Abstract
Background:
Post-operative pain is one of the problems, wherein lack of control on it has many side-effects such as tachycardia, hypertension, myocardial ischemia, decreased alveolar ventilation, and poor wound healing.
Aims:
In this study, we evaluated the pre-operative administration of pregabalin sufficiency and security in relieving post-operative pain after lower limb orthopedic surgery and reducing the need for opioids and their possible side-effects.
Materials and Methods:
This study is a randomized, double-blind clinical trial. It was performed on 60 patients under lower limb surgery by spinal anesthesia. Patients were randomly allocated to two groups, one group has received a 150 mg pregabalin capsule 2 h before surgery and the other group has received placebo as a control. In both groups at 2, 6, 12, and 24 h after surgery, the patients were evaluated and the pain score, the score of sedation, incidence of nausea and vomiting was recorded in the checklists. Then, the data were analyzed by SPSS v16.
Results:
Visual analog pain scores at all hours in pregabalin group significantly reduced compared to the placebo group (P < 0.0001). Also, in the pregabalin group nausea and vomiting scores at all hours, sedation levels at 2 h and 6 h post-operatively, and pethidine consumption in all hours have significantly been reduced.
Conclusion:
A single pre-operative oral dose of pregabalin 150 mg is an effective method for reducing post-operative pain and pethidine consumption in patients undergoing orthopedic surgery.
Keywords: Pethidine, post-operative pain, pregabalin, visual analogue scale
INTRODUCTION
Post-operative pain is among the problems, which lack of adequate control on it has many complications. Post-operative appropriate pain control in terms of preventing from complications such as tachycardia, hypertension, myocardial ischemia, decreased alveolar ventilation, and poor wound healing is of special importance. Exacerbation of acute pain may lead to increased sensitivity in neurons and releasing central and peripheral mediators. Advances in the molecular mechanisms has led to the development of multidimensional analgesia and using new pharmaceutical products on post-operative pain control,[1] and pregabalin is one of these new products. Acute pain after surgery has been as predictor of persistent pain after surgery and about 5-50% of patients experience persistent post-operative pain in different varieties of surgical operations.[2] Recent advances in the pathophysiology of pain have provided the possibility of prevention or reduction of excessive excitability of the central nervous system (CNS) associated with post-operative intensified pain.[3] Pregabalin is a new synthetic molecule derived from inhibitor for the neurotransmitter gamma-amino butyric acid. This medicine is δ-2 α ligand having anesthetic, anticonvulsant, anti-excitement, and sleep movement moderating effects. Pregabalin has been effective in the treatment of neuropathic pain, incisional pain, and inflammatory pain and pains induced by formalin. Pregabalin plays its role in treatment of acute post-operative pain by decreasing the excitability of dorsal horn neurons caused by tissue damage.[4] Moreover, since most patients are afflicted with stress and emotion pre-operatively, the anti-excitement effects of pregabalin can be effective.[5] In several studies, pregabalin has been used to reduce the need for opioids,[6] treatment of dental pains,[7] the treatment of pain after spinal fusion surgery,[8] and treatment of pain after laparoscopic cholecystectomy.[9,10,11,12,13,14]
In this study, we investigated pre-operatively prescribing pregabalin effectiveness and harmlessness in reducing pain after lower limb orthopedic surgery, and reduction of pethidine consumption and possible complications.
MATERIALS AND METHODS
This prospective, randomized, double-blind, and placebo controlled clinical study was designed to include 60 adult patients (20-60 years) of either sex, American Society of Anesthesiologists (ASA) physical status I and II, undergoing lower limb surgery under spinal anesthesia in Fatemi Hospital of Ardabil Medical Science University. The study protocol was approved from the institutional ethical committee and is registered at clinicaltrials.gov database (Reference No. IRCT201212254093N5). The written informed consent was obtained from all participants.
Patients with pregnancy, neurological disease, psychiatric disorders, history of seizures, acute or chronic renal disease, history of peptic ulcer, history of allergy to pregabalin or pethidine, taking psychotropic drugs, alcohol or drugs abuse, and daily intake of analgesics were excluded from the study.
