Abstract
Considering the emergence of antibiotic resistance, scientists are interested in using new antimicrobial agents in the treatment of infectious diseases including infections of the enteric systems. Lactic acid bacteria have the great potential to produce antimicrobial compounds that inhibit and control pathogenic bacteria. The aim of this study was to determine the anti-bacterial and anti-adherence properties of Lactobacillus delbrueckii subsp bulgaricus against Escherichia coli. The antibacterial activity of L. delbrueckii was investigated using disc diffusion and spot on lawn methods. In vitro anti-adhesion effect of L. delbrueckii against E. coli was examined using Caco-2 cells. In anti-adhesion assay, three competition conditions including competitive inhibition, adhesion inhibition, and displacement were examined. In spot on lawn method the zone of growth inhibition of E. coli by L. delbrueckii was 21.1 mm. The cell free supernatant of L. delbrueckii showed a good antibacterial activity against E. coli which was mainly related to lactic acid produced by L. delbrueckii. When two bacteria added simultaneously (competitive inhibition) degree of inhibition of E. coli binding by L. delbrueckii was 77%. In adhesion inhibition assay, L. delbrueckii was able to exclude E. coli adherence by around 43.5%. Displacement assay showed that L. delbrueckii had strong displacement ability toward E. coli and reduction of E. coli attachment by bound L. delbrueckii was 81.3%. The results suggest that L. delbrueckii may be able to inhibit E. coli infection in the gut; however more studies including in vivo studies need to be performed.
Keywords: Anti-bacterial, Anti-adherence, Escherichia coli, Lactobacillus delbrueckii
INTRODUCTION
Lactobacillus delbrueckii subsp bulgaricus is a gram-positive bacteria belonging to lactic acid bacteria. This bacterium is a yoghurt starter culture which produces lactic acid and gives yogurt its flavor and textural properties(1). Previous studies have reported that consumption of yoghurt containing viable bacteria (Streptococcus thermophilus and L. delbrueckii) improved lactose digestion and decreased lactose intolerance(2). Recent studies have shown that L. delbrueckii has a potential probiotic function. Guglielmotti and coworkers reported that some commercial strains of L. delbrueckii subsp bulgaricus have shown high hydrophobicity values, β-galactosidase activity, good lysozyme tolerance and poor bile resistance. They indicated that these bacteria also possess antibacterial activity toward tested pathogens and can block the invasion of Salmonella enterica serovar Enteritidis into Caco-2/TC-7 cells(3).
Recently, scientific communities have focused on probiotics as health promoters. Probiotics are live microorganisms which beneficially affect their host by improving the intestinal microbial balance(4). Previous studies reported that some probiotic strains have antagonistic activities against gastro-intestinal pathogens(5,6). Many mechanisms for these observations have been proposed which includeproduction of antimicrobial compounds, change in the environmental conditions such as gut pH, competition for same nutrients and adhesion sites of like pathogens, and stimulation of the immune and non-immune defense mechanisms of the host(7). One of the common virulence strategies of pathogenic strains is adhesion to the host cells which provides a new target for treatment strategies(8). There are many approaches which inhibit bacterial attachment to the host cells. Some studies reported that sub-lethal concentrations of current antibiotics may inhibit bacterial adhesion(9). Today, the widespread use of antibiotics, repeatedly and incorrectly, increases antibiotic resistances causing inefficacy of antibiotics against bacteria which form biofilm and hospital acquired infections. Considering the emergence of antibiotic resistance, scientists are interested in using new antimicrobial agents in the treatment of infectious diseases including infections of the enteric system(10–11).
There is evidences that some probiotics can inhibit gastrointestinal infections by blocking adherence of the pathogens to the intestinal epithelium cells(6,12). However, this effect of probiotics depends on both the specific probiotic strain and the pathogen(13).
