Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic lung disease of unknown etiology. Recent investigations have demonstrated that the impaired immune response is a common characteristic feature of IPF. Unfortunately, no definitive and effective drug treatment is available that could improve or at least inhibit the progressive course of this fatal disease. That is why one of the main priorities of pulmonary fibrosis investigations is to identify novel and effective molecular targets for preventive and therapeutic interventions. caffeic acid phenethyl ester (CAPE) is one of the most interesting bioactive compounds extracted from bee propolis. It has been shown that CAPE has an antioxidant activity and modulatory impact on immune system. Accordingly, the aim of the present study was to investigate the regulatory effects of CAPE on the levels of type I collagen (COL-1) and Interferon-gamma (IFN-γ) in bleomycin (BLM)-induced pulmonary fibrosis. Immunohistochemistry procedure was employed to assess the effects of CAPE on lung tissue. In this study, male Sprague-Dawley rats were divided into 5 groups (n=8) included 1: Positive control group: bleomycin (BLM). 2: Negative (saline) control group. 3, 4: Treatment groups of 1 and 2: BLM+CAPE (5 and 10 μmol/kg/day, respectively). (5: Sham group: CAPE (10 μmol/kg/day). BLM application resulted in significant changes in the level of studied parameters as compared to the controls. CAPE could decrease type I collagen concentration, modulate IFN-γ level, increase the animals′ body weight and decrease the lung index dose-dependently, compared with model group. In conclusion, CAPE may provide a novel therapeutic target for treating pulmonary fibrosis.
Keywords: Pulmonary fibrosis, Bleomycin, CAPE, IFN-γ, Type I collagen
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF), the most common form of the idiopathic interstitial pneumonia, is a chronic, progressive, devas-tating, irreversible, and usually lethal human interstitial lung disease of unknown cause that unfortunately leads to death in a short time after diagnosis(1,2). Currently no definitive pharmacological treatment has been introduced to improve the survival rate of patients with IPF and available therapies are usually futile and associated with considerable adverse side effects(3,4). Thus, there is an urgent need to the development of novel effective agents that could replace or complement current therapies and ameliorate IPF disease.
The complex pathogenesis of IPF is not entirely understood and hypotheses continue to develop steadily. One of these hypotheses that states the important consecutive key events in the development of IPF includes acute lung inflammatory responses after exposure to a harmful agent followed by abnormal tissue repair in damaged area and subsequent destruction of the lung architecture(1). Now the most accepted theory suggests that "IPF is an epithelial/fibroblastic disorder." In other words, epithelial damage and fibroblast activation are two important early events that trigger a cascade of changes leading to disruption of the lung tissue compartments. According to this hypothesis, epithelial injury and activation rather than alveolitis represent the key factors in the pathogenesis of IPF(2). Inefficacy of immunosuppressive therapy in IPF patients has lead to a re-evaluation of the inflammation role in the development of IPF(3). On the other hand, some evidences indicate that fibrosis may develop with a slight inflammation(1). Nevertheless, several studies show the involvement of inflammatory pathways in the pathogenesis of IPF(4,5).
Fibrosis is characterised by proliferation of fibroblasts and an excessive production of extracellular matrix (ECM) components including collagen and proteoglycans which leads to deposition of collagen in the interstitial space of the tissue, and if not controlled can result in organ dysfunction(10). In IPF, gradual deposition of the newly formed collagen accumulates in the lung interstitium and leads to thickening of alveolar septum and pulmonary dysfunction(6).
Approximately 15-20% of the total lung mass consists of collagen. Lung cells synthesize different types of collagen. type I collagen is the main and most abundant structural protein present in lung tissue among other types of collagen. Naturally human lung is composed of about 65% type I collagen. In addition, excessive type I collagen and its accumulation is characteristic of fibrotic lungs(7).
Cytokines play a critical role in wound healing, tissue repair, and fibrogenesis(13,14). Recent investigations have demonstrated that poorly balanced immune response, including imbalance of cytokines with positive (e.g. TGFβ1) and negative (e.g. interferon-γ) fibrogenic effects, is a characteristic feature of human progressive lung fibrosis such as idiopathic pulmonary fibrosis. Interferon-γ (IFN-γ) is a known anti-fibrotic cytokine in animal and human studies which inhibits fibroblast collagen synthesis in vitro and in vivo(8).
Various fibrogenic and pulmonary toxic agents may be used to produce an animal model of pulmonary fibrosis(9,10). Bleomycin (BLM) is a chemotherapeutic antibiotic which has been widely used as an experimental model of human IPF. In our study the IPF animal model was induced by intratracheal instillation of BLM. This animal model of IPF considerably simulates the pathologic events of human fibrotic lung disease and is widely used to evaluate the pathogenic mechanisms and new therapeutic approaches for IPF(11).
CAPE is a major biologically active component of honey bee propolis extracts. In traditional medicine CAPE has been used from ancient times. CAPE has numerous biological activities, including anti-inflammatory(12), immunomodulatory(13), antioxidant(14), and antiviral properties(15). Clinically, CAPE has been exhibited many effects in a wide array of diseases and health conditions(16,17).
