Abstract
Aims:
To evaluate the trend of clinical trials in India over the last 4 years compared to the well-established countries using clinical trial registries since the advent of clinical trial registry of India (CTRI).
Materials and Methods:
The data of clinical trials registered in India, United States (US), and European Union (EU) were obtained from websites of CTRI, clinicaltrial.gov and EU clinical trial registry, respectively from July 20, 2007 to August 29, 2011 for a period of 4 years. Trials registered in Australia, Canada, China, and Japan were obtained from WHO's international clinical trial registry platform for the same period. We used search words for the common diseases such as diabetes, hypertension, etc.,
Results:
The total number of clinical trials registered during the study period was 67,448 across seven study nations. Clinical trials from India constituted only 2.7% of the total number of trials carried out, compared to US constituting 47% of the total number of trials registered, followed by 18% from EU and 11% from Japan. However, India, China, and Japan have been found to show an increase of 3.7%, 5.1%, and 13.1% increase in the number of trials registered in 2011 compared to 2007. In contrast, US and EU showed a decline of 11.3% and 11.95% respectively in the total number of trials registered in 2011 compared to 2007.
Conclusions:
Although India shows gradual increase in trials registered since the advent of CTRI, still it continues to lag behind established countries in clinical research.
Keywords: Clinical trial registry of India, global clinical trials, world health organization's international clinical trial registry platform
INTRODUCTION
Asia contributing to nearly 60% of the world population started venturing into clinical research arena following globalization of clinical trials. Currently, the growth of the clinical research market in Asia is expected to mount faster than United States (US) and Europe. It was estimated that, approximately 30% of the global contract research market supporting clinical research activities, including research and development (R & D) of pharma industries will be outsourced to developing countries by 2008.[1] The swift attention toward the Asian countries may be attributed to the quest of global pharmaceutical companies, in exploring newer avenues to expand their business enterprise.[2] This has been further influenced with the spread of clinical research organizations in Asian countries to cope with the expanding market. The major attractions to conduct clinical trials in Asia is attributed to the increasing prevalence of “Western” diseases like diabetes mellitus, hypertension, dyslipidemia, etc., with the changing dietary pattern and sedentary life style.[3,4]
Among the Asian countries, Japan, Hong Kong, and Singapore are more experienced and already have well-established infrastructure for clinical research. In contrast, countries like India, China, and Korea are actively involved in global clinical trials only in the last few years.[5] In spite of delayed entry, India and China are expected to have tremendous growth potential in clinical research due to their huge disease prevalence and treatment-naïve patient pools. India has the added benefits of vast genetically diverse population, well-equipped hospitals, and highly qualified English-speaking investigators making it one of the preferred destinations for conducting global clinical trials.[6,7] The contract research and manufacturing services in India was expected to increase from $3.8 billion in 2010 to nearly $7.6 billion in 2012.[8]
Recently there has been a claim that India is fast emerging as a favorite clinical trial hub for global pharmaceutical companies.[9] With such claims it becomes mandatory to explore the authentic status of clinical trials in India. Hence this study was designed to evaluate the trend of clinical trials in India over the last 4 years compared to the well-established countries using clinical trial registries since the advent of clinical trial registry of India (CTRI).
MATERIALS AND METHODS
This study was done based on an internet search from different clinical trial registries since the commencement of CTRI in July 20, 2007 to August 29, 2011 for a period of 4 years.[10] Since the study was done to find out the status of clinical trials in India, we chose CTRI as a source of information and hence the date of inception of CTRI was taken as the starting period of the study. Similarly, to learn the pattern of clinical trials registered in US and European Union (EU), data were obtained from the websites of National Institute of Health's clinicaltrials.gov and EU clinical trial registry, respectively.[11,12] Likewise, the data of trials registered in Australia, Canada, China, and Japan were obtained from the WHO's international clinical trial registry platform.[13]
We searched the registries for the total number of clinical trials registered during the above mentioned period along with the trends in the total number of trials registered every year in each region over the study period. In addition we also looked for different search words like diabetes, hypertension, etc., to identify the number of clinical trials done in the major diseases and like pregnancy, elderly, children, etc., to find out the number of trials registered in special populations. We also searched the registries with search words like vaccine and indigenous drugs to have an idea of trials registered in these fields. As far as indigenous drugs are concerned, we included those trials registered with herbal, ayurvedic, siddha, and unani products. The information in each search page was manually counter checked by all the authors to exclude the data that was not relevant to the search word used.
