Abstract
Influenza A infection induces a massive inflammatory response in the lungs that leads to significant illness and increases the susceptibility to secondary bacterial pneumonia. The most efficient way to prevent influenza infection is through vaccination. While inactivated vaccines induce protective levels of serum antibodies to influenza hemaglutinin (HA) and neuraminidase (NA) surface proteins, these are strain specific and offer little protection against heterosubtypic influenza viruses. In contrast, live attenuated influenza vaccines (LAIVs) induce a T cell response in addition to antibody responses against HA and NA surface proteins. Importantly, LAIV vaccination induces a response in a mouse model that protects against illness due to heterosubtypic influenza strains. While it is not completely clear what is the mechanism of action of LAIV heterosubtypic protection in humans, it has been shown that LAIV induces heterosubtypic protection in mice that is dependent upon a Type 1 immune response and requires CD8 T cells. In this study, we show that LAIV-induced immunity leads to significantly reduced viral titers and inflammatory responses in the lungs of mice following heterosubtypic infection. Not only are viral titers reduced in LAIV vaccinated mice, the amounts of inflammatory cytokines and chemokines in lung tissue are significantly lower. Additionally, we show that LAIV vaccination of healthy adults also induces a robust Type 1 memory response including the production of chemokines and cytokines involved in T cell activation and recruitment. Thus, our results indicate that LAIV vaccination functions by inducing immune memory which can act to modulate the immune response to subsequent heterosubtypic challenge by influencing both innate and adaptive responses.
Keywords: Influenza, Vaccination, Heterosubtypic, LAIV
1. Introduction
The response to influenza A infection is characterized by an initial innate immune response followed by an adaptive immune response with T cell infiltrates in the lungs. Influenza infection triggers toll-like receptors and retinoic acid-inducible protein I activation in response to viral RNA [1,2]. This begins a cascade of responses, including the production of inflammatory cytokines and chemokines in the lung and recruitment of innate and then adaptive immune cells. Because of this, the immune response to influenza is very robust and is usually characterized by a significant amount of immunopathology [3,4]. The ultimate result of this immune response and accompanying pathology is that the host is often rendered highly susceptible to secondary bacterial pneumonia, which is a major cause of death during influenza pandemics [5,6].
One effective way to prevent influenza infection is the administration of the yearly influenza vaccine [7]. Inactivated vaccines induce protective immunity via the production of neutralizing serum antibodies against the surface hemaglutinin (HA) and neuraminidase (NA) viral proteins, which are strain specific. Thus, the main drawback of this type of vaccine is that it only induces homotypic immunity to viruses expressing the specific HA and NA molecules in the vaccine. In contrast, immunization with viruses of different subtypes that do not share HA and NA similarities induces heterosubtypic immunity [8–15]. Heterosubtypic immunity does not involve neutralizing antibodies and, thus, does not provide protection from infection. Instead, it does provide cross reactive T cell responses that result in limited viral replication and greatly reduced disease severity and mortality [16]. An example of this is the LAIV vaccination, which induces T cell responses due to the fact that the virus replicates in nasopharyngeal epithelial cells and viral proteins are presented in the context of major histocompatibility proteins, which can then stimulate virus-specific T cells. Since these responding T cells are specific for conserved internal viral proteins as well as surface HA and NA proteins, they contribute to heterosubtypic immunity, protecting from a wider range of influenza viruses.
Even though LAIV vaccines have been in use in the United States since 2003, the actual mechanism of protection of LAIV is not fully understood. It has been shown in mouse models that LAIV vaccination leads to an accelerated recruitment of T cells to the lungs following heterosubtypic influenza infection [10]. We investigated whether this accelerated T-cell recruitment would be associated with accelerated viral clearance and reduced inflammation in the lungs of mice, and found this to be the case. We also examined the immune memory response induced by LAIV vaccination of healthy adult volunteers and found that a robust Type 1 memory response was induced, including production of IFNγ, CCL2, CXCL8 and CXCL10. Our results suggest that LAIV vaccination functions by inducing Type 1 immune memory, which leads to accelerated T cell recruitment, reduced viral titers and reduced lung inflammation following influenza infection.
2. Materials and methods
2.1. Mice
Wild type C57BL/6 mice were purchased from The Jackson Laboratory and bred in the specific pathogen free Trudeau Institute Animal Breeding Facility after embryo rederivation. For these studies, we used 6 week old male mice which were cared for according to Trudeau Institute guidelines. Recumbent mice and mice that lost more than 30% weight were considered moribund and euthanized.
