Abstract
Objective
Circulating angiogenic cells play an essential role in angiogenesis, but are dysfunctional in diabetes characterized by excessive oxidative stress. We hypothesize that oxidative stress-mediated upregulation of thrombospondin-2 (TSP-2), a potent anti-angiogenic protein, contributes to diabetic bone marrow-derived angiogenic cell dysfunction.
Approaches and Results
Bone marrow-derived angiogenic cells (BMACs) were isolated from adult male type 2 diabetic db/db mice and control db/+ (C57BLKS/J) mice. In Matrigel tube formation assay, angiogenic function was impaired in diabetic BMACs, accompanied by increased oxidative stress and NADPH oxidase activity. BMAC angiogenic function was restored by overexpression of dominant-negative (DN) Rac1 or by overexpression of MnSOD. TSP-2 mRNA and protein were both significantly up-regulated in diabetic BMACs, mediated by increased oxidative stress as shown by a decrease in TSP-2 level after overexpression of DN Rac1 or MnSOD. Silencing TSP-2 by its siRNA in diabetic BMACs improved BMAC function in tube formation, adhesion, and migration assays. Notably, the upregulation of TSP-2 was also found in BMACs from streptozotocin-induced type 1 diabetic mice, and normal BMACs with high glucose treatment. let-7f, a microRNA which has been related to endothelial angiogenic function, is found to play key role in TSP-2 increase, but let-7f did not directly interact with TSP-2 mRNA.
Conclusions
The upregulation of TSP-2 mediated by increased oxidative stress contribute to angiogenesis dysfunction in diabetic BMACs.
Keywords: angiogenic cell, thrombospondin-2, oxidative stress, diabetes, angiogenesis
Introduction
Since up to 80% of all deaths in diabetic patients are related to cardiovascular complications 1, 2, there have been many attempts to clarify the cellular and molecular mechanisms for these cardiovascular symptoms in diabetes 2, 3. Aberrant angiogenesis is one of the most serious symptoms associated with diabetes 4, 5. Endothelial progenitor cells (EPCs) could be recruited from bone marrow to the injury site by homing signals to promote endothelial regeneration and neovascularization 6-8, suggesting the potential use of EPCs as clinical therapeutics to improve angiogenesis 9. However, the number of recruited EPCs and their function are decreased under diabetic conditions, implying that impaired EPC function could be critical for defective angiogenesis in diabetes 10-12. Although several studies have given some explanations for diabetic EPC dysfunction, including increased oxidative stress, NADPH oxidase activation, and altered nitric oxide pathway 13, 14, the mechanism underlying the defective angiogenic properties of diabetic EPCs remains largely unknown.
Thrombospondins (TSPs) consist of a family of extracellular glycoproteins, modulating cell-to-cell or cell-to-matrix communication such as cell adhesion, proliferation, and migration 15, 16. In their family of five members, TSP-1 and -2 are known to form a subgroup and possess unique anti-angiogenic characteristics 17. Although the anti-angiogenic property of TSP-1 is well-established in various cell systems 18, 19, the anti-angiogenic function of TSP-2 has not been extensively investigated. Interestingly, evidence from the clinical observations and our previous study demonstrate that oxidative stress is highly increased in EPCs from diabetic subjects 12, 20. Lopes et al. showed that TSP-2 expression could be controlled by oxidative stress in endothelial cells 21, Until now, there have been no studies regarding the role of TSP-2 in EPC function in either normal or disease states including diabetes.
MicorRNAs are small single-stringed RNAs that negatively regulate gene expression by binding to target messenger RNAs 22. In the fields of angiogenesis, it is reported that inhibitor against let-7f reduces endothelial sprout formation 23. However, the regulatory role of let-7f in angiogenesis is not completely understood yet.
Our laboratory has been working with EPCs in cardiovascular diseases including diabetes 12, 24-26. In recent years, the knowledge regarding EPC characterization has evolved 27. Several important phenotypes have been studied, including: 1) expression of both stem cell markers and endothelial cell markers; 2) expression of endothelial cell functional genes; 3) uptake of DiI-Ac-LDL and binding of Lectin; 4) production of pro-angiogenic factors that facilitate vascular formation; and 5) incorporation into the vasculature or formation of tubular structure with lumen, etc 27, 28. The cells studied in our lab, based on their profiling, are better termed as bone marrow angiogenic cells (BMACs).
In the present study, we tested our hypothesis that increased oxidative stress could result in diabetic BMAC dysfunction via up-regulation of TSP-2, contributing to impaired angiogenesis in diabetes. Moreover, the involvement of let-7f was investigated to clarify the role of miRNAs in diabetic BMAC dysfunction. This study reveals a novel insight into the mechanism underlying defective BMAC function in diabetes, which provides an important clue about the potential use of angiogenic cells in therapeutic application for diabetic patients.