Patients meeting the inclusion criteria during the pre- anesthetic evaluation were randomly assigned into two groups of 30 each by using a computer-generated table of random numbers, to receive either a matching placebo (capsules of similar appearance, but lacking the active ingredient of the drug) or pregabalin 150 mg.
The medicine was administered orally approximately 2 h before induction of anesthesia by a staff nurse who was not involved in the study and no other premedication were permitted. None of anesthesiologist, data assessor and data analyzer was aware of the group assignment.
The patients were taken to the operating room and were equally induced by spinal anesthesia with 12-15 mg of Bupivacaine 0.5% injected into the L4-L5 space by using 25-gauge needle and the surgery was performed.
In this study, primary outcomes were severity of post-operative pain, post-operative pethidine requirement and sedation. Secondary outcomes were incidence of post-operative nausea and vomiting (PONV). These outcomes were assessed by an anesthesiologist blinded to group allocation. Assessment of pain was done by a 100 mm visual analogue scale (VAS); 0, no pain; 100, worst imaginable pain. Assessment of pain was done at 2, 6, 12, and 24 h after operation. The Ramsay sedation scale (awake levels were: (1) anxious, agitated, or restless; (2) cooperative, oriented, and tranquil; (3) responds to command; asleep levels were dependent on patient's response to a light glabellar tap or loud auditory stimulus; (4) brisk response; (5) a sluggish response; and 6, no response) was used to assess the sedation; patients with a sedation scale of >4 were considered as sedated.[15]
In the recovery room, patients were given boluses of 50 mg of pethidine, based on an age-stratified IV analgesia protocol, if the pain score was ≥3.
Calculation of sample size was based on the presumption that post-operative VAS scores after pre-operative administration of pregabalin 150 mg could be reduced 15 mm compared the placebo group at all-time points. For the results to be of statistical significance with α = 0.05 and β = 0.8, was calculated to 26 patients in each group. To take care of any drop outs, we enrolled 30 patients in each group.
The data was analyzed using SPSS v. 16 by one-way analysis of variance (ANOVA) statistical tests and t-test for quantitative variables and Chi-square for qualitative ones. P < 0.05 was considered significant.
RESULTS
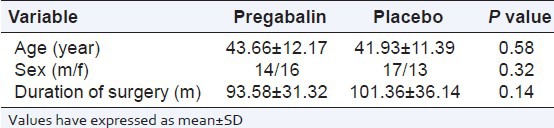
There was no substantial difference among the groups with regard to age, sex, duration of surgery (P > 0.05) [Table 1].
Table 1.
Demographic characteristics in two groups
In addition, the type of fracture in patients was determined in each group. In both groups, the most common type of fracture was femur fracture, and in terms of fracture type there was no significant difference between the two groups’ patients (P > 0.05).
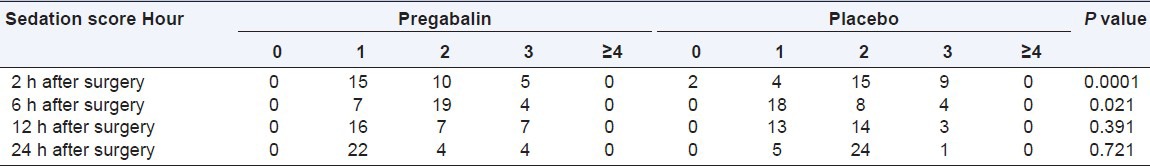
Average pain scores (VAS) at 2,6,12 and 24 h after surgery in pregabalin group was significantly lower than placebo group [Table 2]. The use of pethidine as post-operative analgesic is also significantly lower in pregabalin group than placebo [Table 2]. The incidence of nausea and vomiting in 2, 6, 12, and 24 h after the operation were different among the groups [Table 3]. No significant difference was between the groups in terms of sedation in 12 and 24 h after operation. However, this rate in the hours 2 and 6 was significantly lower in pregabalin group than the control group [Table 4].
Table 2.
Mean visual analogue scale score and mean pethidine consumption during 24 h after surgery

Table 3.
Incidence of post-operative nausea and vomiting during 24 h after surgery
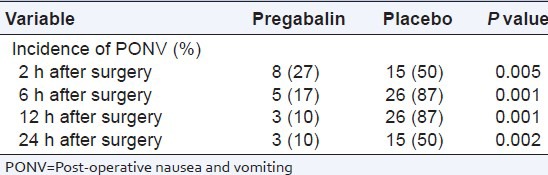
Table 4.