Biological and clinical effects of CAPE prompted us to assume that CAPE may have some effects on idiopathic pulmonary fibrosis. Therefore, the present study was conducted to investigate the possible protective effect of CAPE on pulmonary fibrosis induced by BLM in a rat model. To achieve this aim, the alterations in body weight and lung index of animals were evaluated in different groups at specified time intervals. Immunohistochemical staining technique was also used for α-smooth muscle actin (α SMA) and the level of some relevant biomarkers such as type I collagen and IFN-γ was determined in lung serum samples.
MATERIALS AND METHODS
Chemicals
Bleomycin sulfate (Blenoxane®) was obtained from Bristol-Myers Squibb Co. (Princeton, NJ 08543 USA). Caffeic acid phenethyl ester was purchased from Sigma, St. Louis, MO, USA The rat IFN-γ ELISA kit was an eBioscience product (San Diego, CA, USA). Rat collagen type I (Col I) ELISA kit was obtained from Cusabio Biotech Co. (Wuhan University Science Park, Wuhan, Hubei Province 430223, P.R.China). α-SMA immunohistology kit was purchased from Sigma, St. Louis, MO, USA. All other chemicals used in this study were of the highest grade commercially available.
Animals
Healthy male Sprague-Dawley (SD) rats weighing 180-220 g were purchased from animal house and research center of Jundishapur University of Medical Sciences, Ahvaz, Iran. Animals had free access to standard rat chow and tap water ad libitum. They were kept in a temperature-controlled environment at 23 ± 2°C with an alternating cycle of 12 h light and dark. The animal experimental protocol was approved by the Jundishapur University Animal Care and Use Committee.
Drug treatment groups
Animals were randomly divided into five groups of8 rats each as follows:
1: Positive control (Model) group: received a single intratracheal (i.t.) dose of BLM solution (5 mg/kg/ml).
2: Negative control group: was given a single i.t. dose of saline (maximum permissible volume = 1 ml/kg).
3 and 4: Treatment groups of 1 and 2: each treatment group received CAPE in a dose of 5 and 10 μmol/kg, 7 days before up to 3 weeks after administration of a single i.t. dose of BLM (5 mg/kg/ml).
5: Sham group: CAPE (10 μmol/kg) plus vehicle (without BLM) was administered.
The day of BLM or saline (i.t.) injection was designated as the 7th day. The CAPE doses were given twice daily starting from the day zero and continued for 4 weeks. CAPE was intraperitoneally injected to the animals. Saline was used as the vehicle in preparation of all solutions.
Induction of pulmonary fibrosis by bleomycin
According to the method of Schraufnagel and coworkers(25), rats were anaesthetized with ether, placed on a slanted board and hanged from their upper incisors. Keeping the nose of animal closed and its tongue out, BLM solution (5 mg/kg) was delivered via the mouth into the trachea by a modified needle in a volume of 1 ml/kg animal body weight. Control rats received intratracheal instillations of the same volume of saline. After recovery from anesthesia, rats were returned to their cages.
Isolation of lung tissue samples and serum sampling
At the end of the treatment course, all rats were weighed, blood sampling was performed under light ether anaesthesia by cardiac puncture and the serum was extracted immediately. To avoid repeated freeze-thaw cycles, the serum sample of each animal was divided into several aliquots (about 0.5 ml each). The serum samples then were stored at -70°C for the next steps of the study. As soon as blood sample was taken, the animals were killed with a lethal dose (120 mg/kg, i.p.) of sodium pentobarbitone (Sagatal®). After mid-line sternotomy, whole lung (include both lobes) was dissected out, separated from other tissues, and washed free of blood with ice-cold saline, then the whole lung weight was recorded and placed in a sterile plastic petri dish. Right lungs were preserved in buffered formaldehyde solution (10% w/v) for immunohistochemistry (IHC) assessment. To prepare lung tissue homogenate samples, the left lobe of animal lungs were excised, rinsed with ice-cold saline solution and then quickly frozen in liquid nitrogen before being stored at -70°C. Later, the frozen left lungs were thawed and approximately 500 mg of each tissue sample homogenized quickly in 5 ml of PBS (1×) to yield a 10% w/v tissue homogenate and then stored overnight at -20°C. Two freeze-thaw cycles were performed to break the cell membranes. The homogenates were centrifuged for 5 min at 5000 g. The supernatant was removed immediately, aliquoted and stored at -70°C for biochemical assay.
Biochemical and immunological assays
Type I collagen (as the fibrosis marker) and IFN-γ (a cytokine with known anti-fibrotic activity), were determined using conventional methods using ELISA kits, following the directions of the manufacturer. The samples were read finally by ELISA reader.
