Abstract
Celecoxib is a non-steroidal anti-inflammatory drug (NSAID) developed as a selective inhibitor of cyclooxygenase-2 (COX-2) for the treatment of rheumatoid arthritis disease. Recently some other mechanisms have been identified for anti cancer activity of these agents including induction of apoptosis, inhibition of tumor vascularization, stimulation of antitumor immune responses and inhibition of cellular protein synthesis. The cytotoxic effects of four synthesisized analogues of celecoxib (coded as D, E, F and G) were evaluated against Hela, MDA-MB-231, A-2780-s and HT-29 cancer cells, using MTT assay; Also their induction of apoptosis using DNA fragmentation analysis were studied. MTT assay showed that cell survival percent of COX-2 positive cell lines (HT-29, MDA-MB-231 and Hela; p≤0.05) were decreased significantly after exposure to the tested COX-2 inhibitors while little effect was observed on the COX-2 negative cell line (A-2780-s). Results also showed that A-2780-s and Hela were the most resistant and the most sensitive cell lines to these compounds, respectively. Moreover, in DNA fragmentation assay, induction of apoptosis was confirmed by electrophoretic pattern of separated DNA fragments in Hela cell line. Compounds E and G in comparison with D and F exerted more cytotoxic effect on COX-2 positive cell lines (Hela, HT-29 and MDA-MB-231). This could be due to the hydrophobic substituent (Cl, CH3) located at the para position of phenyl ring leading to more lipophilicity and cell uptake. In addition, these COX-2 inhibitors induced apoptosis on Hela cell-line, which could be considered as one of the cytotoxic mechanisms of these compounds as potential anti cancer agents.
Keywords: Celecoxib, HT-29, MDA-MB-231, A-2780-s, DNA fragmentation, MTT assay
INTRODUCTION
Cyclooxygenase (COX), as a rate limiting enzyme in the synthesis of prostaglandins has two isoforms named (COX-1 and COX-2)(1). Among them, COX-1 is involved in platelet activation, gastrointestinal protection and kidney function and COX-2 is mainly produced in response to tissue damages and pro-inflammatory signals(2,3). Traditional non-steroidal anti-inflammatory drugs (NSAIDs) which inhibit both isoforms of COXs, lead to considerable gastrointestinal side effects(4). In contrast, celecoxib (Celebrex) and rofecoxib (Vioxx) as selective COX-2 inhibitors are devoid of gastric toxic reactions mediated primarily by inhibition of COX-1, and retain high anti inflammatory activity(5). Interestingly, recent reports by Kismet and coworkers(6) showed that celecoxib could also act as a potent chemo-preventive agent used in various types of cancer and could improve tumor response to radiotherapy via different mechanisms including those that make tumor cells more sensitive to radiation(7). At first it was thought that the antineoplastic effects of NSAIDs were due to the inhibition of COXs and production of related prostaglandins(8),(9), while some other evidences indicated that these drugs also act by COX-independent effects(8). Selective cyclooxygenase-2 inhibitors showed a differential ability to inhibit proliferation and induce cell apoptosis(10). The cytotoxic effects and the underlying mechanism of the coxib agents have not been yet fully understood and further studies in this regards are warranted. Therefore, the present study was conducted in order to study the cytotoxic effect and apoptosis induction of new analogues of celecoxib against colon, breast, cervical and ovarian cancer cells.
MATERIALS AND METHODS
Test compounds
Analogues of celecoxib (Fig. 1) were designed and synthesized at the School of Pharmacy, Shaheed Beheshti University of Medical Sciences, Tehran, Iran(11). The compounds were dissolved in dimethyl-sulfoxide (DMSO, Merck, Germany) and freshly diluted in culture medium before the start of experiments. The final DMSO concentration never exceeded 1% and this condition was used as negative control in each experiment.
Fig. 1.

Molecular structure of celecoxib derivatives used in this study. *X: mg of compounds required for preparation of stock solution having 10 mM concentrations (see materials and methods).