Escherichia coli is a gram-negative bacteria which is a member of Enterobacteriaceae family. E. coli can colonize in the body especially in the lower intestine and be transmitted through the oral-fecal route. Pathogenic strains of the bacterium can cause diseases from gastroenteritis to extra-intestinal infections of the urinary tract, pulmonary and nervous system(14).
The aim of the current study was to determine the antibacterial properties of L. delbrueckii against E. coli and also to assess whether intestinal epithelium adhesion and viability of potentially adherent E. coli can be reduced by L. delbrueckii.
MATERIALS AND METHODS
Chemicals
Chemicals used in tissue culture assays were purchased from Gibco (Scotland) via local vendors. Other chemicals and reagents used in this study were of analytical grade and obtained from Merck (Darmstadt, Germany).
Bacterial strains and growth conditions
L. delbrueckii subsp bulgaricus (DSM 20081) was purchased from German Collection of Microorganisms and Cell Cultures (DSMZ, Braunschweig, Germany). L. delbrueckii was grown in Man-Rogosa-Sharpe (MRS) broth at 37°C for 48 h. E.coli (PTCC 1330) was obtained from Persian Type Culture Collection and subcultured on Brain-heart infusion (BHI) broth and incubated at 37°C.
Caco-2 cell culture
The human colon adenocarcinoma cell line, Caco-2, was suplied by Pasteur Institute of Iran, Tehran. It was grown in Dulbecco’s modified Eagle’s Minimal Essential Medium (DMEM) supplemented with 20% (v/v) Fetal Bovine Serum (FBS), penicillin-streptomycin (100 IU/ml and 100 μg/ml, respectively) in a humidified atmosphere containing 5% CO2at 37°C. For adhesion assay, the Caco-2 cells were seeded at a density of 2×105 cells/well in 6-well tissue culture plates. The culture was refed every 2 days to obtain monolayer, and then further cultivated for 7-10 days to obtain differentiated cells. Then, Caco-2 monolayers were incubated with antibiotic-free medium for 24 h to perform the adhesion assay.
Agar spot on lawn method
Three μL of L. delbrueckii overnight culture (1×107 CFU/ml) was spotted on the surface of MRS agar plates and incubated overnight at 37 °C. Next day 200 μL of E. coli overnight culture (1×107 CFU/ml) was added into 7 mL of soft agar (0.7%). This soft agar contained a 1:1 mixture of BHI and MRS. The mixture was overlaid on the MRS agar plate containing the spots of L. delbrueckii. The zone free of bacterial growth observed around the spots was measured in millimeters.
Disk diffusion method
Overnight culture of L. delbrueckii was centrifuged at4000g for 10 min. The cell-free supernatant (CFS) was separated and passed through a 0.22 μ filter. The antibacterial activity of filtrate of CFS was investigated using disc diffusion method. To evaluate the effects of lactic acid and pH on the antibacterial activity of the CFS supernatant, following conditions were examined: 1) pH of CFS was adjusted to 6.5 using 0.1 M NaOH, 2) pH was adjusted to the pH values normally acheived by with L. delbrueckii by addition of enough lactic acid to MRS broth (without bacteria), 3) pH was adjusted to the pH values normally reached by L. delbrueckii by addition of enough HCl to MRS broth (without bacteria), 4) CFS without any treatment and 5) MRS broth. Sterile paper discs were located on BHI agar plates inoculated with E. coli. Samples of f50 μl were added to paper discs and incubated overnight at 37°C. The following day, the zone of inhibitionin millimeters was measured.
Adhesion assays
The overnight culture of E. coli and L. delbrueckii were centrifuged and after washing with ringer solution, the bacterial pellets were resuspended with DMEM medium (pH 4.5). Bacterial adhesion on Caco-2 cells was evaluated using 6-well plates. One ml of bacterial suspension (0.5 × 105 colony forming unit (CFU/ml)) was added to each well containing Caco-2 monolayer and incubated at 37°C for 1 h. Unbound bacteria were eliminated by three times washing with phosphate buffered saline (PBS).