Sedation score of patients during 24 h after surgery
It wasn’t observed any other adverse events such as dizziness, headache, and visual disturbance in patients of both groups.
DISCUSSION
In this study, we observed that pre-operative pregabalin (150 mg) was effective in reducing of post-operative pain along with post-operative pethidine consumption in subjects undergoing lower limb orthopedic surgery.
Experimental models of neuropathic and inflammatory pain have shown that amino butyric acid analogues such as gabapentin and pregabalin contain analgesic components and anti-nociceptive. It is suspected, that CNS sensitivity may lead to post-operative pain growth.[16] Administering gabapentin before surgery, before inflammatory trauma, or surgical stimulation may reduce the degree of sensitivity of the CNS.[17] In contrast to gabapentin, pregabalin which is an alternative to increase its solubility in fat and causes permeability in the blood-brain barrier, include better pharmacokinetic properties and due to lack of hepatic metabolism has less drug interactions.
In this study, the patients were assessed in terms of PONV incidence rate and sedation score during the 24 h after surgery. In pregabalin group, PONV incidence rate, as well as the sedation score was lower than the control group. In several studies the presence of complications among the patients are different. So that in Hill study[7] on the patients who had surgery on their teeth, and in Paech et al. study[18] who administered the drug after gynecologic surgeries, some complications such as vomiting, nausea, and abdominal pain were higher in pregabalin group while in the study of Agarwal[12] on patients undergoing laparoscopic cholecystectomy, and in Mathiesen et al. study[19] on patients who had undergone hysterectomy these complications were lower in pregabalin group.
In this study, the pain severity was determined based on VAS score and it was found that pregabalin could significantly reduce the mean score of pain in patients. The majority of previous studies have similar results. For example, in Hill,[7] Agarwal et al.,[12] and Schulmeyer et al.[20] studies the post-operative pain severity was reduced as a result of consuming pregabalin while in other studies, including Paech et al.,[18] Jokela et al.[21] and Mathiesen et al.[19] it was found that the group using pregabalin had the same post-operative pain intensity as the control group. One aspect observed in many studies on this drug is reduced post-operative opioid and analgesic consumption in the pregabalin group. In Zhang et al.[6] study in 2011, he indicated that pregabalin significantly reduced post-operative opioid consumption rate in patients. He also showed that pregabalin consumption can reduce some opioid side effects such as nausea and vomiting. Durkin et al.[9] showed in their study pregabalin consumption reduces opioid consumption in patients with chronic neuropathic pains. Additionally, Post et al.[10] observed that the use of pregabalin reduced the opioid consumption after hip arthroplasty surgical operation. Our results were consistent with these studies.
CONCLUSION
A single pre-operative oral dose of pregabalin 150 mg is an effective method for reducing post-operative pain in patients undergoing orthopedic surgery. The peri-operative administration of pregabalin has a significant opioid-sparing effect in the first 24 h after surgery. PONV and sedation score was reduced with pregabalin administration.