2.2. Viruses
Influenza virus A/PR/8/34 (H1N1), the Enders strain of Sendai virus and cold-adapted influenza virus c.a.A/Alaska/72/CR9 (LAIV) were grown, stored, and titered as previously described [10,17,18]. Vaccination was administered intranasally (i.n.) to anesthetized mice using 350 TCID-50 LAIV or 250 EID-50 Sendai virus; control groups received PBS. Three weeks following vaccination, all groups received influenza infection i.n. using 400 EID-50 H1N1. At the indicated time points (day 1, 3 or 5), viral burden in whole lung tissue was determined by real-time PCR measuring viral acid polymerase copy number [19]. Unless otherwise indicated, 5 mice/group were used for these studies.
2.3. Real-time PCR
At each time point following H1N1 infection (day 1, 3 or 5), RNA was extracted from lung tissue using the RNeasy Kit (Qiagen) following manufacturer’s instructions. RNA samples were reverse transcribed to generate cDNA, which was amplified with Taqman reagents on the ABI Prism 7700 sequence detection system (Applied Biosystems). Primers and probes were purchased from Applied Biosystems Gene Expression Assays. The genes assayed for each mouse were CXCL9, CXCL11, IFNγ IL-1β, IL-6, IL-12p40, CCL2, CCL3, CCL4 and CXCL1. Levels of gene expression were normalized to levels of GAPDH for each sample and the fold increase in signal was determined using the ΔΔCT calculation recommended by Applied Biosystems. Results shown are log fold changes in gene expression compared to uninfected controls.
2.4. Differential counts
Bronchiolar lavage fluid (BAL) was collected from lungs of animals sacrificed on day 5 following H1N1 challenge. BAL cells were centrifuged, resuspended in 100 μl PBS and cytospin slides were generated. After drying, differential stain (Diff-quick, IMEB Inc.) was applied according to manufacturer’s protocol. Differential counts (200 cells per sample) were performed to distinguish the percent macrophages, polymorphonuclear cells (PMNs) and lymphocytes.
2.5. Study enrollment and vaccination
Eligible subjects gave written informed consent before voluntary enrollment at the Naval Health Research Center in accordance with Institutional Review Board protocol NHRC.2008.0041. Enrollees provided 25 ml baseline and convalescence blood draws prior to and after LAIV vaccination. All participants received the standard dose of the LAIV (FluMist®, MedImmune) for the 2009–2010 season. The initial blood draw was conducted 1 week prior to vaccination. Subsequent blood draws were repeated at 4, 7, and 11 weeks post vaccination. Blood samples were collected in 8.5 ml serum-separator tubes (BD Vacutainer, Becton Dickinson). Tubes were processed according to manufacturer’s instructions. Specimens were shipped overnight at ambient temperature.
2.6. PBMC culturing
PBMCs were isolated from peripheral blood by Histopaque 1077 (Sigma–Aldrich) gradient purification. Cells were cultured at 2 × 106/200 μl in serum-free Aim V medium (Invitrogen Life Technologies) with or without 50 μl of the 2009 Flumist vaccine in 48 well plates. After 24 h at 37 °C, supernatants were harvested and then frozen for later analysis. Cytokine and chemokine production was assessed using the Luminex Human Panel 1 (Millipore). Specific cytokine production was determined by subtracting the cytokine concentration from the medium-alone wells from the Flumist stimulated wells.
2.7. Statistics
For human studies, statistical analysis was performed by comparing the values for each time point to the prevaccination values. For murine studies, analysis was performed by comparing the LAIV vaccinated (or Sendai virus vaccinated) and unvaccinated groups. Significance was determined by unpaired Student’s t test (for murine studies) or paired Student’s t test (for human studies). Values of less than 0.05 were considered significant.
3. Results
3.1. LAIV induces heterosubtypic influenza immunity in a mouse model
Influenza A infection of mice induces an illness that is characterized by high viral titers and weight loss. Heterosubtypic immunity, which is typically achieved by prior influenza infection [8–10], protects from these symptoms of influenza infection. It has been shown that LAIV vaccination abrogates this illness and confers heterosubtypic immunity by several groups [8–10,13–15] and in Fig. 1. Mice were given LAIV i.n. and unvaccinated control groups were given PBS. Three weeks later all groups were challenged i.n. with a sublethal dose of heterosubtypic H1N1 influenza. This time point was chosen in order to determine the rapidity of protection and enhanced response following vaccination, which is important when considering vaccine development for an emerging influenza pandemic. Prior vaccination with LAIV resulted in reduced viral titers and less weight loss following subsequent H1N1 influenza challenge in vaccinated groups (LAIV/H1N1) compared to groups not receiving LAIV (PBS/H1N1) (Fig. 1). This protection was specific since animals vaccinated with Sendai virus, which is a respiratory parainfluenza virus that is not related to influenza A, do not exhibit protection with regards to H1N1 challenge or weight loss. Interestingly, the Sendai virus infected group lost even more weight than the PBS control group, which could be due to the fact that the Sendai virus infection, unlike LAIV, left these animals even more susceptible to the effects of influenza infection. These results indicate that LAIV vaccination can specifically limit heterosubtypic viral titers and protects from influenza-induced weight loss.