RESULTS
The total number of clinical trials registered in the respective registries during the study period was found to be 67,448 across seven study nations. Of this, clinical trials from India constituted only 2.7% of the total number of trials registered while the established countries like US, EU, Japan, and China contributed 47%, 18%, 11%, and 8% of total trials as shown in Figure 1. The average number of clinical trials registered per month during the entire study period from 2007 to 2011 was found to be 35 in India compared to 650, 247, 103, 89, 101, and 144 from US, EU, Canada, Australia, China, and Japan respectively as shown in Figure 2. This was calculated by dividing the total number of clinical trials registered per annum divided by 12 except for the years 2007 and 2011 where the denominators used were 6 and 8 respectively. This was done to maintain uniformity in the data as we did not include the whole year as far as 2007 and 2011 were concerned.
Figure 1.
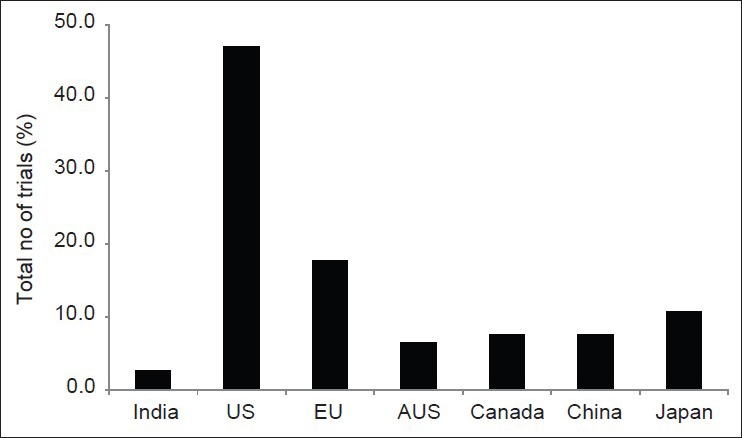
Total number of clinical trials registered in different countries from 2007-2011
Figure 2.
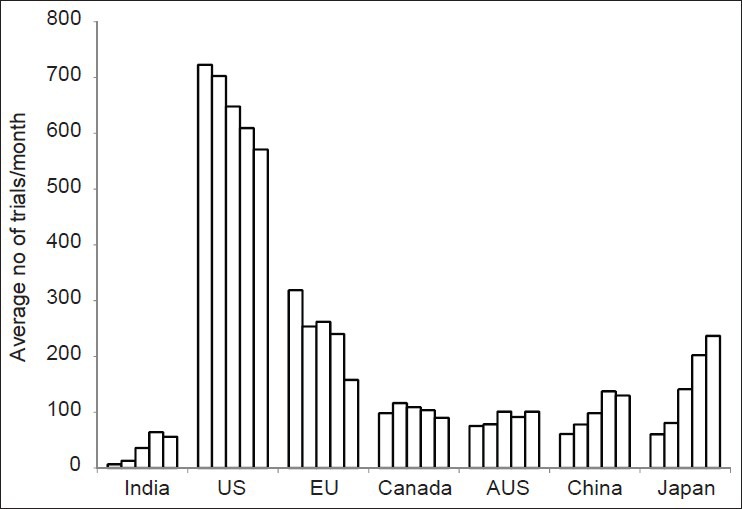
Year wise breakup of clinical trials registered in different regions from 2007-2011
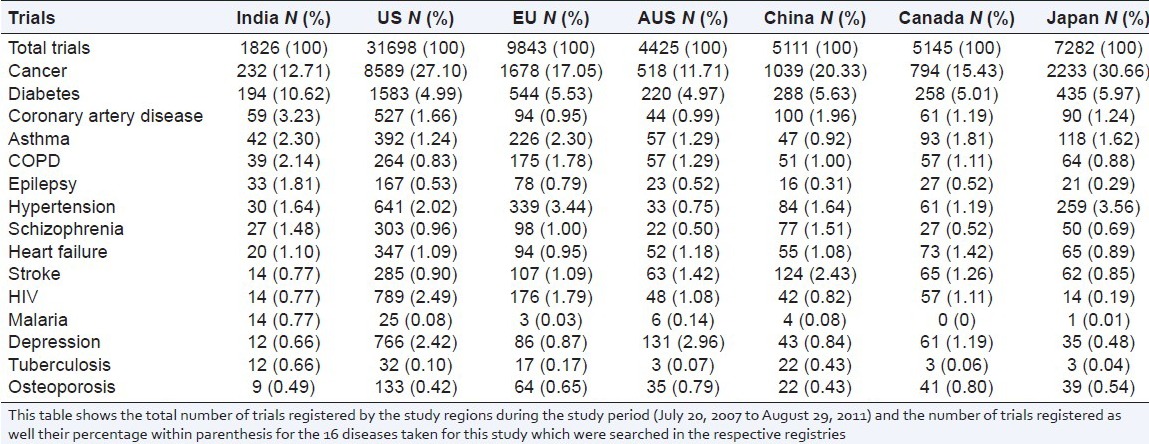
The top 10 diseases with maximum ongoing trials in India was found to be in cancer, diabetes, coronary artery disease (CAD), asthma, chronic obstructive pulmonary disease (COPD), epilepsy, hypertension, schizophrenia, heart failure, stroke, HIV, and malaria as shown in Table 1. Our study has shown that the number of clinical trials registered in infectious diseases like malaria and tuberculosis remain very low in India in spite of the disease burden. However, looking at the total