In our study following type and order of bacterial additions were evaluated: 1) L. delbrueckii alone (L), 2) E. coli alone (E), 3) addition of simultaneous L. delbrueckii and E. coli (L+E) 4) addition of E. coli after L. delbrueckii (L/E) and 5) addition of L. delbrueckii after E. coli (E/L). To evaluate the possibility of substitution of E. coli by L. delbrueckii or vice versa, after three times washing with PBS, second bacteria was added and incubated. Then, Caco-2 cells were disrupted with 0.05% Triton X-100 for 5 min, and bound L. delbrueckii and E. coli were evaluated using plate counting on MRS and violet red bile (VRB) agar (CFU/ml), respectively. The MRS and VRB plates were incubated at 37°C for 48 and 24 h, respectively.
Statistical analysis
Each assay was repeated three times to ensure reproducibility of the results. All data are presented as mean ± standard deviation. Significant differences were calculated by analysis of variance (ANOVA) using SPSS version 16 and Tukey test was used to evaluate the difference between groups. P <0.05 was considered significant.
RESULTS
Agar spot on lawn
Results of spot on lawn method showed that L. delbrueckii inhibited the growth of E. coli and the zone of growth inhibition of E. coli by L. delbrueckii was 21.1 ±3 mm (n=12).
Disk diffusion
The antibacterial activity of CFS of L. delbrueckii was investigated using disc diffusion method (Table 1). The zone of inhibition for pH adjusted MRS broth with lactic acid and CFS (unadjusted pH) were significantly more than that of the negative control (PBS). The zone of inhibition for pH adjusted MRS with HCl was not significantly larger than that of the negative control (PBS). The results also showed that antibacterial effect of CFS did not significantly differ from that of pH adjusted MRS broth with lactic acid. After neutralizing pH to 6.5, antibacterial effect of CFS diminished and zone of inhibition for pH adjusted CFS was equal to that of the negative control (Fig. 1).
Table 1.
Antibacterial activity of L. delbrueckii against E. coli using disc diffusion method. (n=9)
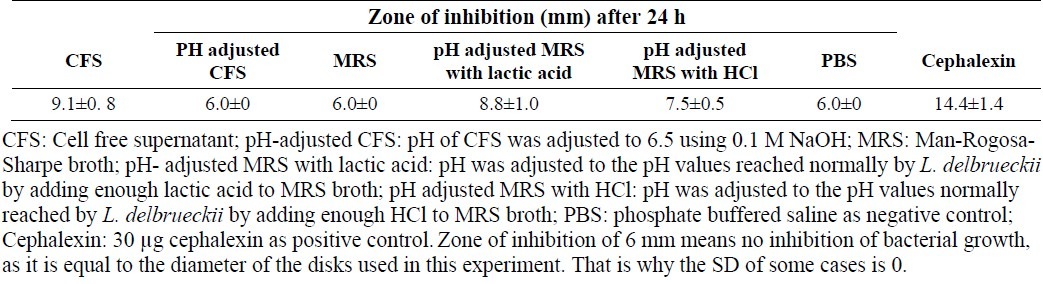
Fig. 1.
Evaluation of antibacterial activity using disk diffusion method. 1: positive control (cephalexin); 2: negative control (PBS); 3: cell free supernatant (CFS) without any treatment; 4: MRS broth whose pH was adjusted to the pH values normally reached by each L. delbrueckii (pH of CFS) by adding enough lactic acid; 5: CFS whose pH was adjusted to 6.5 using 0.1 M NaOH; and 6 : MRS broth.
Adhesion assays
In vitro adhesion of L. delbrueckii was examined using Caco-2 cells. Adhesion of L. delbrueckii to Caco-2 cells observed using light microscopy after Gram-staining (Fig. 2). The number of adhered bacteria (CFU/ml) was dependent on the amount of added cells (Fig. 3) and their coloration was liner (r2= 0.96).
