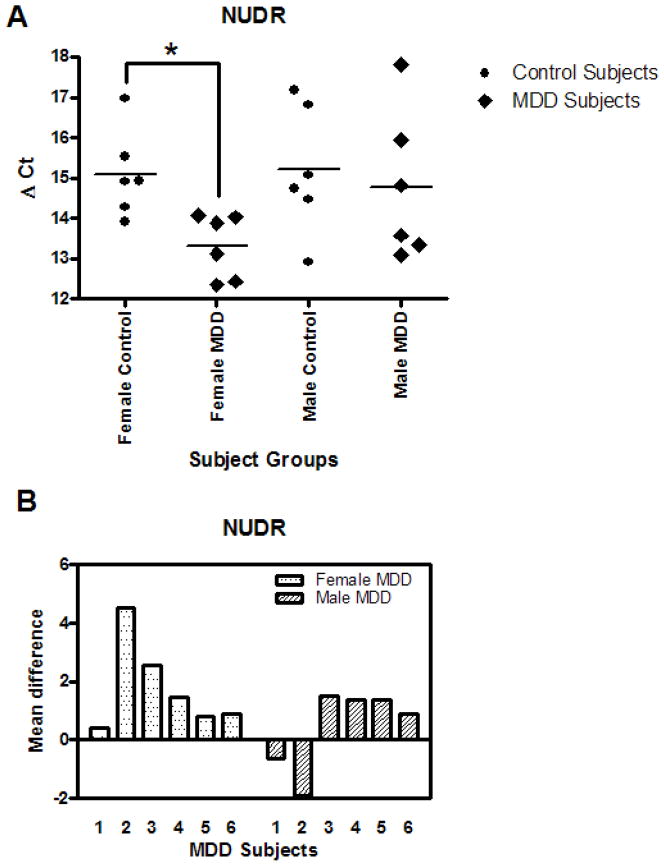
The mRNA expression for NUDR is summarized in Figs. 3A and B. The mean mRNA level of NUDR was increased significantly by 3.43 fold (p=0.0088) in the female MDD subjects as compared to their matched female control subjects while there was no difference in male MDD subjects relative to matched control subjects (Table 3 and Table 4). The mean change in NUDR expression from individually matched control subjects revealed that NUDR mRNA in DR neurons was increased in every female MDD subject while only four male MDD subjects showed increased levels (Fig. 3B). Pooling data across genders, the increase in NUDR mRNA in MDD subjects did not reach statistical significance (2.31 fold, p=0.0700).
Figure 3.
A. NUDR mRNA levels in DR neurons of 6 female and 6 male control and MDD subjects expressed as mean Δ Ct values. B. NUDR mRNA levels of female and male MDD subjects expressed as Δ Ct mean difference values from paired controls.
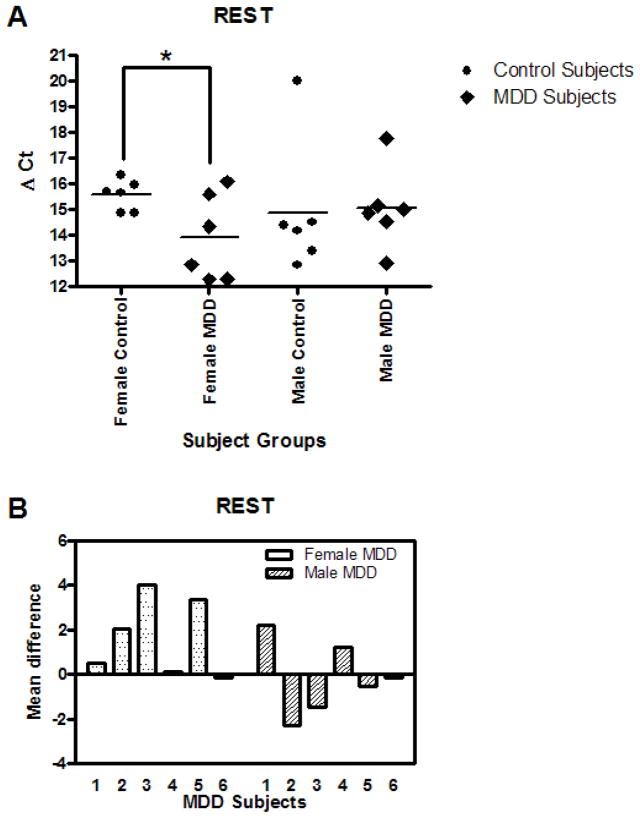
The mRNA expression for REST is summarized in Figs. 4A and B. REST mRNA expression in DR neurons was significantly increased by 3.15 fold (p=0.0458) in the female MDD subjects compared to their matched control subjects. There was no alteration in REST mRNA in the male MDD subjects relative to matched controls. Comparison of individually matched pairs revealed that the mean REST mRNA level was increased in five of the six depressed female subjects compared to the matched control subjects (Fig. 4B). There was no difference in REST mRNA levels in the combined depressed and control groups. Also, Freud-1 mRNA was not changed in depressed group when analyzed gender-wise or across genders (Table. 3, 4 and 5).