Type I collagen measurement in lung tissue homogenate
The microtiter plate provided in this kit had been pre-coated with an antibody specific to Col I. Standards or samples were then added to the appropriate microtiter plate wells with a biotin-conjugated antibody specific for Col I. Avidin conjugated to Horseradish Peroxidase (HRP) was added to each microplate well and incubated. Then TMB (3,3′,5,5′ tetramethyl-benzidine) substrate solution was added to each well. The enzyme-substrate reaction was terminated by the addition of a sulphuric acid solution and the color change was measured spectrophotometrically at a wavelength of 450 nm ± 2 nm. The concentration of Col I in the samples was then determined by comparing the O.D. of the samples to the standard curve.
Determination of IFN-γ content in serum
Briefly, in IFN-γ elisa assay the required number of microwell strips was determined. Standards (with serial concentrations), sample diluent (as blank) or samples were added in duplicate, to designated standard, blank and sample microwells. Biotin-conjugate was added to all microwells. Microwell strips were incubated 2 h at room temperature (18° to 25°C). After washing of microwell strips with wash buffer, streptavidin-HRP was added to all microwells. Microwell strips were incubated 1 h at room temperature. Microwell strips were emptied and washed. Then, TMB substrate solution was added to all wells. The microwell strips were incubated for about 10 min at room temperature. Stop solution was added to all microwells. colour intensity was measure at 450 nm.
Immunohistochemical demonstration of α-smooth muscle actin
The α-SMA immunohistochemistry kit provides procedure and materials for immunohistochemical demonstration of α-SMA in paraffin-embedded rat tissue sections. Briefly, in this procedure an antigen-specific primary antibody was applied to deparaffinize tissue sections. Following a brief wash, the sections were incubated with a biotinylated secondary antibody. Upon the addition of an ExtrAvidin®-Peroxidase reagent, a stable avidin-biotin complex was formed with the bound biotinylated antibody. Substrate was made of hydrogen peroxide and the chromogen 3-amino-9-ethylcarbazole (AEC). The sites of antibody deposition were visualized by the addition of freshly prepared substrate. The bound peroxidase catalyzed the oxidation of the AEC to form a reddish-brown insoluble precipitate at the antigen sites.
Alterations in the body weights of animals
The rats of different groups were weighed at the beginning, middle, and the end of experiments and the changes in rats′ body weight were determined.
Evaluation of lung index in different groups
The lungs were removed, trimmed of extraneous tissue, rinsed, and weighed. The ratio of net lung weight (mg) to the body weight (g) for each rat was used as the lung index.
Statistical analysis
The results were expressed as Mean ± SEM. Statistical analysis of parametric variables were carried out by one-way analysis of variance (ANOVA) followed by appropriate post hoc tests including multiple comparison tests (Tukey’s test). The non-parametric variables were evaluated using the Mann-Whitney test. All analyses of data were performed using SPSS 18 software and probability values of 0.05 or less were considered to be statistically significant.
RESULTS
Type I collagen concentrations profile in lung homogenates
Twenty-one days after BLM instillation in model group, the level of type I collagen in lung homogenate samples increased significantly (301 ± 13.6 pg/ml), compared with saline control group (220 ± 11.3 pg/ml) (P<0.001). CAPE could significantly reduce the concentrations of type I collagen in samples. However, 10 μmol/kg of CAPE had a more pronounced effect (225 ± 9.5 pg/ml) (Fig. 1).
Fig. 1.
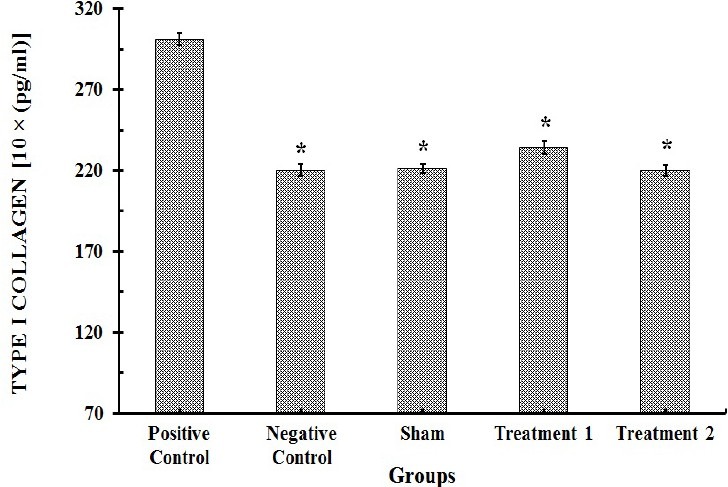
Type I collagen contents in lung tissue homogenate specimens (n=8). Each value represents Mean ± SEM. Significant difference versus positive control group is shown by * (P>0.001).
Interferon-γ serum concentrations profile
The serum level of IFN-γ was significantly decreased (121 ± 5.1 pg/ml) by BLM administration on 28th day of the study and this effect was abrogated by CAPE treatment However, 10 μmol/kg of CAPE could restore the serum level (178 ± 6.7 pg/ml) of IFN-γ close to the normal value (177 ± 8 pg/ml). There were no significant differences in the IFN-γ concen-trations between negative control group and sham group, which shows that systemic administration of CAPE is safe (Fig. 2).