Materials and Methods
Materials and Methods are available in the online-only Supplement. http://atvb-submit.aha-journals.org/atvb_files/2013/04/13/00172476/01/172476_1_data_set_245351_ml7m 6x.pdf
Results
Angiogenesis is significantly impaired in BMACs isolated from type 2 diabetic mice
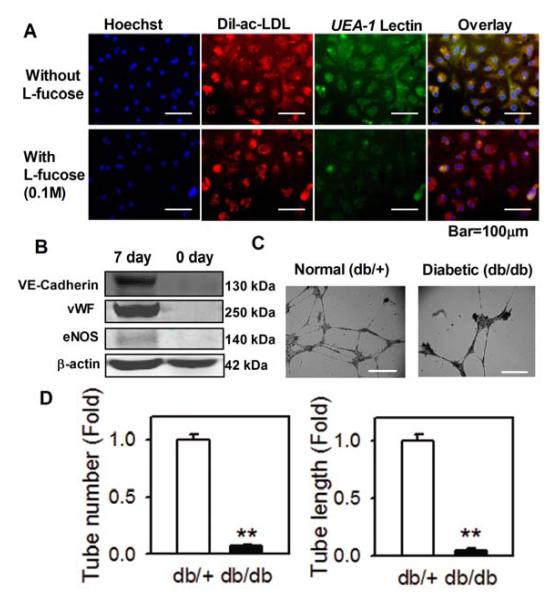
Seven-day cultured BMACs were identified by the uptake of acetylated LDL and lectin binding12, 24-26. These cells showed triple positive fluorescence (Figure 1A). The binding of UEA-1 lectin was abolished by its hapten sugar fucose (Figure 1A). Compared with freshly isolated cells, cells on the 7th day after isolation showed increased expression of endothelial functional molecules, including von Willebrand factor (vWF), vascular endothelial (VE)-Cadherin and endothelial nitric oxide synthase (eNOS) (Figure 1B). Our flow cytometry analysis also indicated that seven-day cells displayed higher expression of stem cell markers and endothelial cell makers than freshly isolated cells (Table 1). These cells are heterogenous, and small fraction of these cells have possibility of being endothelial cells based on CD144 expression. In 3D collagen gel, these cells are capable of forming tubular structures (Figure 1C). To compare the angiogenesis capacity between BMACs isolated from normal (db/+) and type 2 diabetic (db/db) mice, BMACs were plated onto Matrigel and their ability to form tubes and networks was evaluated. BMACs from diabetic mice had significantly impaired tube formation ability, as shown by both tube number and tube length (Figure 1D).
Figure 1.
Impairment of BMAC function in type 2 diabetic mice.
A. BMACs were triply stained by Ac-Dil LDL, UEA-1 lectin and DAPI in fluorescence microscopy. UEA-1 lectin binding was abolished by its hapten sugar fucose. ; B.Expression of VE-Cadherin, vWF, and eNOS in cultured cells on 7th day compared with freshly isolated cells. n=4. C. BMAC cells formed tubular structure (as indicated by arrow) in a 3D collagen cell culture system. D. Tube formation in normal and diabetic BMACs were examined in Matrigel tube formation assay. After replating to Matrigel, the cells and tubes were observed under microscopy. n=4 per group. **p<0.01 vs. db/+. Data were expressed as mean ± SEM and analyzed using Student’s t-test.
Table 1.
Flow cytometric analysis of cell surface markers of bone marrow mononuclear cells after 7-day culture in EGM-2 and freshly isolated mononuclear cells (N=5 per group).
| Percentage (%) | Day 7 | Day 0 |
|---|---|---|
| SCA-1 | 63.61 ± 4.85 | 14.40 ± 2.80 |
| CD34 | 44.36 ± 1.36 | 22.37 ± 1.18 |
| FLK-1 | 20.94 ± 2.13 | 3.38 ± 0.38 |
| CD144 | 8.68 ± 0.94 | 2.06 ± 0.09 |
| CD11b | 47.06 ± 3.95 | 73.72 ± 2.09 |
| CD45 | 8.51 ± 1.38 | 76.35 ± 5.42 |
| CD115 | 49.44 ± 5.66 | 3.51 ± 0.56 |
| Sca/Flk | 15.26 ± 0.83 | 3.20 ± 0.53 |
Impaired angiogenesis in diabetic BMAC is restored by inhibition of NADPH oxidase
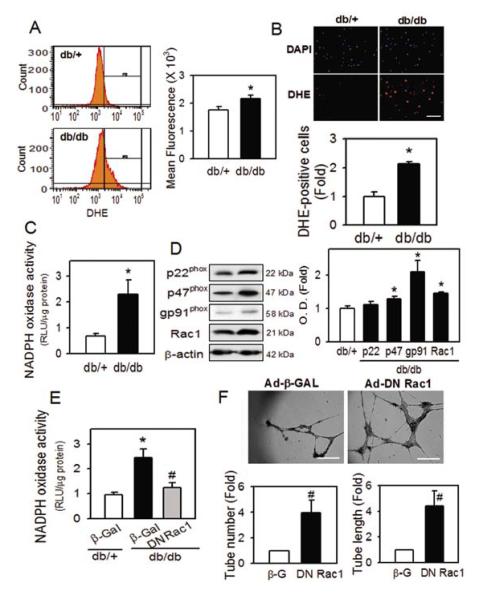
To investigate the mechanism underlying diabetic BMAC dysfunction, we measured the extent of oxidative stress using dihydroethidium (DHE). As shown in Figure 2, the DHE signal in diabetic BMACs was significantly increased compared to normal BMACs, in both flow cytometric (Figure 2A) and fluorescence microscopic (Figure 2B) analysis. To clarify how oxidative stress is increased in diabetic BMACs, we measured NADPH oxidase activity, which is known as one of the enzyme systems most responsible for reactive oxygen generation in vascular tissue, using lucigenin-enhanced chemiluminescence. NADPH oxidase activity was highly increased in diabetic BMACs (Figure 2C), and the protein levels of p47phox, gp91phox and Rac 1, important NADPH oxidase subunits, were significantly enhanced in diabetic BMACs (Figure 2D). To further investigate the possible contribution of increased NADPH oxidase activity, we transfected adenoviral vector-mediated of the dominant negative Rac1 (DN Rac1), which can retard the function of endogenous Rac1 or its control vector expresses β-galactosidase (β-Gal). Overexpression of DN Rac1 decreased NADPH oxidase activity in diabetic BMACs (Figure 2E, Left), and significantly restored impaired ability for tube formation (Figure 2F, Right).