number of trials registered during this period in India, malaria and tuberculosis trials contributed 0.8% and 0.7% of the total trials respectively. This was well ahead of the percentage of the trials registered for malaria and tuberculosis in other study regions.
Table 1.
Disease wise distribution of clinical trials in different countries
With respect to special population, pediatric and geriatric trials were found to be less in India compared to other countries as shown in Table 2. Canada and Australia were found to be involved in more pediatric trials while US and Japan in geriatric trials [Table 2]. In vaccine trials, 49 trials were registered in India during the study period which was the highest after US (783) and EU (155). India ranked third in the vaccine trials among the study regions next to US and EU. However, on comparing the number of vaccine trials registered in each country with respect to their total trials registered, the percentage of vaccine trials was found to be higher in India (2.7%) compared to US (2.5%) and EU (1.6%) [Table 3]. The number of indigenous clinical trials registered in India was found to be highest (2.7%) compared to the other study regions, including China, which was involved in only (0.6%) of the indigenous trials.
Table 2.
Clinical trials in special population
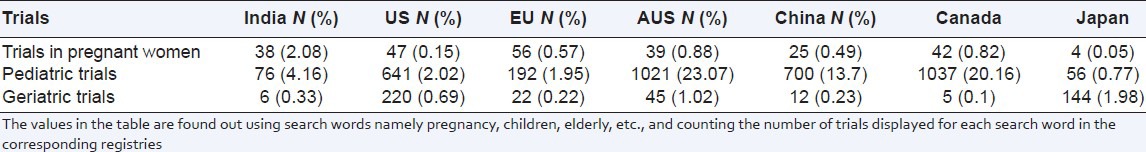
Table 3.
Clinical trials with special interventions

Our study established that there was an overall increase in the percentage of clinical trials registered in India during 2011 compared to 2007 [Figure 3]. Similar results were seen in countries like Japan, China and Australia while US and EU showed a fall in the percentage of trials registered in 2011 compared to that in 2007 [Figure 3].
Figure 3.
