Abstract
Objective:
The present study was designed to investigate the antidepressant potential of N-n-butyl-3-ethoxyquinoxalin-2-carboxamide (6p), a novel 5-HT3 receptor antagonist in rodent behavioral models of depression.
Materials and Methods:
The compound 6p was examined in various behavioral models like forced swim test (FST), tail suspension test (TST), mechanistic models [5-hydroxytryptophan (5-HTP)-induced head twitch and reserpine-induced hypothermia (RIH)], and in chronic surgery model-olfactory bulbectomy in rats.
Results:
Compound 6p (1, 2, and 4 mg/kg, i.p.) exhibited antidepressant-like effect in FST and TST after acute treatment without having an effect on baseline locomotor activity. Moreover, 6p (2 mg/kg, i.p.), potentiated the 5-HTP–induced head twitch responses in mice and inhibited the RIH in rats. Chronic treatment (14 days) with 6p (1 and 2 mg/kg, p.o.) and paroxetine (10 mg/kg, p.o.) in rats significantly reversed the behavioral anomalies induced by bilateral olfactory bulbectomy using open field exploration.
Conclusion:
The preliminary studies reveal that compound 6p exhibits antidepressant-like effect in behavioral rodent models of depression.
KEY WORDS: 5-HT3 receptor antagonists, antidepressant, forced swim test, quinoxaline, serotonin
Introduction
Behavioral, neurochemical, electrophysiological, and molecular analyses provided substantial evidence that rationalizes the correlation between serotonin type-3 receptor inflection and rodent depressive-like behavior.[1] Serotonin type 3 (5-HT3) receptor subtypes are pentameric ion channels belonging to the superfamily of Cys-loop receptors.[2] Receptor activation either leads to fast excitatory responses or modulation of neurotransmitter release, depending on their neuronal localization.[3] 5-HT3 receptors are expressed in the central nervous system in regions involved in the vomiting reflex, perception of pain, the reward system, cognition, depression and anxiety control. In the periphery, they are present on a variety of neurons and immune cells. 5-HT3 receptors are known to be involved in emesis, pain disorders, drug addiction, psychiatric and GI disorders.[4]
Regardless of the chemical structures, most of the clinically existing antidepressant drugs exert their action by elevating the level of monoamine(s) in synapse, either directly or indirectly.[5] The older antidepressant drugs such as monoamine oxidase inhibitors and tricyclic antidepressants are unfortunately well known for their drug–food/–drug interaction and side effects rather than their therapeutic efficacy.[6] Introduction of “low side-effect antidepressants,” such as selective serotonin reuptake inhibitors (SSRIs) for the treatment of depression, enhance the patient's compliance toward pharmacotherapy of depression.[7] Moreover, these molecules are less effective than the older drug molecules and take 4–6 weeks to produce therapeutic effects.[8]
Targeting 5-HT3 receptor will be of notable interest for the development of newer antidepressants. Earlier, the beneficial effects of 5-HT3 receptor antagonists (ondansetron, granisetron, etc.) were well established for treatment of nausea and vomiting (with fewer or negligible side-effect profile) in pre-clinical and clinical studies.[9]
Acute administration of ondansetron, a selective 5-HT3 receptor antagonist, significantly reduces glucose utilization in the limbic regions of the rat brain, especially the median raphe nucleus, which is associated with depression.[9] Moreover, systemic administration of tropisetron prevented restraint stress-induced dopamine release in the nucleus accumbens and prefrontal cortex in rats, which indicates that 5-HT3 receptors mediate stress-dependent activation of dopaminergic neurotransmission.[9]
The involvement of 5-HT3 receptors in depression and anxiety is complemented by studies of 5-HT3 knockout mice, which revealed the regulation of 5-HT3 (3A subtype) in depression- and anxiety-related behaviors.[10] Evidence for the relevance of 5-HT3 antagonists in the treatment of depression stems from clinical trials in which patients suffering from complex disorders such as fibromyalgia and bulimia showed improvement of the co-morbid depression.[11,12]
Despite increased interest among the clinical neurosciences, information regarding the antidepressant activity of serotonin type-3 modulators is still lacking. Thus, selecting the test sensitive to antidepressant drugs, the present study was designed to investigate the antidepressant potential of N-n-butyl-3-ethoxyquinoxalin-2-carboxamide (6p) in rodent acute and chronic models of depression.