Figure 2.
Role of oxidative stress in diabetic BMAC dysfunction.
ROS generation was determined by flow cytometry (A) or fluorescence microscope (B) after DHE staining. Scale bar: 200 μm. C. NADPH oxidase activity was detected in luminometer. D. NADPH oxidase subunits were examined using western blot. E. Effects of dominant negative Rac1 (Ad-DN Rac1) over-expression on NADPH oxidase activity. F. Effects of dominant negative Rac1 (Ad-DN Rac1) over-expression on diabetic BMAC function. Scale bar: 500 μm. Ad-β-Gal was used as control. A:n=8, B:n=4 C:n=4, D: n=4-7, E: n=4, F:n=5, *p<0.05 vs. db/+, #p<0.05 vs. db/db β-Gal. Data were expressed as mean ± SEM and analyzed with Student’s t-test.
The anti-angiogenic protein thrombospondin-2 (TSP-2) is significantly upregulated in diabetic BMACs
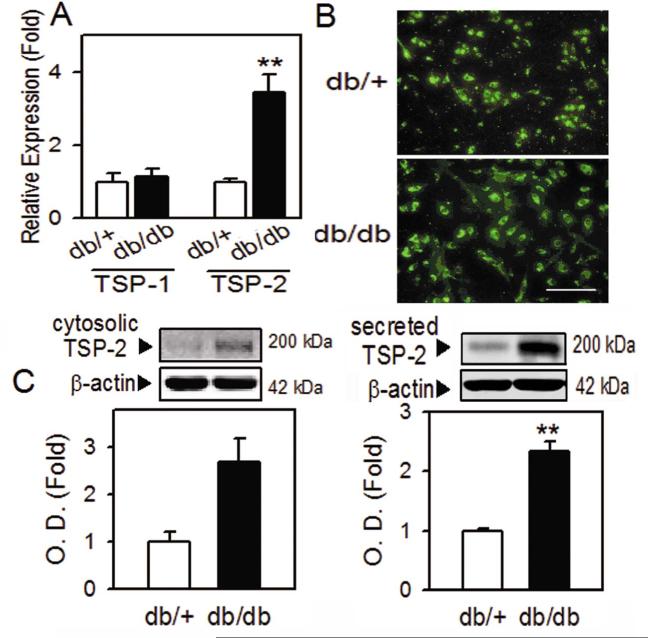
To identify the target protein of oxidative stress in impaired diabetic BMAC angiogenesis, we examined anti-angiogenic TSP-2 levels, based on a previous study showing that TSP-2 could be regulated by oxidative stress in normal endothelial cells 21. Real time PCR results revealed that TSP-2 mRNA was highly increased in diabetic BMACs, while TSP-1 was not changed (Figure 3A). The increased TSP-2 in diabetic BMACs was also found by immunostaining (Figure 3B). Usually, TSP-2 is known to be synthesized and secreted to extracellular media to exert its function in cell-cell or cell-matrix interaction 15. In western blot analysis, newly synthesized cytosolic form in diabetic BMACs was increased (Figure 3C, left panel), which is well correlated with mRNA level. The main functional form of secreted TSP-2 was also significantly increased, as analyzed in conditioned media after diabetic BMAC incubation (Figure 3C, right panel).
Figure 3.
Upregulation of TSP-2 in diabetic BMACs.
A. TSP-1 or TSP-2 mRNA in normal and diabetic BMAC was measured by qRT-PCR. B. Expression of TSP-2 was determined in fluorescence microscope. C. Cytosolic (left panel) or secreted (right panel) TSP-2 protein level was detected in western blot. A:n=3-6, B:n=3, C: n=4-6, Scale bar: 200 μm, *p<0.05, **p<0.01 vs. db/+. Data were expressed as mean ± SEM and analyzed using Student’s t-test or one-way ANOVA followed by Duncan’s test to determine the significant differences between treatment groups.