Figure 4.
A. REST mRNA levels in DR neurons of 6 female and 6 male control and MDD subjects expressed as mean Δ Ct values. B. REST mRNA levels of female and male MDD subjects expressed as Δ Ct mean difference values from paired controls.
SERT and TREK-1
The transcription level of SERT in DR neurons was not changed in depressed subjects relative to matched control subjects within or across genders. Similarly, no difference in TREK-1 mRNA was found in depressed subjects relative to matched control subjects within or across genders (Table 3, 4 and 5).
Discussion
The present study represents the first report using laser-capture microdissection and Q-PCR to quantify mRNA transcripts in individual TPH2-immunofluorescent DR neurons of female and male MDD subjects and gender-matched control subjects. The results revealed that mRNA expression of NUDR, REST and the 5-HT1D receptor was significantly increased in DR serotonin-containing neurons of female subjects diagnosed with MDD relative to matched female control subjects, but the mRNA transcripts measured were unaltered in midbrain DR neurons of male depressed subjects compared to male controls.
NUDR is the human homolog of Drosophila DEAF-1, which has been identified as a DNA-binding protein and gene regulator (Huggenvik et al. 1998). Recent investigations have shown that NUDR represses both human and rat 5-HT1A receptor promoter luciferase constructs and is colocalized with 5-HT1A receptors in the prefrontal cortex, hippocampus and raphe nuclei (Lemonde et al. 2003). While stable expression of NUDR reduced the expression and binding of 5-HT1A receptors and mRNA levels in raphe cells suggesting that NUDR negatively regulates 5-HT1A receptor (Lemonde et al. 2003), a subsequent study revealed that NUDR functions as a transcriptional enhancer in non-serotonergic cells (Czesak et al. 2006). Therefore, NUDR may have a dual function depending on the synaptic localization of 5-HT1A receptors. A recent study using a gene knockout strategy reported that NUDR−/− embryos display neural tube exencephaly at the mid-hindbrain region which caused death at birth (Hahm et al. 2004), this may suggest that NUDR may play an important neurodevelopment role in the early formation of midbrain structures.
We previously documented a gender-specific reduction in protein levels of NUDR and 5-HT1A receptors in the prefrontal cortex (PFC) of women with depression, but not in depressed men (Szewczyk et al. 2009). The present observation is consistent with this previous report of alterations in NUDR only in depressed females, but an increase in NUDR mRNA was found in the DR compared to a decrease in NUDR protein in the PFC. Since NUDR is believed to function as a 5-HT1A receptor gene repressor in DR neurons, elevated NUDR mRNA in the depressed female subjects may reflect an adaptive change to increase NUDR synthesis and activity to down-regulate over-expression of somatodendritic 5-HT1A autoreceptors in the DR of depressed subjects. Increased 5-HT1A receptor binding sites has been previously reported in the DR of MDD or depressed suicide subjects (Stockmeier et al. 1998; Boldrini et al. 2008). Similarly, the mean level of 5-HT1A receptor mRNA in DR neurons was increased by 3.73-fold in the depressed female subjects, however the data did not reach statistical significance (p=0.0619). The trend towards an increase in 5-HT1A receptor mRNA in depressed female DR neurons is in agreement with a 5-HT1A receptor binding assay where increased 5-HT1A receptor binding was found in the DR of both male and female depressed suicides as compared to gender-matched control subjects (Boldrini et al. 2008), likewise an earlier study reported 5-HT1A receptor binding sites were increased in the subnuclei of the DR of suicide victims with MDD compared with controls (Stockmeier et al. 1998).