Cell culture
Cells were obtained from Pasteur Institute (Tehran, Iran). HT-29, Hela and A-2780-s were grown in RPMI 1640 medium and MDA-MB-231 cells maintained in high glucose Roswell Park Memorial Institute medium (RPMI) (Gibco, Scotland) supplemented with 10% Fetal Bovine Serum (FBS, Gibco, Scotland).
Cell viability assay
The thiazolyl blue (MTT, Merck, Germany) assay has been used in many experiments for assessment of cell viability, and this reaction is used as the end point in a rapid drug-screening assay(12). Briefly, cells were seeded at density of 1 × 105 cells/mL in 96-well tissue culture plates and were resuspended in 10 mL complete culture medium and allowed to attach for 24 h. After this period, cells were incubated with increasing concentrations of compounds (0.001, 0.01, 0.1, 1 mM) for 48 h separately. MTT solution (20 μL) was then added to each well and plate was incubated for 3 h at 37 °C. During this period, living cells produced blue insoluble formazan from the yellow soluble MTT. The reaction was stopped by addition of DMSO (150 μL/wells) and the contents of the wells were dissolved during 2-3 min. MTT formazan product was detected by measuring absorbance with an ELISA plate reader (Awareness, USA) at 540 nm(13). All tests were performed intriplicate and in three different days. The absorbance of the formazan treated wells in the visible region correlates with the number of viable cells as follows:
Viable cells (%) = [T-B)/ (C-B] ×100
where, C is the absorbance of control, T is the absorbance of treated samples, and B is the absorbance of the blank.
Apoptosis assay
Using Apoptotic DNA Ladder Kit (Roche Diagnostics GmbH, Mannheim, Germany), Hela cells (2×106 cells/well) were seeded in two 6 well plates and allowed to adhere for 24 h and then treated with 400 μL of compound D and E (0.001 mM). Plates were then incubated for 48 h at 37 °C in humidified atmosphere of 95% air and 5% CO2. After trypsinization, cells were washed with 200 μL of PBS. Apoptotic cells were incubated with 200 μL of lysis/binding buffer in 15-25 °C for 10 min. After incubation, the lysed sample was mixed with 100 μL isopropanol and pipetted into a filter tube containing glass fleece. DNA which was bound to the filter tube was isolated from the lysate through centrifugation of the sample (1 min; 8000 rpm; twice) which was followed by a final high speed spin (13000 rpm; 1 min; then 10 sec in RT). The flow-through liquid containing unbound lysate components was then discarded. After washing the bound DNA, the filter was inserted into 1.5 ml-centrifuge tube, 200 μL warmed (70 °C) elution buffer was then added and the eluted DNA was collected by centrifugation (1 min; 8000 rpm; RT). 20 μL of DNA eluted sample was mixed with 4 μL of loading buffer, electrophoresed (Akhtarian, Iran) on 0.8% agarose gels at 90 V for 1.5 h and visualized using a UV transilluminator and then photographed(14).
Statistical analysis
One-way analysis of variance (ANOVA) and Scheffe post hoc were used for data analysis. All results were expressed as mean ± SD and P < 0.05 was considered as statistically significant.
RESULTS
Cell viability assay
Four malignant cell lines were incubated with different concentrations of compounds (0.0001, 0.001, 0.01, and 0.1 mM) in wells against doxorubicin (0.1 mM) as positive control for 48 h, and their cytotoxicities were evaluated using MTT assay protocol. Viable cells were determined by trypan blue exclusion assay and percentage of survival was calculated assuming viable untreated cells to 100 %. The values represent the mean of three independent experiments each performed in triplicate (error bars; SD).
As shown in Fig 2–4 all of the tested compounds showed significant inviability compared to the negative control (p<0.05) in COX-2 positive cell lines (Hela, MDA-MB-231 and HT-29). Hela cell line was the most sensitive cells to the tested compounds (Fig. 2) whereas, A-2780-s cell was the most resistant cell line (Fig. 5).
Fig. 2.