TSP-2 upregulation in diabetic BMACs is mediated by increased oxidative stress in diabetic BMACs
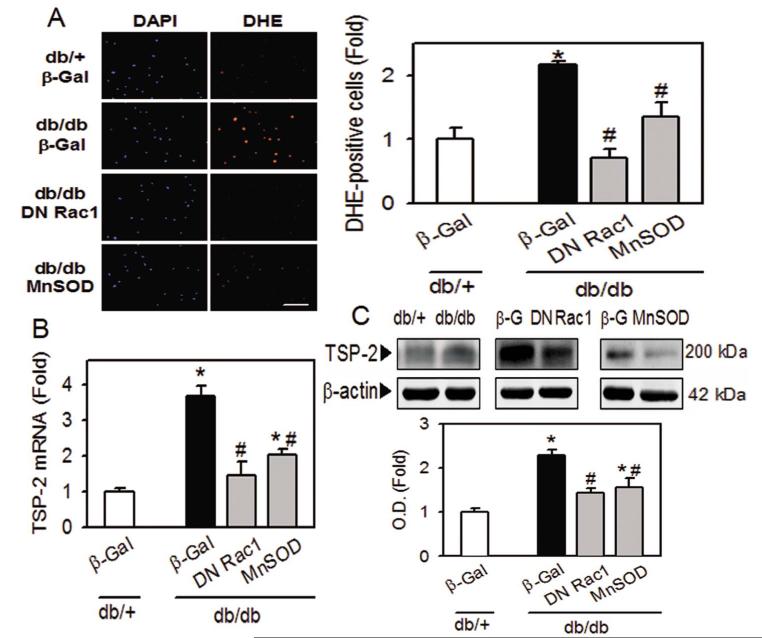
To examine the relationship between increased oxidative stress and TSP-2 upregulation in diabetic BMACs, we determined TSP-2 level after modulating oxidative stress using the adenoviral vector overexpressing DN Rac1 or MnSOD. The adenoviral vector overexpressing dominant negative Rac1 interferes with endogenous Rac 1, the major component of NADPH, hence, the NADPH activity was significantly decreased after Ad-DN Rac1 transfection in db/db BMACs (Figure 2D). MnSOD is the main antioxidant enzyme found in BMACs 29. Adenovirus overexpressing MnSOD is expected to decrease oxidative stress in cells. As shown in Figure 4A, increased oxidative stress in diabetic BMACs was reversed after overexpression of DN Rac1 or MnSOD. Upregulation of TSP-2 mRNA and protein level were significantly diminished after inhibition of oxidative stress (Figure 4B and 4C), demonstrating that increased oxidative stress was the main cause for the upregulation of TSP-2 in diabetic BMACs.
Figure 4.
Regulation of TSP-2 by oxidative stress in diabetic BMACs.
A. Effect of Ad-DN Rac1 or Ad-MnSOD on oxidative stress in BMACs. B and C. Expression of TSP-2 was determined in mRNA level (B) and protein level (C) after modulation of oxidative stress. A:n=4, B:n=3-6, C:n=4-8, Scale bar: 200 μm, *p<0.05 vs. db/+, #p<0.05 vs. db/db β-Gal. Data were expressed as mean ± SEM and analyzed with one-way ANOVA followed by Duncan’s test to determine the significant differences between treatment groups.
Upregulated TSP-2 plays a key role in impairment of diabetic BMAC function
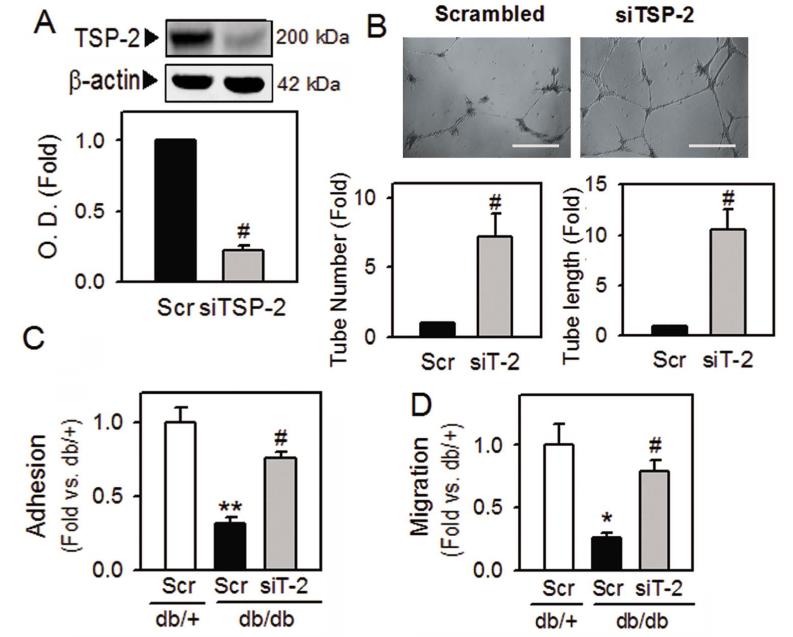
To determine the role of increased TSP-2 in diabetic BMAC dysfunction, we introduced silencing RNA against TSP-2 (siTSP-2) to diabetic BMACs. The viability and morphology of BMACs after siRNA transfection were not affected (data not shown). The protein level of TSP-2 was significantly decreased after 72 hrs of transfection (Figure 5A). In Matrigel tube formation assay, siTSP-2 restored the impaired BMAC function, as shown by an increase in tube number and tube length (Figure 5B). Besides tube formation ability, adhesion and migration ability, which are the key features of BMAC angiogenic function, were significantly impaired in diabetic BMACs, but were remarkably improved by inhibition of TSP-2 using its siRNA (Figure 5C and 5D).
Figure 5.
Improvement of diabetic BMAC function after silencing TSP-2.
A. TSP-2 protein level was measured 72 hrs after transfection of silencing RNA against TSP-2 (siTSP-2; siT-2) to confirm the silencing efficiency. Scrambled siRNA (Scr) was used as a negative control. B to D. The effects of siTSP-2 on diabetic BMAC function were determined in tube formation (B), adhesion (C), and migration (D). A:n=5, B:n=4, C:n=3, D:n=3, Scale bar: 500 μm, *p<0.05, **p<0.01 vs. db/+, #p<0.05 vs. db/db Scr. Data were expressed as mean ± SEM and analyzed with paired Student’s t-test or one-way ANOVA followed by Duncan’s test to determine the significant differences between treatment groups.