Fig. 2.
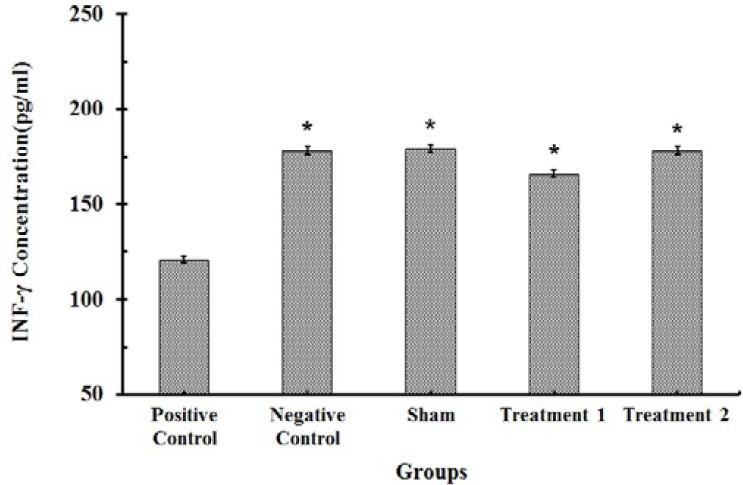
IFN-γ contents in serum of rats (n=8). Each value represents Mean ± SEM. Significant difference versus positive control group is shown by * (P<0.001).
Effect of CAPE on body weight changes during the study
Treatment of rats with BLM caused a marked decrease (P<0.001) in their body weight as compared to the saline treated control group on 7th, 14th, and 21th days. Generally, there was a body weight loss in all BLM-receiving groups. Administration of CAPE (5 and 10 μmol/kg) led to a significant increase (P<0.001) in body weight as compared to the BLM group (Fig. 3). The weight loss was lasted for the whole 4-weeks of experimental period in BLM treated groups, although the rats′ body weight was recovered gradually in the CAPE treatment groups, especially in the group receiving higher dose of CAPE (10 μM/kg) and body weight values in this group were highly close to normal values.
Fig. 3.
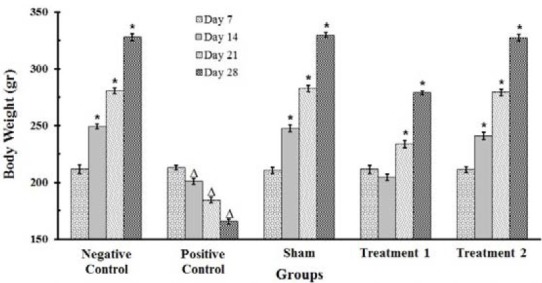
Changes in the body weight of rats (n=8). Each value represents Mean ± SEM. Significant difference versus positive and negative control groups is shown by * and ▵ respectively, (P<0.001).
Effect of CAPE on lung index
As shown in Fig. 4, treatment of rats with BLM resulted in a significant increase (P<0.001) in lung index and wet lung weight as compared to the negative control group on 28th day of the study course. Administration of CAPE (5 and 10 μmol/kg) led to a considerable decrease (P<0.001) in lung index and wet lung weight compared with the BLM group, suggesting that the extent of BLM induced pulmonary pathologic changes are alleviated by treatment with this agent.
Fig. 4.
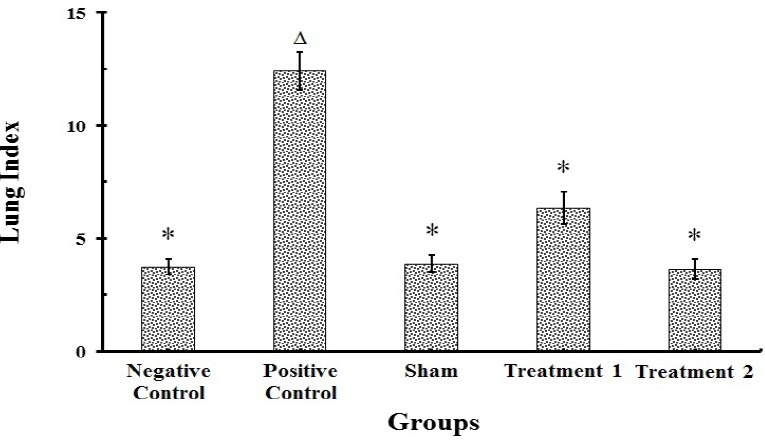
Effects of BLM and/or CAPE on the lung index of rats (n=8). Each value represents mean ± SEM. Significant difference versus positive and negative control groups is shown by * and ▵ respectively, (P<0.001)
Immunohistochemical examination of lung tissues
α-SMA positive cells have significantly increased in positive control group, compared with vehicle control group, particularly in areas where there was an increase in the severity of fibrosis and the density of fibroblasts (Fig. 5.a), indicating that the animal model of pulmonary fibrosis was well established (Fig. 5.b).