Fig. 2.
Adhesion of L. delbrueckii to Caco-2 cells observed using light microscopy after Gram-staining
Fig. 3.
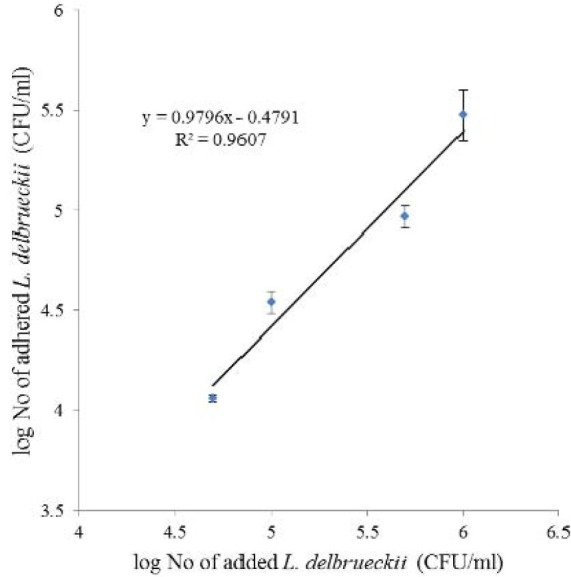
The effect of added bacteria (CFU/ml) on the number of adhered L. delbrueckii to Caco-2 cells. (n=3)
Adhesion value was determined according to the following equation:
% Adhesion = (Adhered bacteria / Added bacteria) × 100
The adherence values for L. delbrueckii and E. coli were 23.7% and 46%, respectively which proved that E. coli bind more effectively to Caco-2 cells than L. delbrueckii. In anti-adhesion assay, three competition conditions including competitive inhibition, adhesion inhibition, and displacements were examined. The numbers of adhered bacteria under different competition conditions are shown in Figs 4a and 4b for L. delbrueckii and E. coli, respectively. The results of competitive inhibition revealed that adhesion values of two bacteria reduced significantly (P < 0.05) when L. delbrueckii and E. coli added simultaneously (Fig. 4). E. coli showed more reduction (77%) in its adhesion value compared to L. delbrueckii (53%). In displacement assay, bacteria were first allowed to attach to Caco-2 cells before addition of second bacteria, and then the rate of reduction in the attachment of first bacteria was measured. In E/L procedure, attachment of E. coli was decreased by L. delbrueckii form 4.35 to 3.63 log CFU/ml (Fig. 4a) and in L/E procedure adherence of L. delbrueckii was reduced by E. coli from 4.07 to 4.05 log CFU/ml (Fig. 4b). Displacement assay showed that L. delbrueckii substituted significantly (P <0.05) the attached E. coli (E/L) while L. delbrueckii displacement by E. coli (L/E) was not statistically significant. In adhesion inhibition, ability of pre-adhered bacteria to exclude attachment of second bacteria was evaluated. The degree of adhesion inhibition of E. coli by pre-adhered L. delbrueckii was 43.5 % and reduction of L. delbrueckii attachment by bound E. coli was 28.6%.
Fig. 4.