The expression of TSP-2 is increased in BMACs under high glucose conditions or in BMACs isolated from type 1 diabetic mice
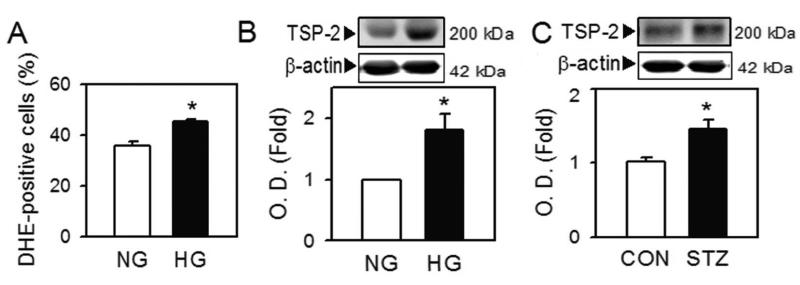
To further explore the relevance of TSP-2 upregulation in diabetic BMACs, we employed two representative diabetic models. We simulated high glucose conditions in diabetic patients with an in vitro model, by incubating normal BMACs in normal (5 mM glucose and 25 mM mannitol) and high glucose media (30 mM glucose) for 7 days 30. High glucose itself increased cellular oxidative stress in normal BMACs (Figure 6A). Notably, TSP-2 level was also significantly upregulated (Figure 6B). A similar increase in TSP-2 level was observed in BMACs isolated from streptozotocin-induced type 1 diabetic mice (Figure 6C). These data suggest that the upregulation of TSP-2 can be observed throughout the different types of diabetes.
Figure 6.
TSP-2 up-regulation in high-glucose condition and type 1 diabetic BMAC.
A and B. After BM-BMACs from normal mice were incubated in normal (NG; glucose 5 mM, mannitol 25 mM) or high (HG; glucose 30 mM) glucose media for 7 days, ROS generation (A) and TSP-2 protein (B) were determined by flow cytometry and western blot, respectively. C. TSP-2 level in BMACs from streptozotocin-induced type 1 diabetic mice was measured. A:n=5, B: n=5, C: n=5, *p<0.05 vs. corresponding control. Data were expressed as mean ± SEM and analyzed using Student’s t-test.
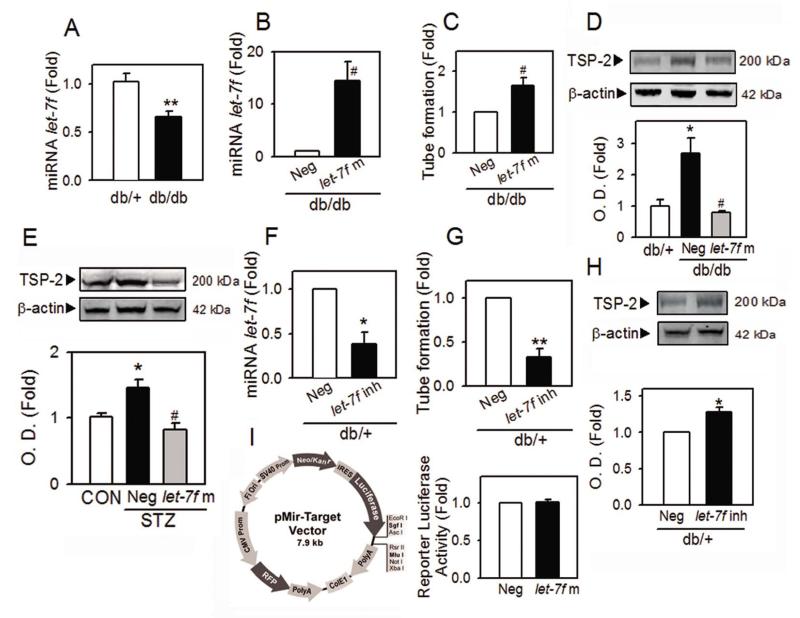
MicroRNA let-7f contributes to TSP-2 upregulation and dysfunction in diabetic BMACs
To investigate TSP-2 upregulation and impaired function of diabetic BMACs are under regulation of microRNA (miRNA), we examined the level of let-7f, a representative miRNA related to angiogenic function in endothelial cells. The level of let-7f is significantly decreased in diabetic BMACs (Figure 7A). When let-7f was overexpressed by transfection of let-7f mimic (Figure 7B), impaired tube formation of diabetic BMACs was rescued (Figure 7C) and the increased protein level of TSP-2 was reduced to the normal level (Figure 7D). Notably, the restoration of upregulated TSP-2 level by let-7f was also observed in BMACs isolated from type 1 diabetic animals (Figure 7E), showing that TSP-2 was regulated by let-7f in diabetic BMACs. Consistently, the impaired angiogenic function and the increased level of TSP-2 were observed in normal BMACs after inhibition of endogenous let-7f by transfection with let-7f inhibitor oligonucleotides (Figure 7F to 7H). However, let-7f does not directly interact with the 3′UTR of TSP-2 mRNA, as found in the luciferase assay using reporter vector inserted with 3′UTR of human TSP-2 (THBS2) mRNA (Figure 7I).