Repressor element-1 silencing transcription factor (REST) is another negative transcriptional regulator of 5-HT1A receptors. It binds to the repressor element-1 (RE-1) site adjacent to the dual repressor element (DRE) upstream of the promoter region in the 5-HT1A receptor gene sequence of rat, mouse and human (Lemonde et al. 2004). Hence REST binding contributes to silencing of 5-HT1A receptors in some neuronal and non-neuronal cells (Schoenherr and Anderson 1995; Lemonde et al. 2004). We found a gender-specific increase in REST mRNA expression in DR neurons of female MDD subjects as compared to their matched control subjects but there were no changes in the male subject groups. In theory, the increase in REST in DR neurons may result in increased binding to the 5-HT1A RE-1 promoter site and lead to a decrease in 5-HT1A autoreceptor expression. The decrease in 5-HT1A autoreceptors may reduce inhibitory tone and increase the synaptic concentrations of serotonin. However, it is interesting that both REST and NUDR are two transcriptional repressors of 5-HT1A receptors that are elevated in DR neurons of depressed women, but yet 5-HT1A receptor mRNA remains elevated in depressed women although not statistically significant. Perhaps these changes reflect an adaptive molecular response triggering transcriptional repressor mechanisms to suppress 5-HT1A autoreceptor expression in DR neurons of depressed women and facilitate 5-HT neurotransmission, but the elevation in the transcriptional repressors is not sufficient or functional to completely abolish the enhanced inhibition via increased expression of somatodendritic 5-HT1A receptors.
In addition to 5-HT1A somatodendritic autoreceptors, 5-HT1B and 5-HT1D presynaptic terminal autoreceptors also play a key role in regulating serotonin neurotransmission. The 5-HT1B and 5-HT1D receptors are highly expressed in dorsal raphe nuclei in rats (Davidson and Stamford 1995; Bonaventure et al. 1998) and in human (Bidmon et al. 2001). The finding that 5-HT1B and 5-HT1D receptors can form homo- and heterodimers with each other (Xie et al. 1999) further add to the complexity of the 5-HT1B and 1D autoreceptors action. The increase in 5-HT1D receptor mRNA in DR neurons of depressed women is supported by previous rodent studies measuring the 5-HT1B receptor mRNA (rodent equivalent of the human 5-HT1D). For example, 5-HT1B receptor mRNA was elevated (25%) in the DR of learned helplessness rats compared to controls (Neumaier et al. 1997) albeit the magnitude of change was less compared to our observations. It is also interesting that rats treated for 21 days with fluoxetine reduced 5-HT1B receptor mRNA expression in the DR by 83% of control, whereas rats treated for 7 days and then allowed 7 days of drug washout, had 5-HT1B receptor mRNA levels in the DR return to control levels (Neumaier et al. 1996a). It is important to note that methodological differences between Neumaier et al. (1997) using in situ hybridization histochemistry and our study using RT-PCR must be considered when comparing fold-changes in mRNA expression. The gender-specific increase in the mean levels of 5-HT1D receptor mRNA in DR neurons of female MDD subjects as compared to their matched control subjects is potentially a very important observation that may further elucidate the molecular mechanisms associated with diminished serotonin neurotransmission in depression. Over-expression of presynaptic 5-HT1D autoreceptors in depressed women may reflect excessive inhibitory tone on serotonin neurons leading to reduced synaptic levels of 5-HT. Such a hypothesis is supported by our observations of elevated NUDR and REST mRNA in DR neurons of depressed women to dampen the inhibitory tone on DR neurons by acting to repress 5-HT1A autoreceptors.
There has been only one previous report published on midbrain 5-HT1D receptors in suicide victims where a significant decrease was found in 5-HT1D binding affinity in the depressed suicides (Arranz et al. 1994). Another study reported increased 5-HT1D receptor binding sites in globus pallidus, but these alterations where restricted to those suicides who died by violent means (Lowther et al. 1997). It will be important for future studies to confirm and further investigate the over-expression of 5-HT1D receptors in depression.