Using the three-component pharmacophore model,[13] a series of 5-HT3 receptor antagonists have been designed, synthesized, and screened for their 5-HT3 antagonist potential [Table 1]. The compounds were tested for their ability to inhibit the 5-HT3 receptor in isolated guinea pig ileum and the pA2 values were determined against 2-methyl-5-hydroxytryptamine with ondansetron as a reference drug.[9]
Table 1.
Physical constants and pharmacological data of 3-ethoxyquinoxalin-2-carboxamides
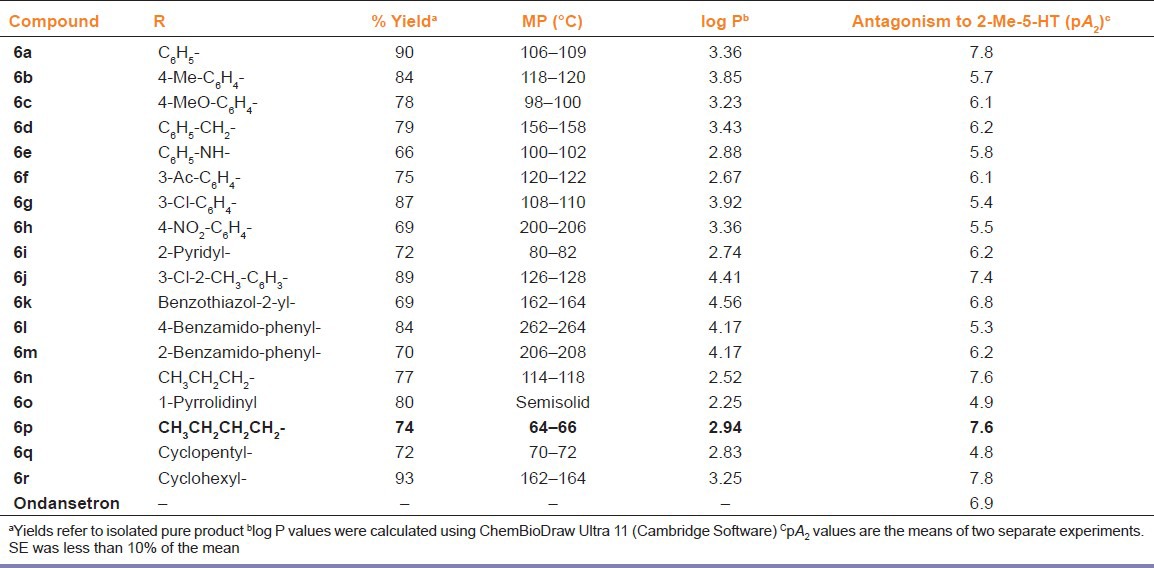
Rodent models of depression have been utilized to assess the antidepressant-like efficacy of various drugs.[14] Some of these tests neglect the aspect of face validity but have a strong predictive validity.[15] Hence, a battery of behavioral tests were adopted for the study, which included acute models like forced swim test (FST)[16] and tail suspension test (TST)[17] and mechanistic models like 5-hydroxytryptophan (5-HTP)-induced head twitch response and reserpine-induced hypothermia (RIH).[9] Evaluation of chronic effect of the compound on olfactory bulbectomized rats[18] provided significant information on the antidepressant activity of 6p, which was identified for this study based on pA2 and log P values.
In the present study, compound 6p which exhibited good log P (2.94) and pA2 values (7.6) [Table 1], greater than the standard 5-HT3 receptor antagonist, ondansetron (pA2 6.9),[19,20] was selected for the preliminary antidepressant screening in the standard rodent models of depression as mentioned above.