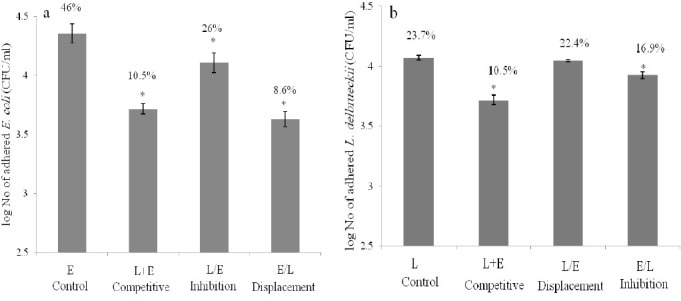
Adhesion of L. delbrueckii(a) and E. coli(b) to Caco-2 cells. The Caco-2 cell was incubated with DMEM medium containing 0.5×105 CFU/ml of bacteria. L: L. delbrueckiialone; E: E. colialone; L+E: L. delbrueckii and E. coli added simultaneously; L/E: E. coli added after L. delbrueckii; and E/L: L. delbrueckii added after E. coli. Asterisk (FNx01) indicates the means which were significantly different (P<0.05) from the control (Adhesion of L. delbrueckii alone (L) was considered as a control for adhesion assay L. delbrueckii and adhesion of E. coli alone (E) was considered as a control for adhesion assay E. coli. (n=9)
DISCUSSION
Lactic acid bacteria are the most widely used bacteria as starter cultures and have the great potential to produce antimicrobial compounds(15). The antimicrobial activity of lactic acid bacteria has been attributed to the production of different antimicrobials such as lactic acid, acetic acid, hydrogen peroxide, carbon dioxide, bacteriocins and other low molecular mass compounds with antimicrobial activity(16).
Using spot on lawn method which shows direct interaction of live bacteria, the antagonistic effect of L. delbrueckii against E. coli was evaluated. The findings indicated that L. delbrueckii had a good inhibitory effect on the E. coli growth. Also, antibacterial activity of the supernatant of L. delbrueckii was determined using disc diffusion method which showed CFS of L. delbrueckii had a good antibacterial effect. This activity was diminished when pH of CFS was adjusted to 6.5 indicating that antimicrobial compounds produced by L. delbrueckii were acidic compound or need low pH for their optimum activities. To evaluate the effects of pH and lactic acid on antibacterial activity, CFS and pH adjusted MRS broth with lactic acid and HCl were compared. These findings indicated that lactic acid was the most potent inhibitor produced by L. delbrueckii. Our findings are in agreement with studies of Guglielmotti and coworkers. and De Keersmaecker and coworkers. According to their studies the antimicrobial activity of some commercial strains of L. delbrueckii was mainly related to the lactic acid, and not to the pH value(3). In other study, Vanderleyden and coworkers reported strong antimicrobial activity of L. rhamnosus GG against Salmonella that was mediated by lactic acid(17).
Since it is difficult to study bacterial adherence in vivo, especially in humans, adhesion has been evaluated using in vitro model. In vitro adhesion of L. delbrueckii was performed using Caco-2 cell line. This cell line is one of the most widely used cell lines for studies related to probiotic and pathogens adhesion to intestinal epithelium(18).
In the present study, the number of adhered bacteria (CFU/ml) was linearly related to the amount of added cells. Similar results were reported by other groups indicating concentration dependent kinetics of adhesion(19). In anti-adhesion assay, the ability of L. delbrueckii to inhibit attachment of E. coli or vice versa was examined under three competitive conditions. When E. coli and L. delbrueckii were added simultaneously, degrees of inhibition were 77% and 53%, respectively.
Lee and cowrkers reported 20-50% reduction in the attachment of strains of E. coli and S. Typhimurium to Caco-2 cells by L. rhamnosus(20). In another study, Lee and coworkers proposed that degree of adherence of two competitor bacteria depends on the affinity of adhesive molecules expressed on the surface of bacteria to adhesion receptor present on the surface of host cells that they are competing for(21). In adhesion inhibition assay, L. delbrueckii was able to reduce E. coli and L. delbrueckii adherence by around 43.5% and 28.6%. Similar findings were reported for in vivo study in gnotobiotic piglets which indicated competitive exclusion of E. coli by L. gasseri K7(22). The possible mechanisms involved in inhibition of adherence of E. coli by L. delbrueckii are competition for common adhesion receptors, effects of substances present in the supernatant of L. delbrueckii and steric hindrance of adhered L. delbrueckii(23). However, the precise details of the proposed mechanisms are not understood. Displacement assay showed that L. delbrueckii has strong displacement ability toward E. coli and reduction of E. coli attachment by bound L. delbrueckii was 81.3%. In other displacement assay, attachment of Staphylococcus aureus in human intestinal mucus was inhibited 39-44% by L. rhamnosus GG, Lactococcus lactis subsp. lactis and Propionibacterium freudenreichii subsp. shermanii.(19) This displacement activity of probiotic bacteria may be explained by production of antimicrobial compounds or anti-adhesion factors and also competition for the same adhesion receptors(24). It is found that the anti-adhesion factors were able to degrade carbohydrate receptors of pathogens, to establish a biofilm and to induce production of biosurfactants and receptor analogues.