The TPH2 gene promoter sequence also contains an RE-1 element and REST has been shown to regulate transcription of TPH2. Patel and colleagues (2007) showed that in transfected C6-glioma cells dominant-negative inhibition of REST produced a 4.4 fold up-regulation of TPH2 mRNA compared to a reference gene which demonstrated the silencing effect of NRSE/REST on TPH2 transcription. Increased REST repression of TPH2 in depression may be maladaptive, but we did not observe any significant changes in any of the TPH isoforms in DR neurons of depressed subjects nor is there any evidence in the literature to support reduced transcription of TPH2 in depressed subjects. Our findings on TPH isoforms in DR neurons of MDD subjects are in contrast to those of Arango and colleagues (Bach-Mizrachi et al. 2008) that have reported an increase in TPH2 mRNA expression using in situ hybridization in depressed suicide subjects in the caudal level of the DR and median raphe nuclei. The only DR subnucleus that exhibited an increase in TPH2 mRNA expression was the caudal subnucleus. The same group also found a higher number and density of TPH-immunoreactive neurons in the mid-rostrocaudal DR region and elevated TPH immunoreactivity in DR region at the level of trochlear decussation in depressed suicides (Underwood et al. 1999; Boldrini et al. 2005). In the context of elevated REST (a TPH2 repressor) mRNA transcripts in women with depression and the lack of change in TPH2 mRNA in the DR of depressed women and men, the increase in TPH2 mRNA reported in DR of depressed suicides by Bach-Mizrachi et al. (2008) is difficult to interpret mechanistically. It is conceivable that based on the functional heterogeneity of the DR reported in rodents (Abrams et al. 2004; Lowry et al. 2008) that alterations in TPH2 mRNA are restricted to the caudal DR in depressed suicide subjects. However, given the considerable evidence of alterations in prefrontal cortical serotonergic markers in major depressed subjects (Austin et al. 2002; Szewczyk et al. 2009; Stockmeier et al. 2009) and that the majority of forebrain 5-HT terminals arise from the midbrain dorsal raphe nucleus which contains more than 90% of the DR 5-HT neurons (Baker et al. 1990; 1991; Tork 1990), the rationale for choosing to examine the mid-rostral DR in the present study is strongly justified.
TPH2-T is a truncated form of TPH2 and is a functional single nucleotide polymorphism in the TPH2 gene (Haghighi et al. 2008). This polymorphism results in low efficiency of normal splicing and produces a truncated TPH2-T isoform by alternative splicing. TPH2-T lacks the catalytic domain and hence TPH2 enzyme activity that may reduce serotonin production. Therefore, to understand the involvement of truncated TPH2-T, we measured the TPH2-T mRNA and found there is no difference in mRNA expression of TPH2-T in depressed subjects suggesting that TPH2-T does not play a major role in the pathophysiology of depression. This is the first study which measured TPH2-T mRNA transcripts in human DR neurons and established that the TPH2 truncated form does not appear to play a role in major depressive disorder.
The previous study by Bonkale et al. 2004 reporting no changes in TPH2 protein combined with the present report of no changes in TPH2 mRNAs in the DR of depressed subjects represent two independent studies conducted on two separate cohorts of MDD and control subjects which consistently demonstrates a lack of change in TPH2 biosynthesis in the dorsal raphe nuclei of subjects diagnosed with major depressive disorder. These studies are at odds with those of Arango and colleagues (Bach-Mizrachi et al. 2008; Underwood et al. 1999; Boldrini et al. 2005) reporting increased TPH2 expression in the raphe of depressed suicides and therefore raise questions regarding the subject characteristics of their depressed cases or perhaps methodological differences.
Of the remaining transcripts measured in the DR of depressed subjects (Freud-1, SERT, TREK-1, BDNF, TrkB, ERα and ERβ), none of these mRNAs were significantly altered in depressed subjects within or across genders. With the exception of SERT, no previous studies have examined mRNA expression of these transcripts in DR neurons of depressed subjects. For example, postmortem studies of depressed suicides revealed a greater percentage of DR neurons expressing higher grain density for SERT mRNA (Arango et al. 2001). In contrast, a recent study found no difference in SERT mRNA in the DR between those who committed suicide and control subjects (Anisman et al. 2008). Similarly, earlier studies found no difference in SERT binding sites as well as SERT mRNA levels in the DR of depressed subjects as compared to matched control subjects (Little et al. 1997; Bligh-Glover et al. 2000; Klimek et al. 2003). Our finding of no change in SERT mRNA in DR neurons of MDD subjects is consistent with these previous studies.