Fig. 5.
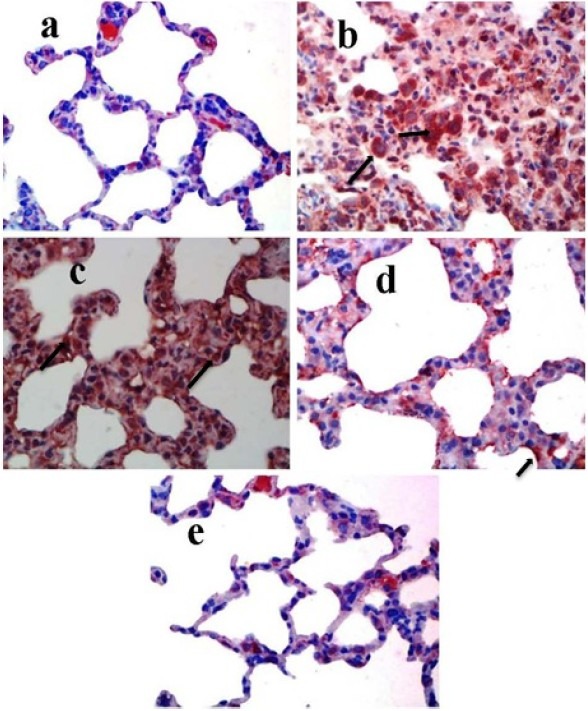
Immunohistochemical staining of rat lung tissue sections using α-SMA antibody. Original magnification of all images is ×40. (a) Negative control group. (b) positive control group. (c) BLM + CAPE (5 μmol/kg) group. (d) BLM + CAPE (10 μmol/kg) group.(e) Sham group.
In contrast, α-SMA immunoreactivity decreased dramatically in tissue sections of the CAPE treated groups (Fig. 5.c and 5.d). These results suggest that the administration of CAPE could decrease the expression of α-SMA and the severity of fibrosis in BLM-induced pulmonary fibrosis. Further studies examining severity of disease in treatment groups, such as monitoring of the changes in animals body weight, were concurred with the immunohistochemical assay. BLM upregulated the expression of α-SMA and CAPE administration could ameliorate the situation.
DISCUSSION
Alveolar epithelial cell and/or alveolar capillary cell damage seems to be the early stage in the pathogenesis of IPF. This event, promotes recruitment of circulating immune cells into the lung. These cells, then release cytokines which stimulate target cells, typically fibroblasts, to replicate and synthesize increased amounts of collagen. While most cytokines are thought to be profibrotic, it has become obvious that there also exists a family of anti-fibrotic cytokines, such as IFN-γ(19).
Previous studies have shown that IFN-γ inhibits fibroblast collagen synthesis in vitro(20) and attenuates BLM-induced lung fibrosis when administered to mice(21). Diminished IFN-γ reported in patients with IPF could enhance collagen accumulation(22). The Th1 response includes increased expression of IFN-γ and in general promotes tissue restoration. In contrast, the classic Th2 cytokines tend to promote fibroblast activation, matrix development, and hence fibrosis(23).
Aberrant vascular remodeling is a central hallmark of IPF progression. The imbalance in local expression of CXC chemokines or α-chemokines (the two N-terminal cysteines are separated by one amino acid, represented in this name with an “X”) seems to be crucial for promotion of aberrant angiogenesis in IPF. Members of the CXC chemokine family whose expression are upregulated by IFN-γ are angiostatic(24).
In a study conducted by Park and colleagues, IFN-γ showed a trend toward increase at CAPE treated group(13). Likewise, Gremy and coworkers(25) showed that ileal mucosa treated with CAPE shifted from an irradiation-induced Th2-like pattern of gene expression toward Th1 gene expression through an apoptotic sensitization pathway. However, some studies suggest that CAPE inhibits IFN-γ secretion and proliferation via inhibition of NF-kB and Akt signaling(26,27). Thus, the aim of this study was to evaluate the effects of CAPE on IFN-γ (as Th1 cytokine) regulation in BLM-induced lung fibrosis model. The results of present study showed significant decrease in IFN-γ concentration in the model group compared with vehicle negative control group. This effect was abrogated in CAPE treated animals which increased the level of IFN-γ close to vehicle group. However, CAPE with a 10 μM/kg dose could further compensate the levels of IFN-γ. The decreased concentration of IFN-γ in the model group, 21 days after BLM injection (i.t.), seems to be in line with the sequence of events in BLM-induced pulmonary fibrosis. Such that the initial elevation of pro-inflammatory cytokines (IFN-γ) is followed by increased expression of pro-fibrotic markers, with a peak around the 14th day. The “switch” between inflammation and fibrosis appears to occur around the day 9 after BLM(28). Several studies have shown that nuclear factor-κB (NF-κB) regulates a large number of genes involved in inflammation(29), it is also considered to be closely correlated with BLM lung toxicity(30). Therefore, decreased concentration of IFN-γ in the model group on day 28th of the study course appears to be according to BLM induced-shift in the pattern of cytokines which is regulated by NF-κB. On the other hand, CAPE is a potent inhibitor of NF-κB activation(31). Thus, in CAPE treated groups, the concentration of IFN-γ may increase close to vehicle group through CAPE inhibitory effect on BLM induced-NF-κB activation. Other mechanisms may also be involved, such as the effects caused by p38 MAPK pathway.