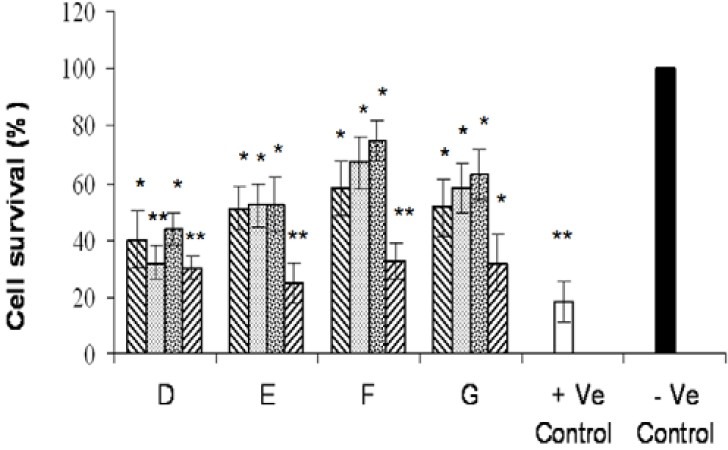
Effects of various tested compounds on the proliferation of COX-2 positive (Hela) cell line. Cells were exposed for 72 h to different concentrations (0.001, 0.01, 0.1, 1 mM) of compounds as indicated. Untreated cells incubated in the presence of vehicle (1% DMSO) and were used as negative control. Doxorubicin (0.1 mM) was used as positive control. For statistical significance one-way ANOVA was used to analyze the differences between each sample and negative control (*P<0.05, **P<0.01).
Fig. 4.
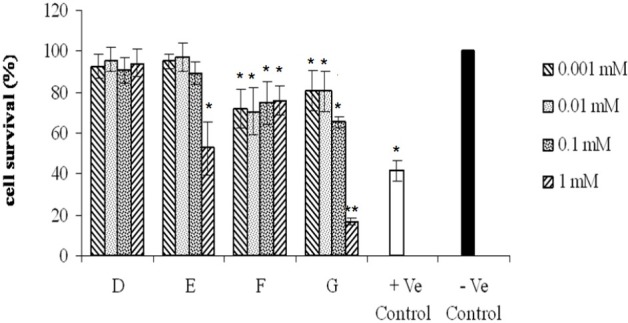
Effects of various tested compounds on the proliferation of COX-2 positive (MAD-MB-231) cell line. Cells were exposed for 48 h to different concentrations (0.001, 0.01, 0.1, 1 mM) of compounds as indicated. Untreated cells incubated in the presence of vehicle (1% DMSO) and were used as negative control. Doxorubicin (0.1 mM) was used as positive control. For statistical significance one-way ANOVA was used to analyze the differences between each sample and negative control (*P<0.05, **P<0.01).
Fig. 5.
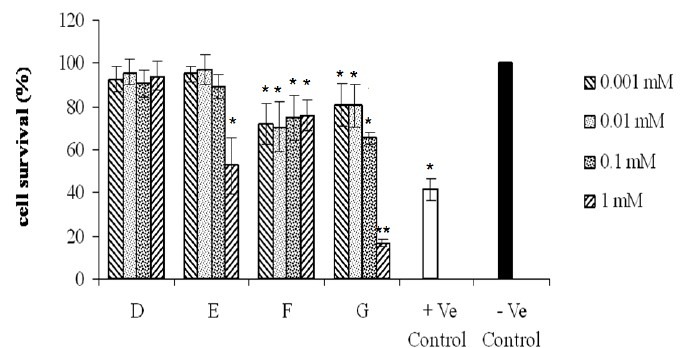
Effects of compounds on the proliferation of COX-2 negative (A-2780-s) cell line. Cells were exposed for 72 h to different concentrations (0.001, 0.01, 0.1, 1 mM) of compounds as indicated. Untreated cells incubated in the presence of vehicle (1% DMSO) and were used as negative control. Doxorubicin (0.1 mM) was used as positive control. For statistical significance one-way ANOVA was used to analyze the differences between each sample and negative control (*P<0.05, **P<0.01).