One potentially important variable to consider in human postmortem mood disorder studies is the confounding affect of antidepressant medication on the dependent measures. While no published studies to date have documented effects of antidepressants on the transcription factors, NUDR and REST, several rodent studies have reported alterations in gene expression of various serotonergic markers in the dorsal raphe. For example, repeated fluoxetine treatment significantly decreased (−38%) 5-HT1A receptor mRNA levels in the anterior raphe area (Le Poul et al. 2000), paroxetine and fluoxetine both reduced 5-HT1B receptor mRNA in the DR by 36% and 27%, respectively (Anthony et al. 2000), whereas fluoxetine significantly reduced expression of TPH2 and SERT mRNAs as determined by RT-PCR in the brainstem (Dygalo et al. 2006). These studies illustrate the importance to consider the patients’ medication history and obtain, when possible, accurate clinical records and toxicological reports for all subjects diagnosed with MDD.
Since toxicology screens were negative for antidepressants for all depressed subjects, it is unlikely that the alterations in the mRNA transcripts in the MDD subjects were influenced by antidepressant medication. However, the potential effects of antidepressants can not be entirely ruled out since several of the MDD subjects had a prescription for an antidepressant at the time of death, and this may raise questions regarding the compliance of these patients and the possible long-term effects of antidepressants on these specific serotonin regulators. It is important to note that having a prescription for a medication does not necessarily imply compliance. In fact, only 25% to 50% of patients with MDD adhere to their antidepressant routine for the length of time recommended by depression treatment guidelines, and close to 50% of depressed patients referred from primary care to specialty care treatment fail to complete the referral (Trivedi et al. 2007).
Gender factor and hormonal effect
The increase in mRNA expression of 5-HT1D receptor, NUDR and REST in female depressed subjects raises the issue of a biochemical alteration specific to women. These results emphasize the facts that women have different biochemical profiles compared to men with respect to the pathophysiology of depression (Carey et al. 1995; Kessler 2003; Baca et al. 2004) and raise the possibility of ovarian hormones playing a role. Currently, there is no available data on the direct effects of estrogen or progesterone on 5-HT1D receptor, NUDR or REST. In a previous rodent study, estrogen selectively decreased 5-HT1B (which is human 5-HT1D counterpart) mRNA in the mid-ventromedial subregion of the DRN, where 5-HT1B mRNA was associated with higher anxiety-like behavior and inversely correlated with TPH2 mRNA levels (Hiroi et al. 2006; Hiroi and Neumaier 2009). These results suggest that estrogen may reduce 5-HT1B autoreceptor and increase TPH2 synthesis in a coordinated fashion, thereby increasing the capacity for 5-HT synthesis and release in distinct forebrain regions that modulate specific components of anxiety behavior (Hiroi et al. 2006). On the other hand increased baseline hippocampal 5-HT levels in only female 5-HT1B knockout mice mirrors and supports our study demonstrating increased 5-HT1D receptor expression and possible diminished serotonin neurotransmission only in depressed women (Jones and Lucki 2005).
Although the precise hormonal stage of the women involved in the study is difficult to ascertain, we have evidence from toxicology screens and from structural interviews that none of the female subjects were receiving hormonal therapy at the time of death. Furthermore, all of the female subjects in our study were over 45 years of age, suggesting that the majority of the women were likely perimenopausal or post-menopausal, so it is unlikely that circulating ovarian hormones had a confounding effect on 5-HT1D receptor, NUDR and REST mRNA expression in our female subjects. But this does not exclude the possibility that long-term fluctuations in ovarian hormones may have played a role in the mechanisms regulating gene expression of 5-HT1D receptor, NUDR and REST in depressed women.
In summary, this report presents evidence of increased gene expression of two novel transcription factors, NUDR and REST, and of 5-HT1D receptors in an isolated population of serotonin-containing dorsal raphe neurons of women with MDD. These findings reveal alterations in specific regulators of serotonin function that may provide clues for understanding the mechanisms associated with diminished serotonin neurotransmission and the higher incidence of depression in women.