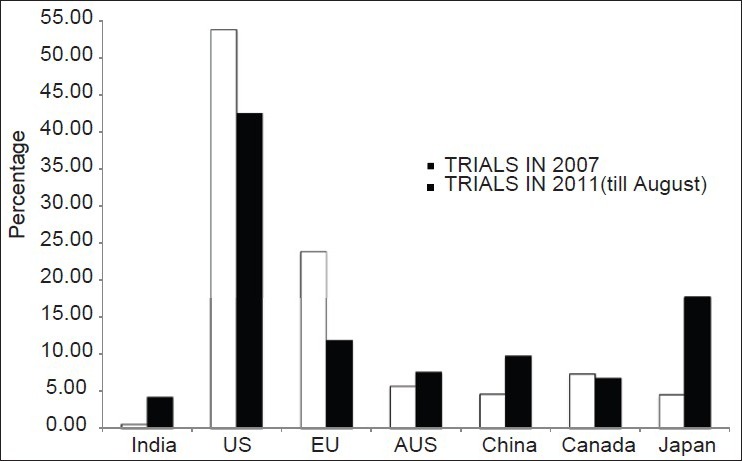
Percentage of clinical trials registered in 2007 and 2011 in various countries
DISCUSSION
The multinational pharmaceutical industries are opting for Asian countries to overcome various challenges in drug discovery and development.[5] It has been well documented that the average costs of conducting phase I/II/III drug trials in US is around $20/50/100 million respectively whereas in Asian countries like India, it is 50-60% of the same in addition to being 75% faster.[1] Further, having English as the primary business and medical language is an extra benefit to conduct global clinical trials in India compared to the other Asian countries.
This study was done to find out the growth of clinical trials in India over the last 4 years compared to the other Asian countries and the developed countries. This was carried out to explore the fact behind the claim that India is emerging as a hub for global clinical trials. Our study found that in the year 2011, India has contributed to only 4.2% of the total clinical trials registered, compared to 42.5%, 11.8%, 6.7%, 7.5%, 9.7%, 17.6% by US, EU, Canada, Australia, China, and Japan respectively as shown in Figure 4. However, India has been found to have an increase of 3.7% increase in the number of trials registered in 2011 compared to 2007 [Figure 5]. Still India is lagging behind the other Asian countries like China and Japan with a rise of 5.12% and 13.14% respectively in the number of trials registered in 2011 compared to 2007. In contrary, US and EU showed a decline of 11.3% and 12% in the total number of trials registered in 2011 compared to 2007 [Figure 5]. The findings of our study is in accordance with the claim that Asian countries like India, China, and Japan are becoming a hub for conducting global clinical trials.[1,14] However, India still has a long way to catch up with other Asian nations in the competition.
Figure 4.
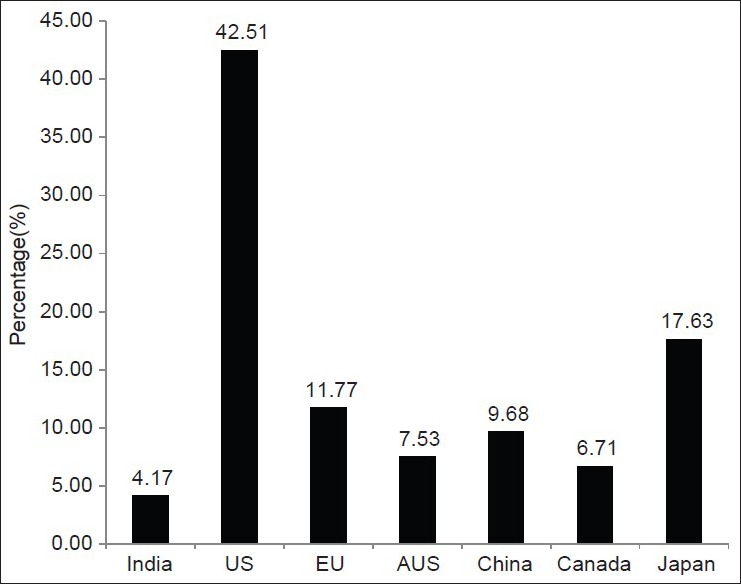
Percentage of trials registered in 2011 (till August) among various nations
Figure 5.