Our findings indicated that L. delbrueckii had a good anti-adhesion activity against E. coli in different competitive conditions. However, the ability of adhesion inhibition may depend on the specific probiotic strains and the pathogens. For example some commercial probiotic strains were not able to inhibit adherence of E. coli, L. monocytogenes and Salmonella typhimurium to human mucus and even increased attachment of these pathogens to intestinal mucus(13).
However, the specific mechanism of action of L. delbrueckii in vivo remains to be elucidated. Some study showed that administration of some of lactobacillus strains can decrease pH of gut and feces(25–26). In human body, in addition to a change in gut pH, the mechanism of inhibitory effect of bacteria may consist of competition for the same nutrients and adhesion sites. For better understanding of mechanism of action of this bacterium it is necessary to design an in vivo study.
CONCLUSION
It can be concluded form this study that L. delbrueckii is effective in inhibition of E. coli adhesion to Caco-2 cells under the conditions tested. The findings suggest that L. delbrueckii may be able to inhibit E. coli infection in the gut; however more studies including in vivo studies need to be performed. As this bacterium is used as a starter culture and considered safe, consumption of yoghurt containing viable L. delbrueckii may help to prevent and treat gastrointestinal infections caused by E. coli.
ACKNOWLEDGMENT
This study was supported by a grant from the Research Council of Isfahan University of Medical Sciences, Isfahan, Iran.
REFERENCES
- 1.Curry B, Crow V. Roginski H, Funquay J, Fox P, editors. Lactobacillus spp.: General characteristics. Encyclopedia of Dairy Science. 2003:1479–1511. [Google Scholar]
- 2.Guarner F, Perdigon G, Corthier G, Salminen S, Koletzko B, Morelli L. Should yoghurt cultures be considered probiotic. Brit J Nutr? 2005;93:783–786. doi: 10.1079/bjn20051428. [DOI] [PubMed] [Google Scholar]
- 3.Guglielmotti DM, Marcó MB, Golowczyc M, Reinheimer JA, Quiberoni AL. Probiotic potential of Lactobacillus delbrueckii strains and their phage resistant mutants. Int Dairy J. 2007;17:916–925. [Google Scholar]
- 4.Oelschlaeger TA. Mechanisms of probiotic actions-A review. Int J Med Microbiol. 2010;300:57–62. doi: 10.1016/j.ijmm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Collado M, Grze kowiak, Salminen S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr Microbiol. 2007;55:260–265. doi: 10.1007/s00284-007-0144-8. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee P, Merkel G, Bhunia A. Lactobacillus delbrueckii ssp. bulgaricus B-30892 can inhibit cytotoxic effects and adhesion of pathogenic Clostridium difficile to Caco-2 cells. Gut Pathog. 2009;1:8–19. doi: 10.1186/1757-4749-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J Nutr. 2000;130:396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- 8.Kelly CG, Younson JS. Anti-adhesive strategies in the prevention of infectious disease at mucosal surfaces. Expert Opin Inv Drug. 2000;9:1711–1721. doi: 10.1517/13543784.9.8.1711. [DOI] [PubMed] [Google Scholar]
- 9.Scheld WM, Zak O, Vosbeck K, Sande M. Bacterial adhesion in the pathogenesis of infective endocarditis. Effect of subinhibitory antibiotic concentrations on streptococcal adhesion in vitro and the development of endocarditis in rabbits. J Clinl Invest. 1981;68:1381–1384. doi: 10.1172/JCI110388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fauci AS, Morens DM. The perpetual challenge of infectious diseases. New Engl J Med. 2012;366:454–461. doi: 10.1056/NEJMra1108296. [DOI] [PubMed] [Google Scholar]