Figure 7.
Involvement of let-7f in TSP-2 upregulation and diabetic BMAC dysfunction.
A. The level of let-7f was measured from BMACs using qRT-PCR after isolation of miRNA. The U6 small nucleolar (sn) RNA was used as the housekeeping small RNA reference gene. B to D. The level of miRNA let-7f (B), tube formation ability (C) and the protein level of TSP-2 (D) were determined after overexpression of let-7f by transfection of let-7f mimic (let-7f m) to BMACs isolated from db /+ or db/db mice. Negative control oligonucleotides (Neg) was used as control. E. The effect of let-7f mimic on TSP-2 level in type I diabetic BMAC was were determined. F to H. The level of miRNA let-7f (F), tube formation ability (G) and the protein level of TSP-2 (H) were determined after inhibition of let-7f by transfection of let-7f inhibitor (let-7f inh) to normal BMACs. I. Luciferase activity was measured after co-transfection of TSP-2 (THBS2) 3′UTR plasmid with let-7f or negative control to HEK 293 cells. The structure of luciferase reporter plasmid was shown in left panel. A:n=6, B: n=4, C:n=4, D:n=4, E:n=4-5, F:n=4, G:n=4, H:n=4, I:n=3,, *p<0.05, **p<0.01 vs. db/+ or corresponding control, #p<0.05 vs. Neg. Data were expressed as mean ± SEM and analyzed using Student’s t-test.
Discussion
In the present study, we demonstrated that increased NADPH oxidase activity and resultant oxidative stress contribute to diabetic BMAC dysfunction, mediated by up-regulation of the anti-angiogenic protein thrombospondin-2 (TSP-2). To our best knowledge, this is the first study showing that TSP-2 could play a key role in BMAC function. Downregulation of TSP-2 by its siRNA significantly restored BMAC functions in tube formation, adhesion, and migration, indicating the critical roles of TSP-2 in angiogenic BMAC function. Furthermore, TSP-2 upregulation was also observed in BMACs under hyperglycemic conditions or isolated from type 1 diabetes, suggesting its potential contribution to BMAC dysfunction in diabetic patients.
In this study, we have investigated the anti-angiogenic role of TSP-2 in BMACs, which has been ignored compared to that of TSP-1. Despite the high degree of structural similarity between TSP-1 and TSP-2, it is reported that they are differently regulated on expression pattern 16, 31, 32, reflecting their distinct roles. The role of TSP-1 in the vascular impairment of the diabetic condition has been suggested by several studies using vascular tissues. Recently, Ii et al. reported that TSP-1 might be important in impairment of EPC-associated neovascularization18. They found that the expression of TSP-1 in diabetic EPCs was similar to that in db/m EPCs under low-glucose conditions, which is consistent with our results (Figure 3A). In this regard, our results showing that TSP-2 is highly upregulated in both types of diabetes and under hyperglycemic condition suggest that TSP-2 could be an important mediator of diabetic vascular impairment, raising the need for further investigation of the role of TSP-2 in diabetic vascular tissues. Supporting our view, a recent study demonstrated that TSP-2 could play a critical anti-angiogenic role in ECs, resulting in inhibition of physiological angiogenesis 33.
Consistent with these present observations, increased oxidative stress has been reported to be the potential mechanism responsible for defective vascular function in diabetic patients and animal models 20, 34, 35. Although here we used DHE, which may not be sufficient to specify between hydrogen peroxide and superoxide anions, many previous studies reported increased ROS generation under diabetic conditions. In vascular tissues isolated from human diabetic patients, NADPH oxidase activity is increased via upregulation of NADPH oxidase subunits, leading to enhanced generation of superoxide anion 35. In BMACs from diabetic patients, NADPH oxidase activity is found to be increased 20, 34, and superoxide generation is significantly increased 20. Supporting our view that increased oxidative stress plays an important role for diabetic EPC dysfunction, a recent review summarized that imbalanced ROS generation could contribute to EPC dysfunction under disease condition. Inhibition of oxidative stress has reversed diabetic EPC dysfunction in previous studies 36. Selective Rac1 inhibition in diabetic mice reversed increased oxidative stress, resulting in improvement of vascular function 37. Inhibition of NADPH oxidase subunit p47phox or scavenging oxidative stress could restore the EPC function 20, and MnSOD expression in diabetic EPC restored impairment of diabetic wound healing 12. Nevertheless, most studies have focused on the oxidative stress itself without demonstrating its possible downstream targets, such as anti-angiogenic protein. In this regard, our study could provide a new insight into the role of increased oxidative stress in cellular signaling including protein expression, under diabetic conditions.