Materials and Methods
Animals
Albino mice (25 ± 2 g), Wistar rats (250 ± 20 g), and Dunkin Hartley guinea pigs (370 ± 20 g) were obtained from Chaudhary Charan Singh Agricultural University, Hissar, Haryana, India. All procedures were carried out in adherence to the guidelines of Institutional Animal Ethics Committee (IAEC) of Birla Institute of Technology and Science, Pilani, India (Protocol No. IAEC/RES/4/1, dated 13.08.08). The animals were kept for at least 1 week before the experiments at optimum temperature (23 ± 2°C) and humidity-controlled (50–60%) animal rooms under a 12:12 h light/dark cycle (light on 6.00–18.00 h) with free access to food and water ad libitum. Behavioral studies were carried out during the light phase (9.00 a.m.–2.00 p.m.). The animals were used only once for each experiment.
Drugs and chemicals
Paroxetine and bupropion were obtained as gift samples from Cipla Pharmaceuticals and IPCA Laboratories Private Limited, India, respectively. Pargyline was purchased from Sigma Chemicals, USA. The drugs for anesthesia, namely, ketamine and xylazine, were purchased from Reidel Neon Labs, Indian Immunologicals (Mumbai, India). The drugs were freshly prepared in distilled water and administered per oral (p.o.) or intra-peritoneally (i.p.) in a constant volume of 10 ml/kg. For interaction studies, the antidepressants/ligands and standards were administered i.p. at 45 and 30 min, respectively, before testing in FST and TST, as per the protocol adopted earlier in our laboratory. The drugs were administered p.o. once a day for 14 days in the chronic treatment schedule.
5-HT3 receptor antagonistic activity
The compounds were tested for their ability to inhibit the 5-HT3 receptor in isolated guinea pig ileum, and the pA2 values were determined against 2-methyl-5-hydroxytryptamine.[20] In order to assess the 5-HT3 receptor antagonistic activity, guinea pigs were sacrificed with ketamine (40 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.). The abdomen was cut open and a length of ileum excised at about 2 cm from the ileo-cecal junction, the longitudinal muscle-myenteric plexus (LMMP), 3–4 cm in length, was prepared and mounted as cited in the literature. The tissue was equilibrated for 30 min under a resting tension of 500 mg with constant aeration in a 40 ml organ bath containing Tyrode solution, maintained at 37°C.
Non-cumulative concentrations of 2-methyl-5-HT were added with a 15-min dosing cycle (to prevent desensitization) and left in contact with the tissue until the maximal contraction had developed. To study the antagonist effect of the test compounds on the response evoked by 2-methyl-5-HT, the compounds were added to the organ bath and left in contact with the tissue for at least 10 min prior to the addition of 2-methyl-5-HT. The contractions were recorded using a T-305 Force transducer coupled to a Student's physiograph (Bio Devices, Ambala, India). Antagonism was expressed in the form of pA2 values, which were graphically determined. The pA2 values of the test compound were compared with the standard antagonist, ondansetron.
Forced swim test
The FST was carried out as described elsewhere[9] with slight modifications.[16] Mice were dropped individually into a Plexiglas cylinder (height 30 cm, diameter 22.5 cm) filled with water to a depth of 15 cm and maintained at 23–25°C. After an initial vigorous activity (2 min), the mice acquire an immobile posture, making only those movements necessary to keep the head above the water. The duration of immobility was recorded during the last 4 min of the 6-min test. The mice were subjected to 15 min training under similar conditions, 24 h before the test.
Tail suspension test
Mice were individually suspended by the tail to a horizontal bar (distance from floor was 50 cm) using scotch tape (distance from tip of tail was approximately 1 cm). Typically, mice exhibited several escape-oriented behaviors interspersed with temporally increasing bouts of immobility.[17,21] The duration of immobility during the 6-min test session was recorded.
Spontaneous locomotor activity
The spontaneous locomotor activity (SLA) was assessed using an actophotometer.[9] The animals were individually placed in a square arena (30 cm × 30 cm) with walls painted black and fitted with photocells just above the floor level. The photocells were checked before the beginning of the experiment. After an initial 2 min familiarization period, the digital locomotor scores were recorded for the next 10 min in a dimly lit room. The arena was cleaned with dilute alcohol and dried between trials.
5-Hydroxytryptophan–induced head twitch response
The method mentioned elsewhere[22] was adopted with slight modifications. The vehicle/drug treated mice were injected with pargyline (75 mg/kg, i.p.) and 5-HTP (5 mg/kg, i.p.), 30 and 15 min prior to drug administration, respectively, and gently placed in separate, clear Plexiglas cages (12 × 12 × 10 cm). The total numbers of head twitches (characterized by abrupt lateral movements) were recorded for the next 15 min.