The biochemical consequences of pulmonary exposure to BLM have provided important insights into the events leading to the final fibrotic state. In BLM model of PF, collagen biosynthesis is clearly altered(32). Investigators have argued that direct effects of BLM on fibroblasts are responsible for the elevated levels of collagen(33). For example, direct exposure of cultured fibroblasts to BLM has been reported to increase the synthesis of type I procollagen(34).
Collagen is the major component of lung extracellular matrix and its deposition directly reflects the grade of lung fibrosis. Fibroblasts persist at the sites of fibrosis in IPF, continuously adding to an environment of aberrant excessive collagen deposition. Interstitial fibroblasts which comprise about 40% of the lung cell population are the major source of type I collagen(35). Other cell types such as smooth muscle cells and endothelial cells also synthesize type I collagen(36). Type I collagen constitutes greater than 65% of the total lung collagen in normal human lungs(37). An excess deposition of ECM including type I collagen in the lung is the hallmark of pulmonary fibrosis(38).
In our study, type I collagen concentration was significantly upregulated as early as 7 days after BLM administration (the data is not shown) and reached its maximal value at the day 28 of the study. CAPE (5 and 10 μmol/kg) could significantly inhibit the BLM-induced upregulation of type I collagen. However, the higher dose of CAPE (10 μmol/kg) was more potent.
α-SMA is a marker of myofibroblast differentiation and its density is usually increased in fibrotic areas, associated with the progress of disease(39). IHC staining of α-SMA confirmed the anti-fibrotic activity of CAPE particularly in higher dose used in this study (10 μmol/kg). In addition, IHC evaluation of α-SMA revealed that CAPE could decrease severity of fibrosis, presence of active myofibroblast, and expression of α-SMA in pulmonary fibrosis.
The results of the present study confirm the results obtained in other studies by Ozyurt and coworkers(40) and Zaeemzadeh and coworkers(41). In these studies CAPE 10 μmol/kg) could represent a well protection against the pathological alterations caused in models of PF. In the study conducted by Ozyurt and coworkers, CAPE had a regulator effect on these parameters: the decrease in total lung OH-proline levels, the increase in CAT and SOD activities and the decrease in MPO activity were seen after CAPE application. CAPE was also more effective in decreasing the tissue levels of NO, MDA and OH-proline than vit E.
The results of the present study clearly show that BLM caused a significant weight loss and an evident increase in lung index, which is consistent with previous studies(42) However, administration of CAPE could dose-dependently modulate IFN-γ level, decrease type I collagen synthesis, increase the animals′ body weight, and decrease the lung index compared with model group.
CAPE may be a novel potential candidate to prevent BLM-induced pulmonary fibrosis. However, more studies must be conducted to clarify the exact mechanism of CAPE in pulmonary fibrosis.
CONCLUSION
In summary, the results of the present study clearly show that BLM caused a significant weight loss and an evident increase in lung index, which is consistent with previous studies48). However, the data reported here reveal that administration of CAPE can dose-dependently attenuate the bleomycin-induced lung fibrosis. The mechanisms underlying this protective action may be attributed to modulate IFN-γ level, decrease type I collagen synthesis, increase the animals′ body weight, and decrease the lung index compared with model group.
These findings suggest that CAPE may be a novel potential candidate to prevent BLM-induced pulmonary fibrosis. However, more studies need to be conducted to clarify the exact mechanism of CAPE in pulmonary fibrosis.
ACKNOWLEDGMENT
This research was funded by a grant from Ahvaz Jundishapur University of Medical Sciences (project No: U 89270). The authors wish to thank them for their financial support.