Fig. 3.
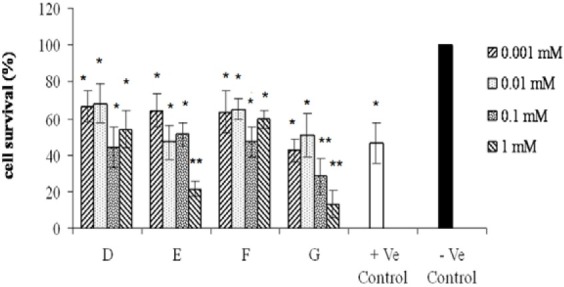
Effects of various tested compounds on the proliferation of COX-2 positive (HT-29) cell line. Cells were exposed for 72 h to different concentrations (0.001, 0.01, 0.1, 1 mM) of compounds as indicated. Untreated cells incubated in the presence of vehicle (1% DMSO) and were used as negative control. Doxorubicin (0.1 mM) was used as positive control. For statistical significance one-way ANOVA was used to analyze the differences between each sample and negative control (*P<0.05, **P<0.01).
Apoptosis assay
To elucidate the cytotoxic mechanism of these compounds, compounds D and E were selected for further DNA fragmentation assay and exposed to the most sensitive cell line, Hela, for 72 h at a concentration of 0.001 mM. As seen in Fig. 6, comparing with ladder and negative control, compounds D and E induced apoptosis in Hela cells.
Fig. 6.
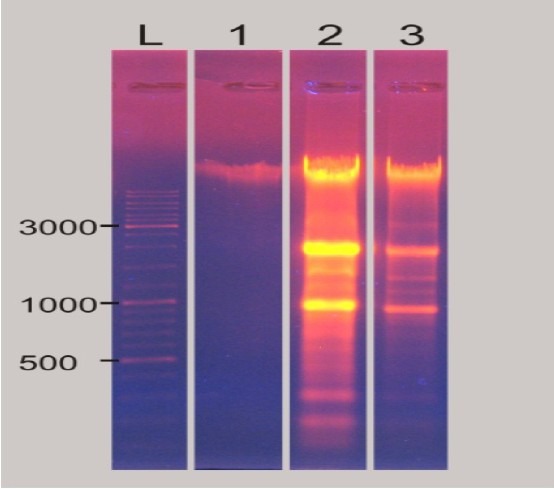
Electrophoretic pattern of separated DNA from treated Hela cells with compound D and E at concentration of (0.001 mM). (L: Ladder, 1: negative control, 2: 20 μL of compound E, 3: 20 μL of compound D).
DISCUSSION
studies have shown that heteroaryl-phenyl-substituted pyrazole derivatives are selective and potent COX-2 inhibitors(15). Celecoxib is one of these agents proposed as a putative chemo-preventive agent enhancing response of tumors to anticancer drugs or radio-response in combination therapy(16–18). These statements are based on the findings that most of cancer progression in colorectal, gastric, esophageal, hepatocellular, pancreatic, lung, breast, skin, cervix and prostate cancer cells, are related to up-regulation of COX-2 which increases the content of PGE2 as one of the main products(6,19). These results suggest that enhanced expression of COX-2 may play a role in the pathogenesis of cancer and COX-2 selective inhibitors can be used for cancer chemoprevention(6), while some other evidences showed these drugs also act by COX-independent mechanisms(8,20). In the present study, cytotoxic effects of four celecoxib derivatives on different human tumor cell lines were tested. Tested compounds showed different effects on each cell line (Figs. 2–4). Cell survival percent in Hela cell-line was the lowest, suggesting Hela to be more sensitive to the test compounds. Compounds E and G exerted potent cytotoxic effects against Hela, HT-29 and MDA-MB-231, COX-2 positive cell lines. Structure activity relationship studies have revealed that the presence of two hydrophobic groups in the structure of COX-2 inhibitors seems essential for better COX-2 inhibitory effect. This is because when they are substituted with polar groups like CH2OH, the COX-2 inhibitory effect is decreased(21). Therefore, the existence of hydrophobic Cl and CH3 substituents at the para position of phenyl ring (R) in G and E compounds could be responsible for their more cytotoxic effects.