Footnotes
Source of Support: Ardabil University of Medical Sciences, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.Vadivelu N, Mitra S, Narayan D. Recent advances in postoperative pain management. Yale J Biol Med. 2010;83:11–25. [PMC free article] [PubMed] [Google Scholar]
- 2.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 3.Buvanendran A, Kroin JS, Kari M, Tuman KJ. Can a single dose of 300 mg of pregabalin reach acute antihyperalgesic levels in the central nervous system? Reg Anesth Pain Med. 2010;35:535–8. doi: 10.1097/AAP.0b013e3181fa6b7a. [DOI] [PubMed] [Google Scholar]
- 4.Gajraj NM. Pregabalin: Its pharmacology and use in pain management. Anesth Analg. 2007;105:1805–15. doi: 10.1213/01.ane.0000287643.13410.5e. [DOI] [PubMed] [Google Scholar]
- 5.Gonano C, Latzke D, Sabeti-Aschraf M, Kettner SC, Chiari A, Gustorff B. The anxiolytic effect of pregabalin in outpatients undergoing minor orthopaedic surgery. J Psychopharmacol. 2011;25:249–53. doi: 10.1177/0269881109106928. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Ho KY, Wang Y. Efficacy of pregabalin in acute postoperative pain: A meta-analysis. Br J Anaesth. 2011;106:454–62. doi: 10.1093/bja/aer027. [DOI] [PubMed] [Google Scholar]
- 7.Hill CM, Balkenohl M, Thomas DW, Walker R, Mathé H, Murray G. Pregabalin in patients with postoperative dental pain. Eur J Pain. 2001;5:119–24. doi: 10.1053/eujp.2001.0235. [DOI] [PubMed] [Google Scholar]
- 8.Kim JC, Choi YS, Kim KN, Shim JK, Lee JY, Kwak YL. Effective dose of peri-operative oral pregabalin as an adjunct to multimodal analgesic regimen in lumbar spinal fusion surgery. Spine (Phila Pa 1976) 2011;36:428–33. doi: 10.1097/BRS.0b013e3181d26708. [DOI] [PubMed] [Google Scholar]
- 9.Durkin B, Page C, Glass P. Pregabalin for the treatment of postsurgical pain. Expert Opin Pharmacother. 2010;11:2751–8. doi: 10.1517/14656566.2010.526106. [DOI] [PubMed] [Google Scholar]
- 10.Post ZD, Restrepo C, Kahl LK, van de Leur T, Purtill JJ, Hozack WJ. A prospective evaluation of 2 different pain management protocols for total hip arthroplasty. J Arthroplasty. 2010;25:410–5. doi: 10.1016/j.arth.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104:1545–56. doi: 10.1213/01.ane.0000261517.27532.80. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal A, Gautam S, Gupta D, Agarwal S, Singh PK, Singh U. Evaluation of a single preoperative dose of pregabalin for attenuation of postoperative pain after laparoscopic cholecystectomy. Br J Anaesth. 2008;101:700–4. doi: 10.1093/bja/aen244. [DOI] [PubMed] [Google Scholar]
- 13.Burke SM, Shorten GD. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg. 2010;110:1180–5. doi: 10.1213/ANE.0b013e3181cf949a. [DOI] [PubMed] [Google Scholar]
- 14.Michaloliakou C, Chung F, Sharma S. Preoperative multimodal analgesia facilitates recovery after ambulatory laparoscopic cholecystectomy. Anesth Analg. 1996;82:44–51. doi: 10.1097/00000539-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woolf CJ, Chong MS. Preemptive analgesia – Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 17.Werner MU, Perkins FM, Holte K, Pedersen JL, Kehlet H. Effects of gabapentin in acute inflammatory pain in humans. Reg Anesth Pain Med. 2001;26:322–8. doi: 10.1053/rapm.2001.25070. [DOI] [PubMed] [Google Scholar]
- 18.Paech MJ, Goy R, Chua S, Scott K, Christmas T, Doherty DA. A randomized, placebo-controlled trial of preoperative oral pregabalin for postoperative pain relief after minor gynecological surgery. Anesth Analg. 2007;105:1449–53. doi: 10.1213/01.ane.0000286227.13306.d7. [DOI] [PubMed] [Google Scholar]
- 19.Mathiesen O, Rasmussen ML, Dierking G, Lech K, Hilsted KL, Fomsgaard JS, et al. Pregabalin and dexamethasone in combination with paracetamol for postoperative pain control after abdominal hysterectomy. A randomized clinical trial. Acta Anaesthesiol Scand. 2009;53:227–35. doi: 10.1111/j.1399-6576.2008.01821.x. [DOI] [PubMed] [Google Scholar]
- 20.Cabrera Schulmeyer MC, de la Maza J, Ovalle C, Farias C, Vives I. Analgesic effects of a single preoperative dose of pregabalin after laparoscopic sleeve gastrectomy. Obes Surg. 2010;20:1678–81. doi: 10.1007/s11695-009-9944-1. [DOI] [PubMed] [Google Scholar]
- 21.Jokela R, Ahonen J, Tallgren M, Haanpää M, Korttila K. A randomized controlled trial of perioperative administration of pregabalin for pain after laparoscopic hysterectomy. Pain. 2008;134:106–12. doi: 10.1016/j.pain.2007.04.002. [DOI] [PubMed] [Google Scholar]