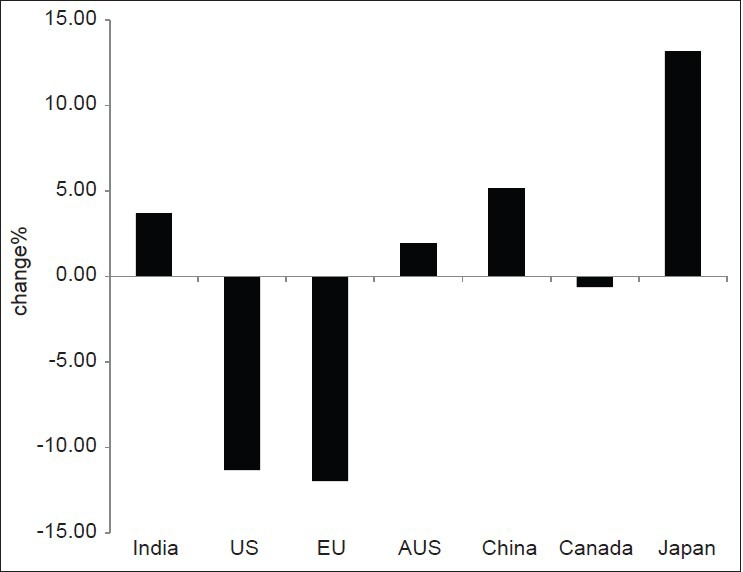
Percentage change in clinical trials registered in 2007 and 2011 in various countries
However, our study found out that in spite of contributing only 2.7% of the global clinical trials among the study countries, the percentage of clinical trials registered in diabetes, CAD, asthma, COPD, epilepsy, schizophrenia, malaria, tuberculosis, pregnancy, vaccines and indigenous drugs were found to be higher in India. This could be attributed to the high prevalence of the disease pattern, increasing population and the availability of treatment naive patients in India.[7] Among the total trials registered during this period, the percentage of trials in vaccines was found to be higher in India with 2.7% followed by US 2.5% and EU 1.6% [Table 3]. Similarly, the trials registered for indigenous drugs in India constituted 2.7% followed by China (0.6%) [Table 3]. This reflects the tradition of the indigenous system of medicine practiced in these countries.[15,16]
Our study also found out that though India is gaining momentum in clinical research in the last few years, it is still lagging behind the developed countries and other Asian nations like Japan and China. One of the reasons for this setback could be under registration of trials due to the lack of awareness among the researchers to register clinical trials in the registry. The other reason could be ignorance and fear among the patients regarding the participation in clinical trials. Studies have already shown that Indian patients are less willing to participate in clinical trials.[14,17] This can be overcome by confiscating the myths regarding clinical trials among the patients, volunteers and public through the conductance of ethically acceptable, well designed trials.[18]
Other possibilities of fewer registered trials in India could be attributed towards the lack of fully equipped clinical trials sites compared to other study regions. Moreover, the concern regarding the ethical principles involved in the conductance of clinical trials and the time delay for approval of trials from Drug Controller General of India could be a major reason for limited trials in India. Hence to be crowned as a hub for global clinical trials, India needs to have more number of well-trained clinical researchers and a system for faster approval process of new drugs.
Limitations of the study
The major limitation of our study is that we could just give a snapshot of the trials registered in the trial registries over the study period across the seven nations. We were not able to estimate the real status of the clinical trials conducted in these countries as there is a possibility of under reporting of clinical trials in the registries. Moreover, since registering of clinical trials in CTRI became mandatory only from 15th June 2009, the number of trials registered before 2009 may not be depicting the real scenario in India. Similar situation may be prevailing in other countries and registries too. Another limitation of our study is that we have restricted the search to only major diseases while leaving out the trials registered pertaining to less common diseases.
CONCLUSION
Our study found that India is showing a slow and gradual increase in the number of clinical trials registered over the last 4 years. The percentage of clinical trials registered in diabetes, CAD, asthma, COPD, epilepsy, schizophrenia, malaria, and tuberculosis are more in India compared to other nations. Still India is lagging far behind the western countries as well as other Asian countries like China and Japan in the total number of trials registered. One of the reasons for this could be lack of awareness among the researchers regarding the registration of clinical trials in clinical trial registries. Another reason could be the availability of a limited number of recognized courses for training physicians and other healthcare professionals involved in the conductance of clinical trials, resulting in shortage of trained manpower. Hence to meet the growing demands of clinical research and to increase the number of registered trials in the country, India needs to train more qualified, ethical clinical investigators, skilled nurses and biostatisticians to suit the clinical trial appraisal. With experienced clinical researchers and technological developments, India is sure to contribute as a major clinical trial destination for the global clinical research in the near future.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.The clinical research profession in India. Clinical research careers. 2007. [Last accessed on 2012 Jan 12]. Available from: http://www. 216.147.199.31/Monitor/2007September/sahoo.pdf .