REFERENCES
- 1.Biswas R, Bunderson-Schelvan M, Holian A. Potential role of the inflammasome-derived inflammatory cytokines in pulmonary fibrosis. Pulm Med. 2011;2011:105707. doi: 10.1155/2011/105707. Epub 2011/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardo A, Selman M, Kaminski N. Approaching the degradome in idiopathic pulmonary fibrosis. Int J Biochem Cell biol. 2008;40:1141–1155. doi: 10.1016/j.biocel.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 3.Gogali A, Wells AU. New pharmacological strategies for the treatment of pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:353–366. doi: 10.1177/1753465810379454. [DOI] [PubMed] [Google Scholar]
- 4.Desai B, Mattson J, Paintal H, Nathan M, Shen F, Beaumont M, et al. Differential expression of monocyte/macrophage- selective markers in human idiopathic pulmonary fibrosis. Exp Lung Res. 2011;37:227–238. doi: 10.3109/01902148.2010.538132. [DOI] [PubMed] [Google Scholar]
- 5.Wuyts WA, Willems S, Vos R, Vanaudenaerde BM, De Vleeschauwer SI, Rinaldi M, et al. Azithromycin reduces pulmonary fibrosis in a bleomycin mouse model. Exp Lung Res. 2010;36:602–614. doi: 10.3109/01902148.2010.492895. [DOI] [PubMed] [Google Scholar]
- 6.Sharma R, Guleria R, Pande JN. Idiopathic pulmonary fibrosis: newer concepts and management strategies. Indian J Chest Dis Allied Sci. 2003;45:31–49. [PubMed] [Google Scholar]
- 7.Matsui R, Goldstein RH, Mihal K, Brody JS, Steele MP, Fine A. Type I collagen formation in rat type II alveolar cells immortalised by viral gene products. Thorax. 1994;49:201–206. doi: 10.1136/thx.49.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmez H, Kardes O, Dogulu F, Kurt G, Memis L, Baykaner MK. Role of antifibrotic cytokine interferon-gamma in the prevention of postlaminectomy peridural fibrosis in rats. Neurosurgery. 2008;62:1351–1357. doi: 10.1227/01.neu.0000333307.02802.04. [DOI] [PubMed] [Google Scholar]
- 9.Hemmati AA, Nazari Z, Samei M. A comparative study of grape seed extract and vitamin E effects on silica-induced pulmonary fibrosis in rats. Pulm Pharmacol Ther. 2008;21:668–674. doi: 10.1016/j.pupt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Takemasa A, Ishii Y, Fukuda T. A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. Eur Respir J. 2012 doi: 10.1183/09031936.00127011. [DOI] [PubMed] [Google Scholar]
- 11.Gong LK, Li XH, Wang H, Zhang L, Chen FP, Cai Y, et al. Effect of Feitai on bleomycin-induced pulmonary fibrosis in rats. Journal of ethnopharmacology. 2005;96:537–544. doi: 10.1016/j.jep.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Borrelli F, Maffia P, Pinto L, Ianaro A, Russo A, Capasso F, et al. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia. 2002;73:S53–63. doi: 10.1016/s0367-326x(02)00191-0. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Lee JK, Kim HS, Chung ST, Eom JH, Kim KA, et al. Immunomodulatory effect of caffeic acid phenethyl ester in Balb/c mice. IntImmunopharmacol. 2004;4:429–436. doi: 10.1016/j.intimp.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73:S21–S9. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 15.Fesen MR, Pommier Y, Leteurtre F, Hiroguchi S, Yung J, Kohn KW. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol. 1994;48:595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]
- 16.Kurauchi Y, Hisatsune A, Isohama Y, Mishima S, Katsuki H. Caffeic acid phenethyl ester protects nigral dopaminergic neurons via dual mechanisms involving heme oxygenase‐1 and brain‐derived neurotrophic factor. Br J Pharmacol. 2012;166:1151–1168. doi: 10.1111/j.1476-5381.2012.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fontanilla CV, Wei X, Zhao L, Johnstone B, Pascuzzi RM, Farlow MR, et al. Caffeic acid phenethyl ester extends survival of a mouse model of amyotrophic lateral sclerosis. Neuroscience. 2011;205:185–193. doi: 10.1016/j.neuroscience.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Schraufnagel DE, Mehta D, Harshbarger R, Treviranus K, Wang NS. Capillary remodeling in bleomycin-induced pulmonary fibrosis. AmJ Pathol. 1986;125:97. [PMC free article] [PubMed] [Google Scholar]
- 19.Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur RespirJ. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbloom J, Feldman G, Freundlich B, Jimenez SA. Transcriptional control of human diploid fibroblast collagen synthesis by gamma-interferon. Biochem Biophys Res Commun. 1984;123:365–372. doi: 10.1016/0006-291x(84)90422-4. [DOI] [PubMed] [Google Scholar]
- 21.Hyde DM, Henderson TS, Giri SN, Tyler NK, Stovall MY. Effect of murine gamma interferon on the cellular responses to bleomycin in mice. Exp Lung Res. 1988;14:687–704. doi: 10.3109/01902148809087837. [DOI] [PubMed] [Google Scholar]