COX-2 inhibitory effects play an important role in anti tumor activities. In the present study, A-2780-s, a non-expressing COX-2 gene cell line originated from ovarian cancer, was exposed to the tested compounds and the results showed that this cell line was the most resistant cell line using MTT assay. These results confirmed that coxib derivatives used here exert their cytotoxic effects via inhibition of COX-2 enzymes. As reported before by Bijman and co-workers HT29, Hela and MDA-MB-231 cells express COX-2 protein under standard culture conditions, while A-2780-s cells is not able to express this enzyme(22). Therefore, little effects of compounds on A-2780-s cell line may be, in part, due to the lack of COX-2 enzyme synthesis.
In agreement with our findings, results of other studies revealed that induction of apoptosis and DNA fragmentation was one of the most reported mechanisms for COX-2 inhibitors(10,23,24). As shown in Fig. 6, compounds D and E fragmented DNA at the lowest doses (0.001 mM). Comparison of the ladder and negative control results confirmed that tested compounds could induce apoptosis after 72 h.
CONCLUSION
Compounds E and G in comparison to D and F exerted more cytotoxic effects on COX-2 positive cell lines (Hela, HT-29 and MDA-MB-231). The increased activity may be due to the presence hydrophobic substituent (Cl, CH3), located at the para position of phenyl ring leading to more lipophilicity, cell uptake and consequently increased cytotoxic effects. Among these compounds, D and E, induced also apoptosis on Hela cell line. Therefore, to propose celecoxib derivatives with optimum anti inflammatory and anti-proliferative activities, compound E with both cytotoxic and apoptotic effects on cancer cell lines could be suggested for further studies.
ACKNOWLEDGMENT
study was financially supported and approved by the research council of the Isfahan University of Medical Sciences (project no. 388495), Isfahan, Iran. Also the authors would like to thank Mrs. Mirian, Mrs. Shafee-zadegan and Mrs. Moazzen for their help and technical support.
REFERENCES
- 1.Marnett LJ, Rowlinson SW, Goodwin DC, Kalgutkar AS, Lanzo CA. Arachidonic acid oxygenation by COX-1 and COX-2, mechanisms of catalysis and inhibition. J Biol Chem. 1999;274:22903–22906. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- 2.Wright JM. The double edged sword of COX-2 selective NSAIDs. CMAJ. 2002;167:1131–1137. [PMC free article] [PubMed] [Google Scholar]
- 3.Williams DA, Lemke TL, Roche VF, Zito SW. Foye’s principles of medicinal chemistry. 6th ed. Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 4.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivity’s for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci USA. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 6.Kismet K, Akay MT, Abbasoglu O, Ercan A. Celecoxib: a potent cyclooxygenase-2 inhibitor in cancer prevention. Cancer Detect Prev. 2004;28:127–142. doi: 10.1016/j.cdp.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Choy H, Milas L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: a rational advance. J Natl Cancer Inst? 2003;95:1440–1452. doi: 10.1093/jnci/djg058. [DOI] [PubMed] [Google Scholar]
- 8.Shiff S, Rigas B. The role of cyclooxygenase inhibition in the antineoplastic effects of nonsteroidal anti inflammatory drugs (NSAIDs) J Exp Med. 1999;190:445–450. doi: 10.1084/jem.190.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pyrko P, Kardosh A, Schönthal AH. Celecoxib transiently inhibits cellular protein synthesis. Biochem Pharmacol. 2008;75:395–404. doi: 10.1016/j.bcp.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki R, Kusunoki N, Matsuzaki T, Hashimoto S, Kawai S. Selective cyclooxygenase-2 inhibitors show a differential ability to inhibit proliferation and induce apoptosis of colon adenocarcinoma cells. FEBS Lett. 2002;531:278–284. doi: 10.1016/s0014-5793(02)03535-4. [DOI] [PubMed] [Google Scholar]