Acknowledgments
We are gratefully acknowledge the work of Drs. James C. Overholser, George J. Jurjus, Herbert Y. Meltzer, and Lesa Dieter, LISW, for psychiatric assessment of the subjects, Lisa Konick and Nicole Herbst in the collection and processing of human tissues and Dr. Bernadeta Szewczyk, Tarsha Harris and Heidi Fitzgibbon for technical assistance. We deeply appreciate the assistance of the next-of-kin of the deceased and the exceptional assistance of the Cuyahoga County Coroner’s Office, Cleveland, OH. This study was supported by a grant from the National Center for Research Resources (RR017701).
Footnotes
None of the authors have conflict of interest.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann NY Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, Merali Z, Poulter MO. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]
- Anthony JP, Sexton TJ, Neumaier JF. Antidepressant-induced regulation of 5-HT(1b) mRNA in rat dorsal raphe nucleus reverses rapidly after drug discontinuation. J Neurosci Res. 2000;61:82–87. doi: 10.1002/1097-4547(20000701)61:1<82::AID-JNR10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- Arranz B, Eriksson A, Mellerup E, Plenge P, Marcusson J. Brain 5-HT1A, 5-HT1D, and 5-HT2 receptors in suicide victims. Biol Psychiatry. 1994;35:457–463. doi: 10.1016/0006-3223(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Austin MC, Whitehead RE, Edgar CL, Janosky JE, Lewis DA. Localized decrease in serotonin transporter-immunoreactive axons in the prefrontal cortex of depressed subjects committing suicide. Neuroscience. 2002;114:807–815. doi: 10.1016/s0306-4522(02)00289-0. [DOI] [PubMed] [Google Scholar]
- Baca E, Garcia-Garcia M, Porras-Chavarino A. Gender differences in treatment response to sertraline versus imipramine in patients with nonmelancholic depressive disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:57–65. doi: 10.1016/S0278-5846(03)00177-5. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Tin A, Ellis SP, Mann JJ, Arango V. Elevated expression of tryptophan hydroxylase-2 mRNA at the neuronal level in the dorsal and median raphe nuclei of depressed suicides. Mol Psychiatry. 2008;13:507–513. 465. doi: 10.1038/sj.mp.4002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, Bakalian MJ, Sibille E, Tamir H, Mann JJ, Arango V. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Törk I. Cytoarchitecture of the human dorsal raphe nucleus. J Comp Neurol. 1990;301:147–161. doi: 10.1002/cne.903010202. [DOI] [PubMed] [Google Scholar]
- Baker KG, Halliday GM, Hornung JP, Geffen LB, Cotton RG, Tork I. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience. 1991;42:757–775. doi: 10.1016/0306-4522(91)90043-n. [DOI] [PubMed] [Google Scholar]
- Baumann B, Bielau H, Krell D, Agelink MW, Diekmann S, Wurthmann C, Trubner K, Bernstein HG, Danos P, Bogerts B. Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychol Med. 2002;32:93–103. doi: 10.1017/s0033291701004822. [DOI] [PubMed] [Google Scholar]
- Bidmon HJ, Schleicher A, Wicke K, Gross G, Zilles K. Localisation of mRNA for h5-HT1B and h5-HT1D receptors in human dorsal raphe. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:364–368. doi: 10.1007/s002100000357. [DOI] [PubMed] [Google Scholar]
- Bligh-Glover W, Kolli TN, Shapiro-Kulnane L, Dilley GE, Friedman L, Balraj E, Rajkowska G, Stockmeier CA. The serotonin transporter in the midbrain of suicide victims with major depression. Biol Psychiatry. 2000;47:1015–1024. doi: 10.1016/s0006-3223(99)00313-3. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. More tryptophan hydroxylase in the brainstem dorsal raphe nucleus in depressed suicides. Brain Res. 2005;1041:19–28. doi: 10.1016/j.brainres.2005.01.083. [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Mann JJ, Arango V. Serotonin-1A autoreceptor binding in the dorsal raphe nucleus of depressed suicides. J Psychiatr Res. 2008;42:433–442. doi: 10.1016/j.jpsychires.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Voorn P, Luyten WH, Jurzak M, Schotte A, Leysen JE. Detailed mapping of serotonin 5-HT1B and 5-HT1D receptor messenger RNA and ligand binding sites in guinea-pig brain and trigeminal ganglion: clues for function. Neuroscience. 1998;82:469–484. doi: 10.1016/s0306-4522(97)00302-3. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Murdock S, Janosky JE, Austin MC. Normal levels of tryptophan hydroxylase immunoreactivity in the dorsal raphe of depressed suicide victims. J Neurochem. 2004;88:958–964. doi: 10.1046/j.1471-4159.2003.02225.x. [DOI] [PubMed] [Google Scholar]
- Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144:311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- Czesak M, Lemonde S, Peterson EA, Rogaeva A, Albert PR. Cell-specific repressor or enhancer activities of Deaf-1 at a serotonin 1A receptor gene polymorphism. J Neurosci. 2006;26:1864–1871. doi: 10.1523/JNEUROSCI.2643-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson C, Stamford JA. Evidence that 5-hydroxytryptamine release in rat dorsal raphe nucleus is controlled by 5-HT1A, 5-HT1B and 5-HT1D autoreceptors. Br J Pharmacol. 1995;114:1107–1109. doi: 10.1111/j.1476-5381.1995.tb13321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–119. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- Dygalo NN, Shishkina GT, Kalinina TS, Yudina AM, Ovchinnikova ES. Effect of repeated treatment with fluoxetine on tryptophan hydroxylase-2 gene expression in the rat brainstem. Pharmacol Biochem Behav. 2000;85:220–227. doi: 10.1016/j.pbb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Fleige S, Pfaffl MW. RNA integrity and the effect on the real-time qRT-PCR performance. Mol Aspects Med. 2006;27:126–139. doi: 10.1016/j.mam.2005.12.003. [DOI] [PubMed] [Google Scholar]
- First MBSR, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; 1996. Structured clinical interview for DSM-IV axis I disorders-patient edition. [Google Scholar]
- Haghighi F, Bach-Mizrachi H, Huang YY, Arango V, Shi S, Dwork AJ, Rosoklija G, Sheng HT, Morozova I, Ju J, Russo JJ, Mann JJ. Genetic architecture of the human tryptophan hydroxylase 2 Gene: existence of neural isoforms and relevance for major depression. Mol Psychiatry. 2008;13:813–820. doi: 10.1038/sj.mp.4002127. [DOI] [PubMed] [Google Scholar]
- Hahm K, Sum EY, Fujiwara Y, Lindeman GJ, Visvader JE, Orkin SH. Defective neural tube closure and anteroposterior patterning in mice lacking the LIM protein LMO4 or its interacting partner Deaf-1. Mol Cell Biol. 2004;24:2074–2082. doi: 10.1128/MCB.24.5.2074-2082.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience. 2009;158:456–464. doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi R, McDevitt RA, Neumaier JF. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: association between gene expression and anxiety behavior in the open field. Biol Psychiatry. 2006;60:288–295. doi: 10.1016/j.biopsych.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Huggenvik JI, Michelson RJ, Collard MW, Ziemba AJ, Gurley P, Mowen KA. Characterization of a nuclear deformed epidermal autoregulatory factor-1 (DEAF-1)-related (NUDR) transcriptional regulator protein. Mol Endocrinol. 1998;12:1619–1639. doi: 10.1210/mend.12.10.0181. [DOI] [PubMed] [Google Scholar]
- Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44:798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- Jones MD, Lucki I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology. 2005;30:1039–1047. doi: 10.1038/sj.npp.1300664. [DOI] [PubMed] [Google Scholar]
- Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- Klimek V, Roberson G, Stockmeier CA, Ordway GA. Serotonin transporter and MAO-B levels in monoamine nuclei of the human brainstem are normal in major depression. J Psychiatr Res. 2003;37:387–397. doi: 10.1016/s0022-3956(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Lasiuk GC, Hegadoren KM. The effects of estradiol on central serotonergic systems and its relationship to mood in women. Biol Res Nurs. 2007;9:147–160. doi: 10.1177/1099800407305600. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Rogaeva A, Albert PR. Cell type-dependent recruitment of trichostatin A-sensitive repression of the human 5-HT1A receptor gene. J Neurochem. 2004;88:857–868. doi: 10.1046/j.1471-4159.2003.02223.x. [DOI] [PubMed] [Google Scholar]
- Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, Sequeira A, Kushwaha N, Morris SJ, Basak A, Ou XM, Albert PR. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23:8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Boni C, Hanoun N, Laporte AM, et al. Differential adaptation of brain 5–HT1A and 5–HT1B receptors and 5-HT transporter in rats treated chronically with fluoxetine. Neuropharmacology. 2000;39:110–122. doi: 10.1016/s0028-3908(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Little KY, McLauglin DP, Ranc J, Gilmore J, Lopez JF, Watson SJ, Carroll FI, Butts JD. Serotonin transporter binding sites and mRNA levels in depressed persons committing suicide. Biol Psychiatry. 1997;41:1156–1164. doi: 10.1016/s0006-3223(96)00301-0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lowther S, Katona CL, Crompton MR, Horton RW. 5-HT1D and 5-HT1E/1F binding sites in depressed suicides: increased 5-HT1D binding in globus pallidus but not cortex. Mol Psychiatry. 1997;2:314–321. doi: 10.1038/sj.mp.4000259. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, Mazumdar S, Mulsant BH, Houck PR, Lopresti BJ, Weissfeld LA, Reynolds CF. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004;29:2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Root DC, Hamblin MW. Chronic fluoxetine reduces serotonin transporter mRNA and 5-HT1B mRNA in a sequential manner in the rat dorsal raphe nucleus. Neuropsychopharmacology. 1996a;15:515–522. doi: 10.1016/S0893-133X(96)00095-4. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Petty F, Kramer GL, Szot P, Hamblin MW. Learned helplessness increases 5-hydroxytryptamine1B receptor mRNA levels in the rat dorsal raphe nucleus. Biol Psychiatry. 1997;41:668–674. doi: 10.1016/S0006-3223(96)00114-X. [DOI] [PubMed] [Google Scholar]
- Patel PD, Bochar DA, Turner DL, Meng F, Mueller HM, Pontrello CG. Regulation of tryptophan hydroxylase-2 gene expression by a bipartite RE-1 silencer of transcription/neuron restrictive silencing factor (REST/NRSF) binding motif. J Biol Chem. 2007;282:26717–26724. doi: 10.1074/jbc.M705120200. [DOI] [PubMed] [Google Scholar]
- Radonic A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267:1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- Schoor O, Weinschenk T, Hennenlotter J, Corvin S, Stenzl A, Rammensee HG, Stevanovic S. Moderate degradation does not preclude microarray analysis of small amounts of RNA. Biotechniques. 2003;35:1192–1196. 1198–1201. doi: 10.2144/03356rr01. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA, Bethea CL. Cognition, mood disorders, and sex hormones. Ilar J. 2004;45:189–199. doi: 10.1093/ilar.45.2.189. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, Overholser JC, Thompson PA, Meltzer HY. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-postmortem evidence for decreased serotonin activity. J Neurosci. 1998;18:7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HB, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockmeier CA, Howley E, Shi X, Sobanska A, Clarke G, Friedman L, Rajkowska G. Antagonist but not agonist labeling of serotonin-1A receptors is decreased in major depressive disorder. J Psychiatric Res. 2009;43:887–894. doi: 10.1016/j.jpsychires.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B, Albert PR, Burns AM, Czesak M, Overholser JC, Jurjus GJ, Meltzer HY, Konick LC, Dieter L, Herbst N, May W, Rajkowska G, Stockmeier CA, Austin MC. Gender-specific decrease in NUDR and 5-HT1A receptor proteins in the prefrontal cortex of subjects with major depressive disorder. Int J Neuropsychopharmacol. 2009;12:155–168. doi: 10.1017/S1461145708009012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Ann N Y Acad Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. discussion 34–35. [DOI] [PubMed] [Google Scholar]
- Tricarico C, Pinzani P, Bianchi S, Paglierani M, Distante V, Pazzagli M, Bustin SA, Orlando C. Quantitative real-time reverse transcription polymerase chain reaction: normalization to rRNA or single housekeeping genes is inappropriate for human tissue biopsies. Anal Biochem. 2002;309:293–300. doi: 10.1016/s0003-2697(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Lin EH, Katon WJ. Consensus recommendations for improving adherence, self-management, and outcomes in patients with depression. CNS Spectr. 2007;12(8 Suppl 13):1–27. [PubMed] [Google Scholar]
- Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:473–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034.1–0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Lee SP, O’Dowd BF, George SR. Serotonin 5-HT1B and 5-HT1D receptors form homodimers when expressed alone and heterodimers when co-expressed. FEBS Lett. 1999;456:63–67. doi: 10.1016/s0014-5793(99)00918-7. [DOI] [PubMed] [Google Scholar]