Interestingly, Tang et al. recently demonstrated that retinal endothelial cells isolated from CYP1B1 knockout mice exhibited impaired migration and capillary morphogenesis by upregulation of TSP-2, which could be reversed by antioxidant, supporting our view that oxidative stress could be the key regulator for TSP-2 expression 38. Our data showed the involvement of oxidative stress in TSP-2 regulation with two different approaches, either by blocking ROS generation, or by upregulating the endogenous antioxidant system (Figure 4). To confirm the role of DN Rac1, we demonstrated that activity of NADPH oxidase or oxidative stress is reversed (Figure 2E and 4A), and both of them are positively correlated with TSP-2 mRNA/protein level (Figure 4B and 4C). To scavenge the ROS, we investigated MnSOD, based on its unique contribution to BMAC anti-oxidant system. The expression of MnSOD, not CuZnSOD or catalase, is found to be 3 to 4 fold higher in BMAC than that in ECs, representing a critical mechanism for intrinsic resistance of BMACs against ROS 29. Decrease of TSP-2 mRNA/protein level after MnSOD overexpression is well correlated with the reverse of increased ROS by MnSOD (Figure 4), reflecting the importance of MnSOD in dys-regulation of ROS in diabetic BMACs. Due to the lack of previous studies about the upstream pathway of TSP-2, the exact mechanism allowing oxidative stress to enhance TSP-2 expression has not yet been clarified. However, the observation that oxidative stress regulates the anti-angiogenic protein of TSP-2 could give a possible explanation for BMAC impairment under high oxidative stress, as found in diverse cardiovascular disease, including diabetes, hypertension, coronary artery disease, or after smoking.
Here we have used in vitro models of adhesion, migration, and tube formation, to evaluate BMAC functions under diabetes. Each of these assays represents the critical stages that occur during BMAC-mediated angiogenesis. Importantly, silencing TSP-2 could improve all of these parameters (Figure 5). These results suggest that TSPs could modulate diverse cellular interactions including cell adhesion and migration are well supported by the literature25. An issue of using Matrigel for tube formation is that it does not indicate tubular structure with lumens. Therefore, more comprehensive approaches are needed to fully represent the angiogenic potential of BMACs. In addition, it would be interesting to look into the regulation of TSP-2 in BMAC proliferation and apoptosis in future, which represent important functional aspects of BMACs. Considering the recent progress that BMACs could be novel targets for cell therapy with possible genetic modulation, further in vivo studies would be necessary to suggest that modulation of TSP-2 could be a possible therapeutic target for vascular dysfunction in diabetes.
In this study, we have investigated the possible contribution of microRNA dysregulation to angiogenic BMAC function. Disturbed level of microRNAs has been suggested to play a role in BMAC dysfunction under pathological condition 25, 39, 40. Consistent with a previous study showing that let-7f is involved in angiogenic function in endothelial cells 23, here we found that the level of miRNA let-7f was decreased under diabetic condition (Figure 7A). Notably, the reduced level of let-7f is a causative factor for TSP-2 upregulation as well as functional impairment both in type 1 and type 2 diabetic BMACs (Figure 7B to 7D). Meanwhile, let-7f does not directly interfere with TSP-2 translation, as shown in luciferase target assay (Figure 7E). Moreover, the potencies for recovery of tube formation ability of diabetic BMACs are different between siTSP-2 and let-7f mimic (Figure 5B and 7C), suggesting the existence of other regulatory pathways for TSP-2 upregulation in diabetic BMACs other than let-7f. Further investigation will be required to clarify how let-7f and/or regulating pathways modulate TSP-2 level in BMACs.
In summary, we demonstrated that increased NADPH oxidase activity and oxidative stress induce upregulation of the anti-angiogenic protein of TSP-2, resulting in impairment of the diabetic BMAC functions of adhesion, migration, and tube formation. With this study, we believe that a novel insight into the role of TSP-2 in BMAC dysfunction is provided, with an important explanation about the oxidative stress-mediated modulation of angiogenesis in diabetes.
Supplementary Material
Significance.
Oxidative stress plays an important role in the defective vascular function in various diseases including diabetes. This study demonstrates that increased NADPH oxidase activity induces upregulation of the anti-angiogenic protein of TSP-2, resulting in impairment of diabetic BMAC functions. These findings provide useful information on oxidative stress-mediated modulation of angiogenesis. Considering recent progress that BMACs could be candidate seed cells for therapy with possible genetic modulation, modulation of TSP-2 could be a possible therapeutic target for vascular dysfunction in cardiovascular diseases.
Acknowledgment
We thank Amy Abramczyk for her technical assistance.
Funding Sources This work was supported in part by NIH/NIGMS R01 GM077352 (to AFC), VA RR&D Merit Awards I01RX000244 and 1I01RX000652 (to AFC), American Diabetes Association Research Award 7-11-BS-23 (to AFC), the National Science Foundation of China (NSFC) Key Research Project 81130004 (to AFC) and NIH 1R21CA158650 (to QDW). Dr. O-N Bae is the receipt of a research fund from the Korea Research Foundation (2012R1A1A3013240). Dr. JM Wang the receipt of the AHA Postdoctoral Fellowship 0920110G and the China Oversea Scholarship 20070320.
Non-standard Abbreviations and Acronyms
- Ad-β-Gal
adenovirus expressing β-galactosidase
- Ad-MnSOD
adenovirus expressing manganese superoxide dismutase
- Ad-DN Rac1
adenovirus expressing dominant negative Rac1
- DHE
dihydroethidium
- Dil-ac-LDL
acetylated low-density lipoprotein Dil complex
- BMAC
bone marrow angiogenic cell
- TSP-2
thrombospondin-2
Footnotes
Disclosure None.