RIH in rats
Rats were gently hand-restrained and the glycerol-lubricated digital thermometer probe was inserted into the rectum. The rectal temperature of the rats treated with reserpine (1 mg/kg, i.p.) was recorded at 30, 60, 90, and 120 min after the drug administration. The differences in the rectal temperature between the baseline and 60th min values were tabulated. On the day preceding the experiment, the rectal temperatures of the rats were assessed in a similar manner in order to habituate the animals to the experimental procedures.[23]
Olfactory bulbectomy
Surgery
Bilateral olfactory bulbectomy (OBX) was carried out as described.[18] In brief, the rat was anesthetized with a combination of ketamine (75 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.). The head was fixed to a stereotaxic frame (Inco, Ambala, India) and the skull exposed by a midline incision. Burr holes (2 mm in diameter) were drilled 8 mm anterior to bregma and 2 mm on either side of the midline at a point corresponding to the posterior margin of the orbit of the eye. The dura was punctured and the olfactory bulbs were removed by suction. Hemostatic sponge was used to prevent excessive bleeding and fill the dead space. The scalp was then sutured and the wound was dabbed with antiseptic solution. Sham surgery was carried out in the same way (including the piercing of the dura mater) except that the olfactory bulbs were left intact. Sulprim (each milliliter containing 200 and 40 mg of sulfadiazine and trimethoprim, respectively) was administered (0.2 ml/300 g, intramuscular) once a day for the first 3 days for both OBX and sham operated rats. The animals were individually housed for the first 3 days following surgery and thereafter in groups of two (one sham and one OBX rat) until the end of the study. The OBX and sham rats were allowed 14 day rehabilitation period during which they were handled by the same experimenter. The surgery, rehabilitation, treatment, and behavioral screening of OBX/sham rats were carried out based on the customized schedule previously reported.[18]
Open field test (OFT) behavior
OBX and sham control rats were subjected to the OFT on 29th day after surgery. The open field exploration was conducted as described elsewhere[24] with substantial modifications. The apparatus consisted of a circular (90 cm diameter) arena with 75-cm-high aluminum walls and floor equally divided into 10 cm squares. A 60-W light bulb was positioned 90 cm above the base of the arena, which was the only source of illumination in the testing room. Each animal was individually placed in the center of the OFT apparatus and the parameters such as ambulation scores (number of squares crossed), number of rearing episodes (when the rat stands upright on its hind paws), and number of fecal pellets were noted for 5 min by a trained observer who was unaware of the specific treatments. After each test, the apparatus was sprayed with dilute alcohol and wiped thoroughly.
Statistical analysis
Data were expressed as mean ± SEM. The single treatment studies were analyzed using a one-way analysis of variance followed by a post-hoc Dunnett test. The interaction studies were analyzed using a two-way analysis of variance followed by a post-hoc Sidak test. P values <0.05 were considered statistically significant.
Results
Based on the pA2 and log P values, compound 6p was taken for extensive behavioral pharmacological testing from amongst the series of compounds.
In FST and TST, the acute treatment with 6p (1, 2, and 4 mg/kg, i.p.) significantly decreased the duration of immobility as compared to vehicle treatment [Figures 1 and 2]. The standard antidepressants, paroxetine (10 mg/kg) and bupropion (20 mg/kg), also significantly reduced the immobility duration in FST and TST, respectively, as compared to the control group. None of the tested doses (except 0.5 and 8 mg/kg, i.p.) influenced the locomotion of mice in actophotometer [Figure 3]. Depletion of brain serotonin induced by reserpine affects the central nervous system as demonstrated by RIH. Administration of reserpine (1 mg/kg, i.p.) elicited a pronounced decrease in core body temperature of rats. This effect was significantly (P < 0.05) reversed by 6p and paroxetine (10 mg/kg) treatments [Figure 4]. 5-HTP-induced head twitches were performed to confirm the involvement of serotonergic pathway. The combination of 5-HTP (5 mg/kg, i.p.) and pargyline (75 mg/kg, i.p.) induced the characteristic head twitch response. The 6p (2 mg/kg, i.p.) and paroxetine (10 mg/kg, i.p.) significantly potentiated the 5-HTP/pargyline induced head twitches, respectively [Figure 5].