- 22.Prior C, Haslam PL. In vivo levels and in vitro production of interferon-gamma in fibrosing interstitial lung diseases. Clin Exp Immunol. 1992;88:280–287. doi: 10.1111/j.1365-2249.1992.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keane MP. The role of chemokines and cytokines in lung fibrosis. Eur Res Rev. 2008;17:151–156. [Google Scholar]
- 24.Strieter RM, Polverini PJ, Kunkel SL, Arenberg DA, Burdick MD, Kasper J, et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J Biol Chem. 1995;270:27348–27357. doi: 10.1074/jbc.270.45.27348. [DOI] [PubMed] [Google Scholar]
- 25.Gremy O, Benderitter M, Linard C. Caffeic acid phenethyl ester modifies the Th1/Th2 balance in ileal mucosa after gamma-irradiation in the rat by modulating the cytokine pattern. World J Gastroenterol : WJG. 2006;12:4996–5004. doi: 10.3748/wjg.v12.i31.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LC, Chu KH, Liang YC, Lin YL, Chiang BL. Caffeic acid phenethyl ester inhibits nuclear factor-kappaB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin Exp Immunol. 2010;160:223–232. doi: 10.1111/j.1365-2249.2009.04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sy LB, Yang LK, Chiu CJ, Wu WM. The immunoregulatory effects of caffeic acid phenethyl ester on the cytokine secretion of peripheral blood mononuclear cells from asthmatic children. Pediatr Neonatol. 2011;52:327–331. doi: 10.1016/j.pedneo.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhary NI, Schnapp A, Park JE. Pharmacologic differentiation of inflammation and fibrosis in the rat bleomycin model. AmJ Respir Crit Care Med. 2006;173:769–776. doi: 10.1164/rccm.200505-717OC. [DOI] [PubMed] [Google Scholar]
- 29.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–12. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurujeyalakshmi G, Wang Y, Giri SN. Taurine and niacin block lung injury and fibrosis by down-regulating bleomycin-induced activation of transcription nuclear factor-κB in mice. J Pharmacol Exp Ther. 2000;293:82–90. [PubMed] [Google Scholar]
- 31.Márquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Muñoz E. Caffeic acid phenethyl ester inhibits T-cell activation by targeting both nuclear factor of activated T-cells and NF-κB transcription factors. J Pharmacol Exp Ther. 2004;308:993–1001. doi: 10.1124/jpet.103.060673. [DOI] [PubMed] [Google Scholar]
- 32.Last JA, Reiser KM. Biosynthesis of collagen crosslinks III In vivo labeling and stability of lung collagen in rats with bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1989;1:111–117. doi: 10.1165/ajrcmb/1.2.111. [DOI] [PubMed] [Google Scholar]
- 33.Cutroneo KR, Sterling KM., Jr The biochemical and molecular basis of bleomycin-induced pulmonary fibrosis. Focus on Pulmonary Pharmacol Toxicol. 1988;1:1–22. [Google Scholar]
- 34.Moseley PL, Hemken C, Hunninghake GW. Augmentation of fibroblast proliferation by bleomycin. J Clini Invest. 1986;78:1150–1154. doi: 10.1172/JCI112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crapo JD, Barry BE, Gehr P, Bachofen M, Weibel ER. Cell number and cell characteristics of the normal human lung. Am Rev Respir Dis. 1982;126:332–337. doi: 10.1164/arrd.1982.126.2.332. [DOI] [PubMed] [Google Scholar]
- 36.Kelly J. In: Lung CellBbiol. Massaro D, editor. New York: M Dekker; 1989. pp. 821–866. [Google Scholar]
- 37.Zhou XM, Zhao Y, He CC, Li JX. Preventive effects of Citrus reticulata essential oil on bleomycin-induced pulmonary fibrosis in rats and the mechanism. Zhong xi yi jie he xue bao = J ChineseIintegr Med. 2012;10:200–209. doi: 10.3736/jcim20120211. [DOI] [PubMed] [Google Scholar]
- 38.Thannickal VJ, Flaherty KR, Martinez FJ, Lynch JP., 3rd Idiopathic pulmonary fibrosis: emerging concepts on pharmacotherapy. Expert Opin on pharmacother. 2004;5:1671–1686. doi: 10.1517/14656566.5.8.1671. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Liu X, Chen S, Wu J, Ye X, Xu L, et al. Tectorigenin inhibits the in vitro proliferation and enhances miR-338FNx01 expression of pulmonary fibroblasts in rats with idiopathic pulmonary fibrosis. J Ethnopharmacol. 2010;131:165–173. doi: 10.1016/j.jep.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Ozyurt H, Sogut S, Yildirim Z, Kart L, Iraz M, Armutcu F, et al. Inhibitory effect of caffeic acid phenethyl ester on bleomycine-induced lung fibrosis in rats. Clin Chim Acta. 2004;339:65–75. doi: 10.1016/j.cccn.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Hemmati AA, Zaeemzadeh N, Arzi A, Jalali T, Rashidi I. Protective Effect of Caffeic Acid Phenethyl Ester (CAPE) on Amiodarone-Induced Pulmonary Fibrosis in Rat. Iran J Pharm Res. 2011;10:321–328. [PMC free article] [PubMed] [Google Scholar]
- 42.Gao J, Huang Y, Li P, Xu D, Li J, Liu Y, et al. Antifibrosis effects of total glucosides of Danggui-Buxue-Tang in a rat model of bleomycin-induced pulmonary fibrosis. J Ethnopharmacol. 2011;136:21–26. doi: 10.1016/j.jep.2011.03.013. [DOI] [PubMed] [Google Scholar]