Drs. Ok-Nam Bae and Jie-Mei Wang share the first authorship.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 2.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 3.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA : the journal of the American Medical Association. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 4.Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. The Journal of clinical investigation. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khurana R, Simons M, Martin JF, Zachary IC. Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation. 2005;112:1813–1824. doi: 10.1161/CIRCULATIONAHA.105.535294. [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 7.Kovacic JC, Moore J, Herbert A, Ma D, Boehm M, Graham RM. Endothelial progenitor cells, angioblasts, and angiogenesis--old terms reconsidered from a current perspective. Trends in cardiovascular medicine. 2008;18:45–51. doi: 10.1016/j.tcm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Shantsila E, Watson T, Lip GY. Endothelial progenitor cells in cardiovascular disorders. Journal of the American College of Cardiology. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 9.Jarajapu YP, Grant MB. The promise of cell-based therapies for diabetic complications: challenges and solutions. Circulation research. 2010;106:854–869. doi: 10.1161/CIRCRESAHA.109.213140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim KA, Shin YJ, Kim JH, Lee H, Noh SY, Jang SH, Bae ON. Dysfunction of endothelial progenitor cells under diabetic conditions and its underlying mechanisms. Archives of pharmacal research. 2012;35:223–234. doi: 10.1007/s12272-012-0203-y. [DOI] [PubMed] [Google Scholar]
- 11.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 12.Marrotte EJ, Chen DD, Hakim JS, Chen AF. Manganese superoxide dismutase expression in endothelial progenitor cells accelerates wound healing in diabetic mice. The Journal of clinical investigation. 2010;120:4207–4219. doi: 10.1172/JCI36858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 14.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. The Journal of biological chemistry. 2008;283:10930–10938. doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annual review of cell and developmental biology. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- 16.Lawler J. The functions of thrombospondin-1 and-2. Current opinion in cell biology. 2000;12:634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 17.de Fraipont F, Nicholson AC, Feige JJ, Van Meir. EG. Thrombospondins and tumor angiogenesis. Trends in molecular medicine. 2001;7:401–407. doi: 10.1016/s1471-4914(01)02102-5. [DOI] [PubMed] [Google Scholar]
- 18.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circulation research. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 19.Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochimica et biophysica acta. 2006;1765:178–188. doi: 10.1016/j.bbcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 21.Lopes N, Gregg D, Vasudevan S, Hassanain H, Goldschmidt-Clermont P, Kovacic H. Thrombospondin 2 regulates cell proliferation induced by Rac1 redox-dependent signaling. Molecular and cellular biology. 2003;23:5401–5408. doi: 10.1128/MCB.23.15.5401-5408.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci. 2008;29:12–15. doi: 10.1016/j.tips.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circulation research. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 24.Wang XR, Zhang MW, Chen DD, Zhang Y, Chen AF. AMP-activated protein kinase rescues the angiogenic functions of endothelial progenitor cells via manganese superoxide dismutase induction in type 1 diabetes. American journal of physiology. Endocrinology and metabolism. 2011;300:E1135–1145. doi: 10.1152/ajpendo.00001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. American journal of physiology. Endocrinology and metabolism. 2010;299:E110–116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie HH, Zhou S, Chen DD, Channon KM, Su DF, Chen AF. GTP cyclohydrolase I/BH4 pathway protects EPCs via suppressing oxidative stress and thrombospondin-1 in salt-sensitive hypertension. Hypertension. 2010;56:1137–1144. doi: 10.1161/HYPERTENSIONAHA.110.160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Suarez J, Fricovsky E, Wang H, Scott BT, Trauger SA, Han W, Hu Y, Oyeleye MO, Dillmann WH. Increased enzymatic O-GlcNAcylation of mitochondrial proteins impairs mitochondrial function in cardiac myocytes exposed to high glucose. The Journal of biological chemistry. 2009;284:547–555. doi: 10.1074/jbc.M808518200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armstrong LC, Bjorkblom B, Hankenson KD, Siadak AW, Stiles CE, Bornstein P. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Molecular biology of the cell. 2002;13:1893–1905. doi: 10.1091/mbc.E01-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke; a journal of cerebral circulation. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- 33.Krady MM, Zeng J, Yu J, MacLauchlan S, Skokos EA, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. The American journal of pathology. 2008;173:879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Werner C, Kamani CH, Gensch C, Bohm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56:2609–2615. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 35.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 36.Fleissner F, Thum T. Critical role of the nitric oxide/reactive oxygen species balance in endothelial progenitor dysfunction. Antioxidants & redox signaling. 2011;15:933–948. doi: 10.1089/ars.2010.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vecchione C, Aretini A, Marino G, Bettarini U, Poulet R, Maffei A, Sbroggio M, Pastore L, Gentile MT, Notte A, Iorio L, Hirsch E, Tarone G, Lembo G. Selective Rac-1 inhibition protects from diabetes-induced vascular injury. Circulation research. 2006;98:218–225. doi: 10.1161/01.RES.0000200440.18768.30. [DOI] [PubMed] [Google Scholar]
- 38.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minami Y, Satoh M, Maesawa C, Takahashi Y, Tabuchi T, Itoh T, Nakamura M. Effect of atorvastatin on microRNA 221 / 222 expression in endothelial progenitor cells obtained from patients with coronary artery disease. European journal of clinical investigation. 2009;39:359–367. doi: 10.1111/j.1365-2362.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- 40.Meng S, Cao JT, Zhang B, Zhou Q, Shen CX, Wang CQ. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. Journal of molecular and cellular cardiology. 2012;53:64–72. doi: 10.1016/j.yjmcc.2012.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.