Figure 1.
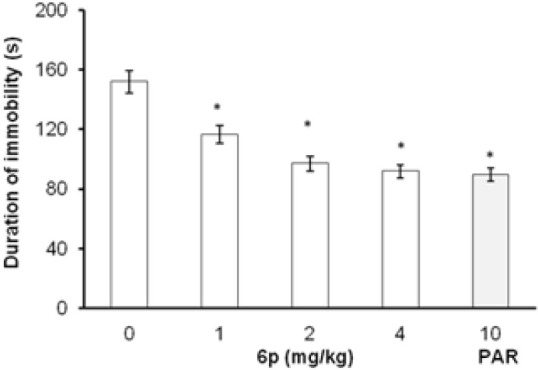
Effect of 6p on the duration of immobility of mice FST. The columns represent mean duration of immobility in seconds (s) and the error bars indicate SEM, n = 8 per group. *P < 0.05 compared with vehicle-treated group. PAR, paroxetine
Figure 2.
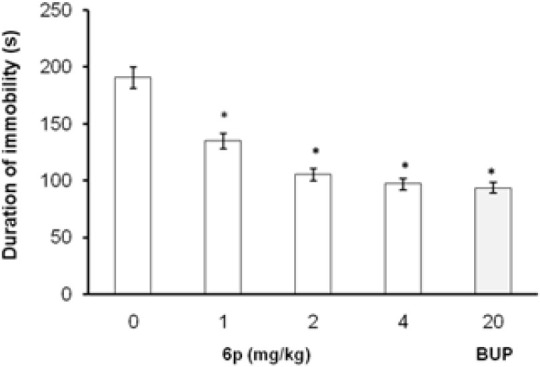
Effect of 6p on the duration of immobility of mice TST. The columns represent mean duration of immobility in seconds (s) and the error bars indicate SEM, n = 8 per group. *P < 0.05 compared with vehicle-treated group. BUP, bupropion
Figure 3.
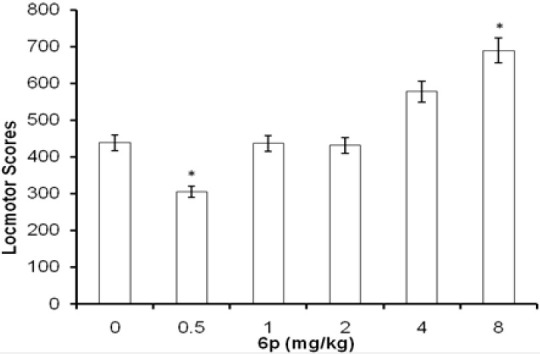
Effect of 6p treatment on spontaneous locomotor activity in mice. Each column represents mean locomotor scores recorded in 10 min observation period. The error bars indicate SEM, n = 8/group. *P < 0.05 compared with vehicle-treated group
Figure 4.
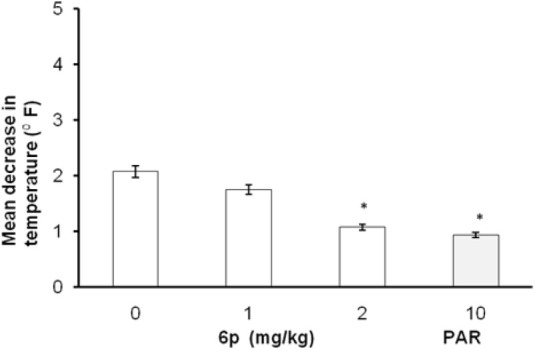
Effect of 6p on reserpine-induced hypothermia in rats. The columns represent mean decrease in temperature (p F) and the error bars indicate SEM, n = 8 per group. *P < 0.05 compared with vehicle-treated group. PAR, paroxetine
Figure 5.