Fig. 1.
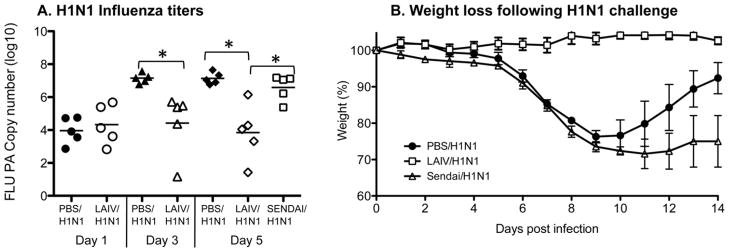
LAIV vaccination induces heterosubtypic protection. Mice were given LAIV, Sendai virus or PBS. After 3 weeks all mice were challenged with a sublethal dose of H1N1. (A) On days 1, 3 and 5 (lungs from Sendai virus vaccinated mice were harvested on day 5 only) after H1N1 challenge the titers of H1N1 in the lungs were determined by real time PCR for the viral acid polymerase gene. Each symbol indicates an individual mouse (*p < 0.05 by unpaired Student’s t test) and bar depicts group mean. (B) Mice were weighed every day following H1N1 challenge. Mean ± SD for N = 5 is shown.
3.2. LAIV induces a reduced inflammatory response upon heterosubtypic influenza challenge
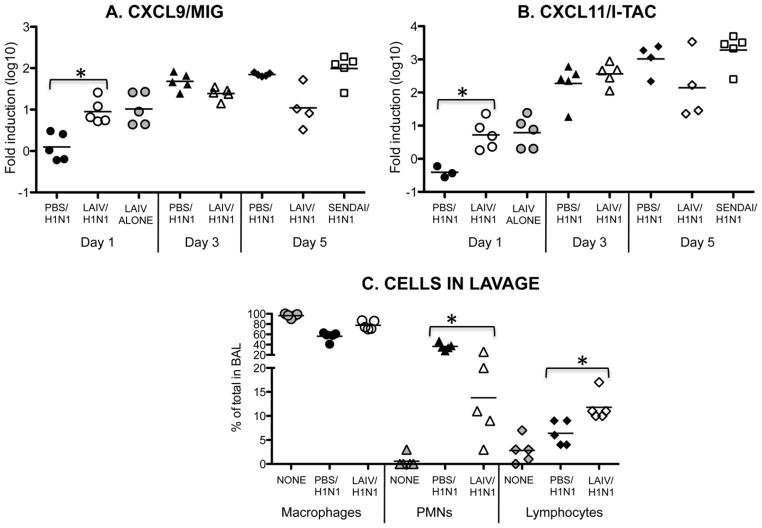
While Dutton and colleagues have shown that this heterosubtypic protection is abrogated when CD8 T cells are depleted [10], the actual mechanism of how LAIV protects is not well understood. The early influx of memory CD8 T cells to the lungs is vitally important for controlling viral titers early. In LAIV vaccinated mice, virus-specific CD4 and CD8 T cells can be found in the lungs as early as day 4 following heterosubtypic challenge [10]. Importantly, early T cell recruitment will result in accelerated reduction of viral titers and reduced levels of lung inflammation, which, in turn, will lead to reduced illness [20]. To examine this further, the levels of chemokine mRNA in the lung tissue of the influenza infected mice was determined. Following LAIV vaccination and H1N1 challenge, there were significantly higher levels of CXCL9 and CXCL11 expression in lung tissue when compared to the PBS group (Fig. 2A and B). Importantly, higher levels of both of these chemokines were also found in LAIV vaccinated mice without H1N1 challenge (LAIV alone group), indicating that this mode of vaccination does in fact change the lung environment for at least 3 weeks. The advantage of this is that these two chemokines are important for T cell recruitment to the lungs following infection [21,22] and we found that by day 5 following H1N1 challenge, there were significantly more lymphocytes in BAL from the LAIV vaccinated group compared to the PBS control group which was not different from uninfected controls (Fig. 2C). Interestingly, there was also a greater percentage of PMNs in the BAL from the PBS control group compared to the LAIV vaccinated group, indicating a higher level of lung inflammation in these animals
Fig. 2.