- 11.Zarghi A, Arfaei S and Ghodsi R. Design and synthesis of new 2, 4, 5-triarylimidazole derivatives as selective cyclooxygenase (COX-2) inhibitors. Med Chem Res. 2011;21:1803–1810. [Google Scholar]
- 12.Sadeghi-aliabadi H, Ghasemi N, Kohi M. Cytotoxic effect of Convolvulus arvensis extracts on human cancerous cell line. Res Pharm Sci. 2008;3:31–34. [Google Scholar]
- 13.Sadeghi-Aliabadi H, Tabarzadi M, Zarghi A. Synthesis and cytotoxic evaluation of two novel anthraqhinone derivatives. Farmaco. 2004;59:645–649. doi: 10.1016/j.farmac.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Dounousi E, Koliousi E, Papagianni A, Ioannou K, Zikou X, Katopodis K, Kelesidis A, Tsakiris D, Siamopoulos KC. Mononuclear leukocyte apoptosis and inflammatory markers in patients with chronic kidney disease. Am J Nephrol. 2012;36:531–6. doi: 10.1159/000345352. [DOI] [PubMed] [Google Scholar]
- 15.Cheng H, Lundy Demello C, Li J, Sakya S, Ando K, Kawamura B, et al. Synthesis and SAR of heteroaryl-phenyl-substituted pyrazole derivatives as highly selective and potent canine COX-2 inhibitors. Bioorg Med Chem Lett. 2006;16:2076–2080. doi: 10.1016/j.bmcl.2006.01.059. [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med. 2006;355:950–952. doi: 10.1056/NEJMe068158. [DOI] [PubMed] [Google Scholar]
- 17.Raju U, Ariga H, Dittmann K, Nakata E, Ang K, Milas L. Inhibition of DNA repair as a mechanism of enhanced radioresponse of head and neck carcinoma cells by a selective cyclooxygenase-2 inhibitor, celecoxib. IJROBP. 2005;63:520–528. doi: 10.1016/j.ijrobp.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Chung ko J, Han wang L, Yuan jhan J, Ci ciou S, Hao hong J, Ting lin S, et al. The role of celecoxib in Rad51 expression and cell survival affected by gefitinib in human non-small cell lung cancer cells. Lung Cancer. 2009;65:290–298. doi: 10.1016/j.lungcan.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Cahlin C, Lönnroth C, Arvidsson A, Nordgren S, Lundholm K. Growth associated proteins in tumor cells and stroma related to disease progression of colon cancer accounting for tumor tissue PGE2 content. Int J Oncol. 2008;32:909–918. [PubMed] [Google Scholar]
- 20.Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. Cox-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742–2744. doi: 10.1096/fj.01-0299fje. [DOI] [PubMed] [Google Scholar]
- 21.Michaux C, Leval X, Julémont F, Dogné J, Pirotte B, Durant F. Structure-based pharmacophore of COX-2 selective inhibitors and identification of original lead compounds from 3D data base searching method. Eur J Med Chem. 2006;41:1446–1455. doi: 10.1016/j.ejmech.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Bijman M, Hermelink C, Van berkel M, Laan A, Janmaat M, Peters GJ, et al. Interaction between celecoxib and docetaxel or cisplatin in human cell lines of ovarian cancer and colon cancer is independent of COX-2 expression levels. Biochem Pharmacol. 2008;75:427–437. doi: 10.1016/j.bcp.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann M, Lorenz HM, Voll R, Grünke M, Woith W, Kalden JR. A rapid and simple method for the isolation of apoptotic DNA fragments. Nucleic Acids Res. 1994;22:5506–5507. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamanaka Y, Shiraki K, Inoue T, Miyashita K, Fuke H, Yamaguchi Y, et al. COX-2 inhibitors sensitize human hepatocellular carcinoma cells to TRAIL-induced apoptosis. Int J Mol Med. 2006;18:41–47. [PubMed] [Google Scholar]