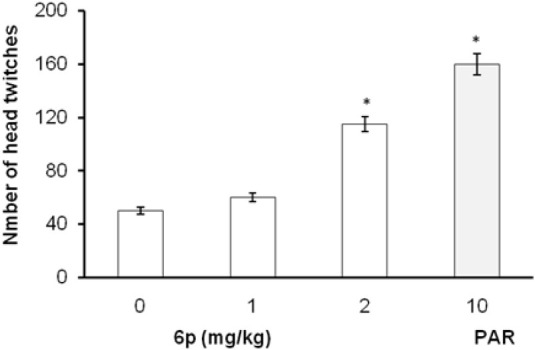
Effect of 6p and PAR on 5-HTP and pargyline-induced head twitch response in mice. The columns represent mean number of head twitches and the error bars indicate SEM, n = 8 per group. *P < 0.05 compared with vehicle-treated group. PAR, paroxetine
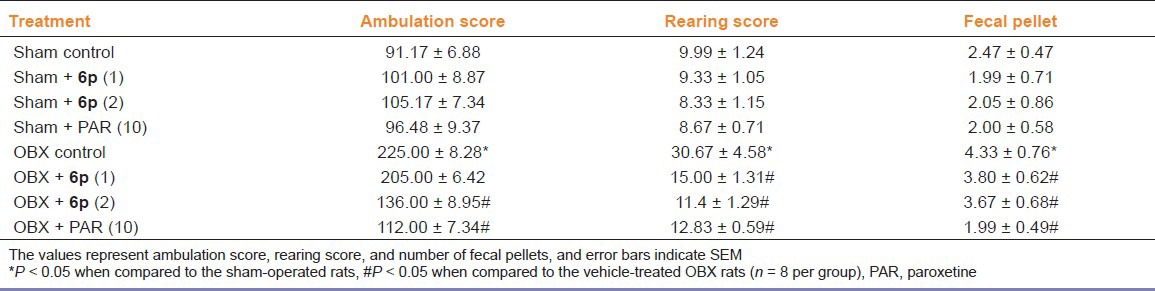
The effects of 6p on the behavior of OBX/sham rats were analyzed in modified open field paradigm [Table 2]. Removal of olfactory bulbs produced a characteristic hyperactivity behavioral pattern characterized by increased ambulation, rearing, and fecal pellets in OBX rats when compared to sham rats in modified OFT. The dose levels of 6p (1 and 2 mg/kg, p.o.) were selected based on the data obtained from FST and TST. Chronic treatment (14 days) with 6p significantly (P < 0.05) reduced the ambulation, rearing, and fecal pellets in OBX rats compared to the vehicle OBX rats. Paroxetine treatment (10 mg/kg, p.o.) also significantly reduced the number of ambulation, rearing, and fecal pellets, as compared to control group.
Table 2.
Effects of paroxetine (10 mg/kg, p.o.) and 6p (1 and 2 mg/kg, p.o.) open field behavior (ambulation/rearing/fecal pellet) in OBX/sham rats
Discussion
The present investigation shows the antidepressant-like effect of 6p in acute and chronic animal models of depression. In addition, 6p was able to potentiate antidepressant-like effect with standard antidepressants in FST.
Animal studies led to the consensus that 5-HT3 antagonists have antidepressant effects by blocking limbic hyperactivity response.[25] Since 5-HT3 receptors are expressed in brain areas implicated in mood and 5-HT3 antagonists are able to pass the blood–brain barrier,[25] they represent potential therapeutic candidates. In the present study, 6p, a selective 5HT3 receptor antagonist, revealed antidepressant-like effect. The psychomotor stimulation/sedation may increase/decrease the locomotor status (global motor activity) of mice in behavioral assays (FST and TST), when interpreting the depressant or antidepressant-like effect of a new chemical entity (NCE).
To eliminate the non-specific motor effects of 6p that could influence the activity in FST and TST, locomotor activity of mice was evaluated using actophotometer. Interestingly, 6p did not influence the basal locomotor scores at 1, 2, and 4 mg/kg, i.p. dose level, and hence, the above-mentioned doses were selected for FST and TST experiments.