LAIV vaccination rapidly induces chemokines involved in enhanced lymphocyte recruitment to lungs. Mice were given LAIV, Sendai virus or PBS. After 3 weeks all mice were challenged with a sublethal dose of H1N1 except for the LAIV alone group (day 1), which did not receive H1N1 challenge. On days 1, 3 and 5 (lungs from Sendai virus vaccinated mice were harvested on day 5 only) following challenge lungs were harvested, cDNA was prepared and real time PCR was performed for (A) CXCL9 and (B) CXCL11. (C) On day 5 following H1N1 challenge of mice vaccinated with LAIV or PBS, the percent of each cell type present in bronchiolar lavage fluid (BAL) was determined by differential staining to distinguish macrophages, polymorphonuclear cells (PMNs) and lymphocytes. Cell types present in uninfected animals (NONE) are also included for comparison. Each symbol indicates an individual mouse (*p < 0.05 in unpaired Student’s t test) and bar depicts group mean.
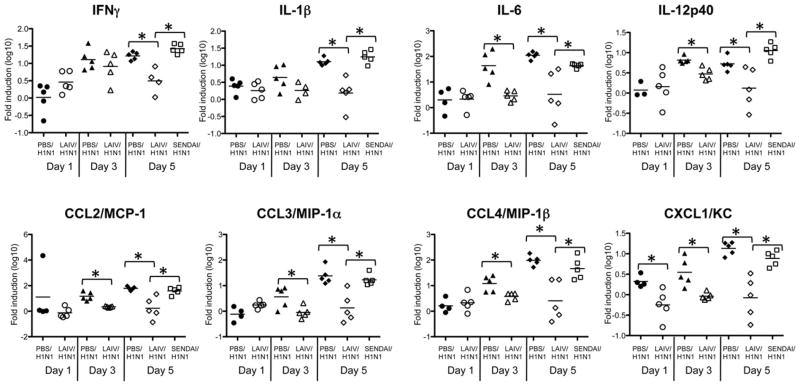
This enhanced inflammation in the PBS control animals was also demonstrated by examining cytokines and chemokines in lung tissue following H1N1 challenge. Inflammatory cytokine production was significantly lower in LAIV vaccinated animals even as early as days 3 and 5 following challenge (Fig. 3). In the LAIV vaccinated group, there were lower levels of the Type 1 cytokines IL-12p40 and IFNγ, as well as the innate cytokines IL-1β and IL-6, which are involved in inflammatory processes following viral infection. In addition in the LAIV vaccinated group, chemokines that induce inflammation were reduced including CCL2, which attracts monocytes and dendritic cells, and CCL3, CCL4 and CXCL1, which attract neutrophils [23–26]. This reduction in inflammation in the LAIV vaccinated group corresponds with the lower viral titers shown in Fig. 1 and is influenza A specific since it does not occur in the Sendai virus/H1N1 group.
Fig. 3.
LAIV vaccination reduces inflammatory cytokines and chemokines in the lungs following influenza challenge. Using the same samples as in Fig. 2, real time PCR was performed for the indicated cytokines and chemokines. Each symbol indicates an individual mouse (*p < 0.05 in unpaired Student’s t test) and bar depicts group mean.
3.3. LAIV induces a Type 1 memory response in humans
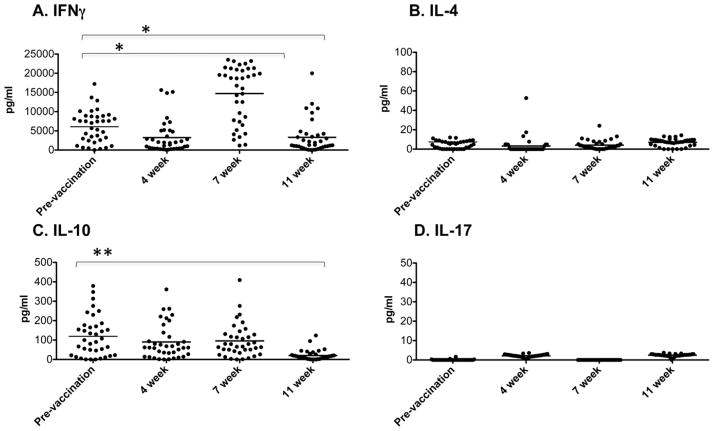
In order to examine the immune memory resulting from vaccination with LAIV, healthy adult volunteers were given the 2009 FluMist® vaccine. Peripheral blood samples were collected from these volunteers prior to vaccination and at 4, 7 and 11 weeks post-vaccination. PBMCs were cultured with or without the LAIV vaccine and culture supernatants were assayed for cytokines. Prior to vaccination, there was production of IFNγ and IL-10 but not IL-4 or IL-17 by PBMC in response to ex vivo stimulation with the LAIV (Fig. 4), indicating the prior presence of some memory cells responsive to this stimulation. Following vaccination, there was a decline in IFNγ production at 4 weeks, a significant increase at 7 weeks and then a decline by 11 weeks. In contrast, IL-10 production was unchanged following vaccination at 4 and 7 weeks but was significantly reduced at 11 weeks and no IL-4 or IL-17 production was observed following vaccination. Thus, since only IFNγ production was specifically enhanced following LAIV vaccination, this is a typical Type 1 immune response [27].