- 2.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–23. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 3.Diamond J. Medicine: Diabetes in India. Nature. 2011;469:478–9. doi: 10.1038/469478a. [DOI] [PubMed] [Google Scholar]
- 4.Gupta R, Guptha S, Agrawal A, Kaul V, Gaur K, Gupta VP. Secular trends in cholesterol lipoproteins and triglycerides and prevalence of dyslipidemias in an urban Indian population. Lipids Health Dis. 2008;7:40. doi: 10.1186/1476-511X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarenga LS, Martins EN. Biopharmaceutical industry-sponsored global clinical trials in emerging countries. Rev Assoc Med Bras. 2010;56:428–33. doi: 10.1590/s0104-42302010000400015. [DOI] [PubMed] [Google Scholar]
- 6.Pandey A, Aggarwal A, Seth S, Maulik M, Bano R, Juneja A. Clinical Trials Registry – India: Redefining the conduct of clinical trials. Indian J Cancer. 2008;45:79–82. doi: 10.4103/0019-509x.44060. [DOI] [PubMed] [Google Scholar]
- 7.Gupta YK, Padhy BM. India's growing participation in global clinical trials. Trends Pharmacol Sci. 2011;32:327–9. doi: 10.1016/j.tips.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Contract research to grow at a healthy clip: ICRA. The Hindu Business line. 2012. [Last accessed on 2012 Oct 06]. Available from: http://www.thehindubusinessline.com/industry-and-economy/article2091126.ece .
- 9.India emerging as hub for clinical trials says ASSOCHAM. Clinuity. 2011. [Last accessed on 2012 Jan 12]. Available from: http://www.clinuity.com/blog/2011/07/india-emerging-as-hub-for-clinical-trials-says-assocham .
- 10.Clinical Trials Registry India. National Institute of Medical Statistics. Indian Council of Medical Research. 2012. [Last accessed 2012 Jan 12]. Available from: http://www.ctri.nic.in/Clinicaltrials/advancesearchmain.php .
- 11.Clinical Trials.gov. A service of the US National Institutes of Health. [Last accessed on 2012 Jan 12]. Available from: http://www.clinicaltrials.gov/ct2/search/advanced .
- 12.EU Clinical Trials Register. 2012. [Last accessed on 2012 Jan 12]. Available from: http://www.clinicaltrialsregister.eu/ctr .
- 13.World Health Organization. International Clinical Trials Registry Platform Search Portal. 2012. [Last accessed on 2012 Jan 12]. Available from: http://www.apps.who.int/trialsearch/AdvSearch.aspx .
- 14.Bhatt A. Registration of clinical trials: An idea whose time has come! Indian J Pharmacol. 2008;40:47–8. doi: 10.4103/0253-7613.41037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandey A, Aggarwal A, Maulik M, Seth SD. Clinical trial registration gains momentum in India. Indian J Med Res. 2009;130:85–6. [PubMed] [Google Scholar]
- 16.Zammar G, Meister H, Shah J, Phadtare A, Cofiel L, Pietrobon R. So different, yet so similar: Meta-analysis and policy modeling of willingness to participate in clinical trials among Brazilians and Indians. PLoS One. 2010;5:e14368. doi: 10.1371/journal.pone.0014368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karen PV. Wherever you do business, we speak the language. China's Clinical Trial Boom Language Connections – A Language Service Provider. PharmaFocus Asia. 2011. [Last accessed on 2012 Oct 06]. Available from: http://www.languageconnections.Com/descargas/China's%20Clinical%20Trial%20Boom.pdf .
- 18.Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on Centella asiatica: A potential herbal cure-all. Indian J Pharm Sci. 2010;72:546–56. doi: 10.4103/0250-474X.78519. [DOI] [PMC free article] [PubMed] [Google Scholar]