FST behavior despair test is most frequently used as an authentic animal model of depression to screen antidepressants as well as to explore the underlying mechanism of action of antidepressants.[9] Acute treatment with 6p significantly decreases the duration of immobility in the FST, reflecting the antidepressant-like effects of this drug. Moreover, earlier investigations have suggested that 5-HT3 receptor is involved in the pathophysiology of depression.[26] The involvement of 5-HT3 receptors in anxiety is complemented by studies of 5-HT3A knockout mice, which revealed that 5-HT3A regulates depression- and anxiety-related behaviors.[26] Moreover, acute treatment with 6p significantly decreased the duration of immobility in TST, reflecting the antidepressant-like effects of the drug. The results from the rodent antidepressant assays indicate that the 5-HT3 receptor antagonists (i) decreased the duration of immobility in FST and TST and (ii) increased the swimming behavior in FST.[26]
In mechanistic models, reserpine, being a non-specific monoamine depleting agent, acts by blocking the monoamine transport into synaptic vesicle. The depletion of brain biogenic amines affects the central nervous system, which is characterized by hypothermia. The decrease in body temperature induced by reserpine was reported to be antagonized by antidepressants.[9] The 6p and paroxetine significantly prevented the hypothermic effect of reserpine exhibiting antidepressant effects in this sensitive model. Postsynaptic 5-HT3 receptor antagonism in serotonergic neurons can facilitate specific binding of 5-HT to other postsynaptic receptors such as 5-HT1B, 5-HT2A, and 5-HT2C, thereby aiding in serotonergic transmission, and antagonized the RIH.[25]
5-HTP, being the immediate precursor of 5-HT, significantly increases the serotonergic transmission, inducing a characteristic head twitch response in mice. The action is mediated via increase in availability of serotonin at 5-HT2A receptor. The antidepressant-like effect of 6p appears to be modulated by an increase in monoamines’ level, particularly 5-HT concentrations in the synapse.[22] In the current investigation, pargyline and 5-HTP–induced head twitch response were significantly increased by 6p and paroxetine treatment.
The rat olfactory bulbectomy model of chronic depression (with adequate face and predictive validity) has been validated as a model of depression over the past 20 years.[24] Olfactory bulbectomy has been proposed to be an agitated hypo-serotonergic model of depression and is used to explore the antidepressant potential of novel agents. OBX rats exhibited a specific, abnormal behavioral pattern in the OFT, characterized by increased ambulation, rearing, and fecal pellets, and this abnormal behavior is reversed by antidepressants.[18] The increased locomotor/exploratory behavior of OBX rat in OFT may be due to exposure to a novel environment.[24] Moreover, the other possible reason for hyperactivity of OBX rats in OFT may be decrease in activity in competing behavior or delay/failure to habituate to novel environment.[18] The 6p enhances the availability of serotonin at postsynaptic receptors. In this regard, potentiate antidepressant-like effect of 6p with conventional antidepressants may be through modulation of serotonergic signal transduction pathway.
Based on aforementioned findings, it is concluded that 6p, a 5-HT3 receptor antagonist, could be a potential candidate for the management of depression. Further studies are warranted to examine the possible mechanism(s) such as the post-receptor action and signal transduction.
Footnotes
Source of Support: Authors thank the University Grants Commission (UGC), India, Birla Institute of Technology and Science (BITS), Pilani, India, and SAIF, Panjab University, Chandigarh, India, for providing financial support, laboratory and analytical facilities, respectively
Conflict Interest: None
References
- 1.Peters JA, Lambert JJ. Electrophysiology of 5-HT3 receptors in neuronal cell lines. Trends Pharmacol Sci. 1989;10:172–5. doi: 10.1016/0165-6147(89)90230-7. [DOI] [PubMed] [Google Scholar]
- 2.Davies PA, Wang W, Hales TG, Kirkness EF. A novel class of ligandgated ion channel is activated by Zn2+ J Biol Chem. 2003;278:712–7. doi: 10.1074/jbc.M208814200. [DOI] [PubMed] [Google Scholar]
- 3.Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels, the impact of receptor structure on function. Biochem Soc Trans. 2004;32:529–34. doi: 10.1042/BST0320529. [DOI] [PubMed] [Google Scholar]