Fig. 4.
LAIV vaccination induces a Type 1 memory response in human subjects. Peripheral blood samples were taken 1 week prior to and 4, 7 and 11 weeks after administration of LAIV. PBMCs were isolated and cultured with the LAIV or with medium alone (2 × 106/200 μl per well). Supernatants were collected after 24 h and cytokine concentrations were determined by the Luminex multiplex system. For each cytokine, background production (from medium alone cultures) was subtracted from the values for the LAIV stimulated cultures. Each symbol represents an individual person (*p < 0.05, **p < 0.0001 by paired Student’s t test comparing each time point to prevaccination values) and bar depicts group mean.
In addition, we found that all of the cytokines shown in Fig. 5 (GM-CSF, IL-1β IFNα and IL-6) express a similar pattern of expression, with some production pre-vaccination, a slight decrease at 4 weeks (some not significant), a significant increase at 7 weeks and then a decline at 11 weeks post-vaccination. This is interesting since IL-6 and IFNα are produced in response to influenza virus [28], while GM-CSF and IL-1β further enhance the Type 1 phenotype of the response to the LAIV vaccination: GM-CSF by inducing Type 1 cytokine production by T cells and IL-1 by driving clonal expansion of the responding T cells [29–31]. Thus, these innate cytokines can enhance and promote virus-specific T cell memory cells.
Fig. 5.
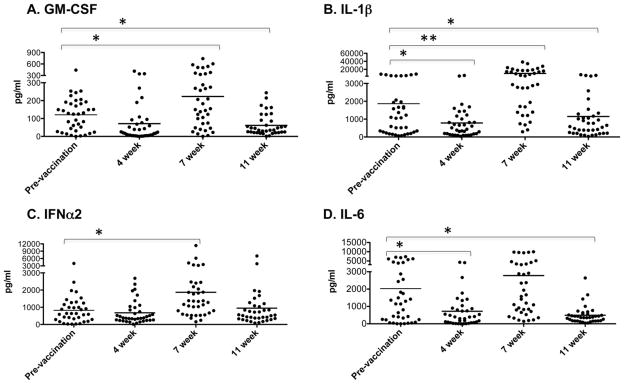
LAIV vaccination induces innate cytokine production. Cytokine production by PBMCs was examined by Luminex as in Fig. 4. Each symbol represents an individual person (*p < 0.05, **p < 0.0001 by paired Student’s t test comparing each time point to prevaccination values) and bar depicts group mean.
3.4. LAIV induces the production of chemokines involved in a recall response
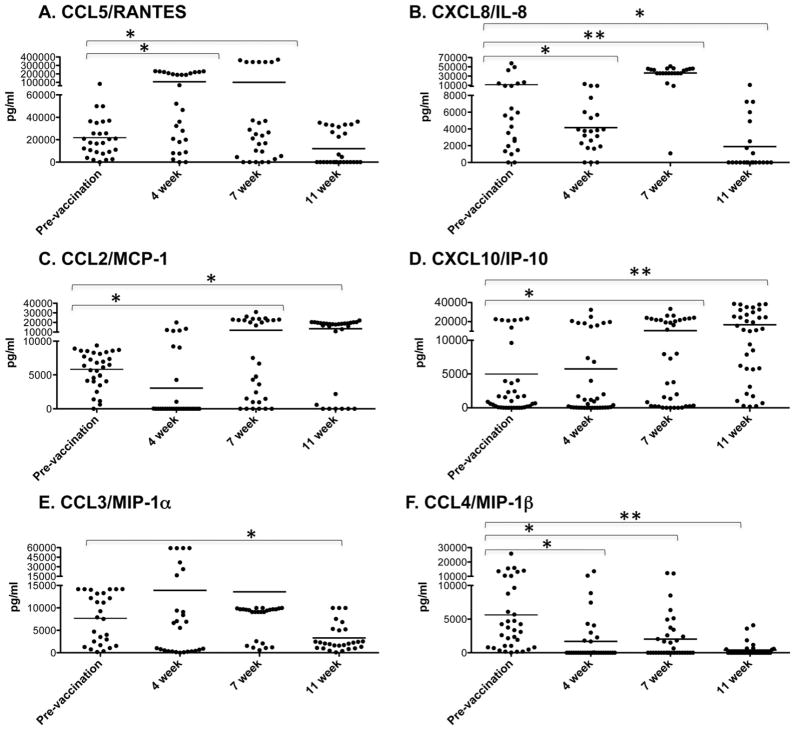
We also examined chemokine production following ex vivo stimulation of PBMCs (Fig. 6). There were three patterns of chemokine production observed. First, CCL5 (T cell recruiting [32]) and CXCL8 (attracts cytotoxic CD8 T cells [33]) showed an increase at 4 and/or 7 weeks followed by a decrease in production at 11 weeks post-vaccination. This pattern indicates that these chemokines are produced relatively early after vaccination and that they wane rather quickly. Second, CCL2 and CXCL10 (both of which recruit T cells and antigen presenting cells [24,34]) showed an increase in production at 7 and 11 weeks compared to pre-vaccination. This pattern indicates that production of these chemokines is sustained and does not wane within the time frame of this study. Lastly, CCL3 and CCL4, which are important for macrophage recruitment and inflammation [25], were unchanged or decreased at all time points compared to pre-vaccination levels. This pattern indicates that these chemokines are not produced to any great extent in response to the LAIV vaccination.