- 11.Venugopal AA, Johnson S. Fidaxomicin: A novel macrocyclic antibiotic approved for treatment of Clostridium difficile infection. Clin Infect Dis. 2012;54:568–574. doi: 10.1093/cid/cir830. [DOI] [PubMed] [Google Scholar]
- 12.Bernet M, Brassart D, Neeser J, Servin A. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collado M, Meriluoto J, Salminen S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol. 2007;45:454–460. doi: 10.1111/j.1472-765X.2007.02212.x. [DOI] [PubMed] [Google Scholar]
- 14.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2009;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 15.Tufail M, Hussain S, Malik F, Mirza T, Parveen G, Shafaat S, et al. Isolation and evaluation of antibacterial activity of bacteriocin produced by Lactobacillus bulgaricus from yogurt. Afr J Microbiol Res. 2011;5:3842–3847. [Google Scholar]
- 16.Šuškoviæ J, Kos B, Beganoviæ J, Leboš Pavunc A, Habjaniè K, Matošiæ S. Antimicrobial activity-The most important property of probiotic and starter lactic acid bacteria. Food Technol Biotechnol. 2010;48:296–307. [Google Scholar]
- 17.De Keersmaecker SCJ, Verhoeven TLA, Desair J, Marchal K, Vanderleyden J, Nagy I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol Lett. 2006;259:89–96. doi: 10.1111/j.1574-6968.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 18.Sambuy Y, De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biolo Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 19.Vesterlund S, Karp M, Salminen S, Ouwehand AC. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiol. 2006;152:1819–1826. doi: 10.1099/mic.0.28522-0. [DOI] [PubMed] [Google Scholar]
- 20.Lee YK, Puong KY, Ouwehand AC, Salminen S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J Med Microbiol. 2003;52:925–930. doi: 10.1099/jmm.0.05009-0. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y, Puong K. Competition for adhesion between probiotics and human gastrointestinal pathogens in the presence of carbohydrate. Brit J Nutr. 2002;88:101–108. doi: 10.1079/BJN2002635. [DOI] [PubMed] [Google Scholar]
- 22.Matijašiæ BB, Stojkoviæ S, Salobir J, Malovrh Š, Rogelj I. Evaluation of the Lactobacillus gasseri K7 and LF221 strains in weaned piglets for their possible probiotic use and their detection in the feces. Anim Res. 2004;53:35–44. [Google Scholar]
- 23.Lee Y, Lim C, Teng W, Ouwehand A, Tuomola E, Salminen S. Quantitative approach in the study of adhesion of lactic acid bacteria to intestinal cells and their competition with enterobacteria. Appl Environ Microbiol. 2000;63:3692–3697. doi: 10.1128/aem.66.9.3692-3697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lievin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser J, et al. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut. 2000;47:646–652. doi: 10.1136/gut.47.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biasco G, Paganelli G, Brandi G, Brillanti S, Lami F, Callegari C, et al. Effect of lactobacillus acidophilus and bifidobacterium bifidum on rectal cell kinetics and fecal pH. Ital J gastroenterol. 1991;23:142. [PubMed] [Google Scholar]
- 26.Romond MB, Ais A, Guillemot F, Bounouader R, Cortot A, Romond C. Cell-free whey from milk fermented with Bifidobacterium breve C50 used to modify the colonic microflora of healthy subjects. J Dairy Sci. 1998;81:1229–1235. doi: 10.3168/jds.S0022-0302(98)75683-8. [DOI] [PubMed] [Google Scholar]