Fig. 6.
LAIV vaccination induces chemokines involved in memory T cell and DC recruitment. Chemokine production by PBMCs was examined by Luminex as in Fig. 4. Each symbol represents an individual person (*p < 0.05, **p < 0.0001 by paired Student’s t test comparing each time point to prevaccination values) and bar depicts group mean.
4. Discussion
Despite vaccination efforts, influenza infects over 24 million people in the United States annually with dramatic consequences for public health [35]. Inactivated influenza vaccines induce protection via the generation of neutralizing antibodies directed to the HA and NA molecules on the surface of the virus. While these antibodies usually function well to block viral infection, the HA and NA molecules are strain specific and antibodies directed against them are not protective if there is antigenic drift and antigenic shift or if a new strain of influenza virus appears. Thus, the interest in vaccines that induce heterosubtypic immunity to a wide range of influenza virus strains has been prevalent. Heterosubtypic immunity is due to both T cells and non-neutralizing antibodies and does not prevent infection but does limit viral replication and greatly reduces disease severity and mortality [10,14–16,36].
While the LAIV has been in use in the United States for a number of years, its mode of action is not totally understood. In this study, we have explored how LAIV vaccination can lead to protective heterosubtypic immunity in a mouse model. Our results show that LAIV vaccination reduces viral titers early during infection and prevents weight loss. This is associated with the enhanced expression of chemokines involved in recruitment of T cells into lung tissue following heterosubtypic influenza challenge. We also found that in LAIV vaccinated animals there was reduced expression of inflammatory cytokines and chemokines in the lungs compared to unvaccinated groups. Thus, the effects of the LAIV vaccine are to boost early recruitment of T cells, which can then clear virus more rapidly. This accelerated clearance of virus would then result in lower levels of inflammation in the lung. Presumably, lower levels of inflammation will also lead to reduced pathology and reduced susceptibility to secondary infections. We construe from these studies that LAIV vaccination induces heterosubtypic immunity and that immune memory resulting from this LAIV vaccination can act to modulate the immune response to subsequent challenge by influencing both innate and adaptive responses.
This study went on to examine responses in LAIV vaccinated people. While there was some pre-existing responsiveness found in the prevaccination samples, we found that there was a robust Type 1 memory response, characterized by production of IFNγ and chemokines involved in T cell and dendritic cell recruitment following vaccination. By examining ex vivo restimulation of PBMCs, we were able to show that this response was specific to the vaccinating LAIV. The memory response to the LAIV peaked at 7 weeks post-vaccination and included the production of GM-CSF and IL-1β in addition to IFNγ. Both GM-CSF and IL-1β are important for cytokine production by and proliferation of responding T cells and reinforce the Type 1 memory response to the vaccine [29–31]. In addition, IFNα and IL-6, both of which are produced in response to influenza virus [28], also peaked at 7 weeks post-vaccination.
Chemokines involved in T cell recruitment and activation were also produced following LAIV vaccination. We found three patterns of chemokine production. CCL5 and CXCL8 were produced early following vaccination and then waned by 11 weeks. CCL5, which binds CCR5, plays a key role in the early memory CD8+ T cell response to respiratory virus infections [18]. CXCL8, which binds the IL-8 receptor, identifies a CD8 T cells subset that is enriched in perforin, granzyme B, and IFNγ, and has high cytotoxic potential which is important for anti-viral responses [33]. Thus, these two chemokines are likely involved in early memory and/or late effector responses following vaccination. In contrast, CCL2 and CXCL10 were generated at later time points and did not wane during this study. CCL2, which binds to CCR2 on monocytes and dendritic cells, acts to direct them from the blood to the site of infection, where they can activate responding T cells and promote a Type 1 immune response [24,26]. CXCL10, which binds CXCR3, directs memory T cells to site of infection [37]. Thus, these two chemokines are likely more important for long term memory following vaccination. Finally, CCL3 and CCL4 are not induced above prevaccination levels following vaccination and levels are quite low by 11 weeks. Both of these chemokines are involved in neutrophil recruitment and are important for inflammatory responses [25], which are not likely to be important for sustained immune memory. Thus, the patterns of chemokines produced serve to promote a memory T cell response, which can then serve to confer protection from subsequent influenza infection.
5. Conclusions
In a mouse model, LAIV induced heterosubtypic immunity results in faster virus clearance. This is associated with reduced weight loss and inflammatory cytokine and chemokine expression in the lungs. In humans, LAIV vaccination induces a potent Type 1 response characterized by IFNγ production and expression of chemokines involved in T cell and APC recruitment. Thus, LAIV vaccination can act to modulate the immune response to subsequent challenge by influencing both innate and adaptive responses.
Acknowledgments
This project was funded by Department of Defense contract W911QY-09-C-0014. We would like to thank Dr. Larry Johnson for assistance with statistical analysis and Dr. Brian Murphy for providing the c.a.A/Alaska/72/CR9 virus.
Abbreviations
- HA
hemaglutinin
- NA
neuraminidase
- LAIV
live attenuated influenza vaccine
References
- 1.Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, Uematsu S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007 Oct 7;179:4711–20. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 2.Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, et al. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007 Mar 6;178:3368–72. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- 3.La Gruta NL, Kedzierska K, Stambas J, Doherty PC. A question of self-preservation: immunopathology in influenza virus infection. Immunol Cell Biol. 2007 Feb-Mar;85:85–92. doi: 10.1038/sj.icb.7100026. [DOI] [PubMed] [Google Scholar]
- 4.Peiris JS, Hui KP, Yen HL. Host response to influenza virus: protection versus immunopathology. Curr Opin Immunol. 2010 Aug 4;22:475–81. doi: 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brundage JF, Shanks GD. Deaths from bacterial pneumonia during 1918–1919 influenza pandemic. Emerg Infect Dis. 2008 Aug 8;14:1193–9. doi: 10.3201/eid1408.071313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill JR, Sheng ZM, Ely SF, Guinee DG, Beasley MB, Suh J, et al. Pulmonary pathologic findings of fatal 2009 pandemic influenza A/H1N1 viral infections. Arch Pathol Lab Med. 2010 Feb 2;134:235–43. doi: 10.5858/134.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couch RB. Seasonal inactivated influenza virus vaccines. Vaccine. 2008 Sep;26(Suppl 4):D5–9. doi: 10.1016/j.vaccine.2008.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton KA, Misplon JA, Lo CY, Brutkiewicz RR, Prasad SA, Epstein SL. Heterosubtypic immunity to influenza A virus in mice lacking IgA, all Ig, NKT cells, or gamma delta T cells. J Immunol. 2001 Jun 12;166:7437–45. doi: 10.4049/jimmunol.166.12.7437. [DOI] [PubMed] [Google Scholar]
- 9.Liang S, Mozdzanowska K, Palladino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. Effector mechanisms and their longevity. J Immunol. 1994 Feb 4;152:1653–61. [PubMed] [Google Scholar]
- 10.Powell TJ, Strutt T, Reome J, Hollenbaugh JA, Roberts AD, Woodland DL, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007 Jan 2;178:1030–8. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 11.Schulman JL, Kilbourne ED. Induction of partial specific heterotypic immunity in mice by a single infection with influenza A virus. J Bacteriol. 1965 Jan;89:170–4. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yetter RA, Lehrer S, Ramphal R, Small PA., Jr Outcome of influenza infection: effect of site of initial infection and heterotypic immunity. Infect Immun. 1980 Aug 2;29:654–62. doi: 10.1128/iai.29.2.654-662.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armerding D, Rossiter H, Ghazzouli I, Liehl E. Evaluation of live and inactivated influenza A virus vaccines in a mouse model. J Infect Dis. 1982 Mar 3;145:320–30. doi: 10.1093/infdis/145.3.320. [DOI] [PubMed] [Google Scholar]
- 14.Lo CY, Wu Z, Misplon JA, Price GE, Pappas C, Kong WP, et al. Comparison of vaccines for induction of heterosubtypic immunity to influenza A virus: cold-adapted vaccine versus DNA prime-adenovirus boost strategies. Vaccine. 2008 Apr 17;26:2062–72. doi: 10.1016/j.vaccine.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Tannock GA, Paul JA. Homotypic and heterotypic immunity of influenza A viruses induced by recombinants of the cold-adapted master strain A/Ann Arbor/6/60-ca. Arch Virol. 1987;92(1–2):121–33. doi: 10.1007/BF01310067. [DOI] [PubMed] [Google Scholar]
- 16.Epstein SL, Price GE. Cross-protective immunity to influenza A viruses. Expert Rev Vaccines. 2010 Nov 11;9:1325–41. doi: 10.1586/erv.10.123. [DOI] [PubMed] [Google Scholar]
- 17.Murphy BR, Chanock RM, Clements ML, Anthony WC, Sear AJ, Cisneros LA, et al. Evaluation of A/Alaska/6/77 (H3N2) cold-adapted recombinant viruses derived from A/Ann Arbor/6/60 cold-adapted donor virus in adult seronegative volunteers. Infect Immun. 1981 May 2;32:693–7. doi: 10.1128/iai.32.2.693-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlmeier JE, Miller SC, Smith J, Lu B, Gerard C, Cookenham T, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008 Jul 1;29:101–13. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jelley-Gibbs DM, Dibble JP, Brown DM, Strutt TM, McKinstry KK, Swain SL. Persistent depots of influenza antigen fail to induce a cytotoxic CD8 T cell response. J Immunol. 2007 Jun 12;178:7563–70. doi: 10.4049/jimmunol.178.12.7563. [DOI] [PubMed] [Google Scholar]
- 20.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol. 2001 Jan 1;158:119–30. doi: 10.1016/s0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farber JM. Mig IP-10 CXC chemokines that target lymphocytes. J Leukoc Biol. 1997 Mar 3;61:246–57. [PubMed] [Google Scholar]
- 22.Tensen CP, Flier J, Van Der Raaij-Helmer EM, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R, et al. Human IP-9: a keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3) J Invest Dermatol. 1999 May 5;112:716–22. doi: 10.1046/j.1523-1747.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 23.Baggiolini M. Activation and recruitment of neutrophil leukocytes. Clin Exp Immunol. 1995 Jul;101(Suppl 1):5–6. doi: 10.1111/j.1365-2249.1995.tb06151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994 Apr 9;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menten P, Wuyts A, Van Damme J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002 Dec 6;13:455–81. doi: 10.1016/s1359-6101(02)00045-x. [DOI] [PubMed] [Google Scholar]
- 26.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009 Apr 4;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carter LL, Dutton RW. Type 1 and type 2: a fundamental dichotomy for all T-cell subsets. Curr Opin Immunol. 1996 Jun 3;8:336–42. doi: 10.1016/s0952-7915(96)80122-1. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser L, Fritz RS, Straus SE, Gubareva L, Hayden FG. Symptom pathogenesis during acute influenza: interleukin-6 and other cytokine responses. J Med Virol. 2001 Jul 3;64:262–8. doi: 10.1002/jmv.1045. [DOI] [PubMed] [Google Scholar]
- 29.Eksioglu EA, Mahmood SS, Chang M, Reddy V. GM-CSF promotes differentiation of human dendritic cells and T lymphocytes toward a predominantly type 1 proinflammatory response. Exp Hematol. 2007 Aug 8;35:1163–71. doi: 10.1016/j.exphem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Res. 2006 Feb 2;16:126–33. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009 Apr 17;106:7119–24. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song A, Nikolcheva T, Krensky AM. Transcriptional regulation of RANTES expression in T lymphocytes. Immunol Rev. 2000 Oct;177:236–45. doi: 10.1034/j.1600-065x.2000.17610.x. [DOI] [PubMed] [Google Scholar]
- 33.Hess C, Means TK, Autissier P, Woodberry T, Altfeld M, Addo MM, et al. IL-8 responsiveness defines a subset of CD8 T cells poised to kill. Blood. 2004 Dec 12;104:3463–71. doi: 10.1182/blood-2004-03-1067. [DOI] [PubMed] [Google Scholar]
- 34.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. 2002 Apr 7;168:3195–204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 35.Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 Jun 27;25:5086–96. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Lamere MW, Lam HT, Moquin A, Haynes L, Lund FE, Randall TD, et al. Contributions of antinucleoprotein IgG to Heterosubtypic immunity against influenza virus. J Immunol. 2011 Apr 7;186:4331–9. doi: 10.4049/jimmunol.1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kohlmeier JE, Cookenham T, Miller SC, Roberts AD, Christensen JP, Thomsen AR, et al. CXCR3 directs antigen-specific effector CD4+ T cell migration to the lung during parainfluenza virus infection. J Immunol. 2009 Oct 7;183:4378–84. doi: 10.4049/jimmunol.0902022. [DOI] [PMC free article] [PubMed] [Google Scholar]