- 4.Richardson BP, Engel G, Donatsch P, Stadler PA. Identification of serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985;316:126–31. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- 5.Girish MB, Bhuvana K, Nageshraju G, Sarala N. A novel atypical antidepressant drug. Agomaeltine-A review. Int J Pharm Biomed Res. 2010;1:113–6. [Google Scholar]
- 6.Shelton RC. Classification of Antidepressants and Their Clinical Implications. Primary Care Companion. J Clin Psychol. 2003;5:27–32. [Google Scholar]
- 7.Ayuso-Gutierrez JL. Old and New Antidepressants. Where are we? World J Biol Psychol. 2002;3:112–4. doi: 10.3109/15622970209150611. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar R, Mahesh R, Minasri B. Antidepressant-like effects of serotonin type-3 antagonist, ondansetron. an investigation in behavior-based rodent models. Behav Pharmacol. 2008;19:29–40. doi: 10.1097/FBP.0b013e3282f3cfd4. [DOI] [PubMed] [Google Scholar]
- 9.Devadoss T, Pandey DK, Mahesh R, Yadav SK. Effect of acute and chronic treatment with QCF-3 (4-benzylpiperazin-1-yl)(quinoxalin-2-yl)methanone, a novel 5-HT 3 receptor antagonist, in animal models of depression. Pharmacol Rep. 2010;62:245–57. doi: 10.1016/s1734-1140(10)70263-2. [DOI] [PubMed] [Google Scholar]
- 10.Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT 3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/s0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]
- 11.Haus U, Varga B, Stratz T, Spath M, Muller W. Oral treatment of fibromyalgia with tropisetron given over 28 days. influence on functional and vegetative symptoms, psychometric parameters and pain. Scand J Rheumatol Suppl. 2000;113:55–8. doi: 10.1080/030097400446652. [DOI] [PubMed] [Google Scholar]
- 12.Faris PL, Eckert ED, Kim SW. Evidence for a vagal pathophysiology for bulimia nervosa and the accompanying depressive symptoms. J Affect Disord. 2006;92:79–90. doi: 10.1016/j.jad.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 13.Hibert MF, Hoffmann R, Miller RC, Carr AA. Conformation-activity relationship study of 5-HT3 receptor antagonists and a definition of a model for this receptor site. J Med Chem. 1990;33:1594–600. doi: 10.1021/jm00168a011. [DOI] [PubMed] [Google Scholar]
- 14.Bourin M. Is it possible to predict the activity of a new antidepressant in animals with simple psychopharmacological tests? Fund Clin Pharmacol. 1990;4:49–64. doi: 10.1111/j.1472-8206.1990.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 15.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behav Pharmacol. 1997;8:523–32. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 17.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test. a new method for screening antidepressant drugs. Psychopharmacology. 1985;85:367–70. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 18.Kelly JP, Wyrnn AS, Leonard BE. The olfactory bulbectomized rat as a model of depression. an update. Pharmacol Ther. 1997;74:299–316. doi: 10.1016/s0163-7258(97)00004-1. [DOI] [PubMed] [Google Scholar]
- 19.Paton WD, Zar AM. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968;194:13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacKay D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated? J Pharm Pharmacol. 1978;30:312–3. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- 21.Bourin M, Chenu F, Ripoll N, David DJ. A proposal of decision tree to screen putative antidepressants using forced swim and tail suspension tests. Behav Brain Res. 2005;164:266–9. doi: 10.1016/j.bbr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Martin P, Massol J, Soubrie P, Puech AJ. Effects of triiodothyronine (T 3) on the potentiation by antidepressants of L-5-hydroxytryptophan-induced head-twitches in mice. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13:735–48. doi: 10.1016/0278-5846(89)90061-4. [DOI] [PubMed] [Google Scholar]
- 23.Englert LF, Ho BT, Taylor D. The effects of (-)-delta9-tetrahydrocannabinol on reserpine- induced hypothermia in rats. Br J Pharmacol. 1973;49:243–52. doi: 10.1111/j.1476-5381.1973.tb08369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoomissen JV, Kunrath J, Dentlinger R, Lafrenz A, Krause M, Azar A. Cognitive and locomotor/exploratory behavior after chronic exercise in the olfactory bulbectomy animal model of depression. Behav Brain Res. 2011;222:106–16. doi: 10.1016/j.bbr.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression. A probable neuronal target? J Psychopharmacol. 2010;24:455–69. doi: 10.1177/0269881109348161. [DOI] [PubMed] [Google Scholar]
- 26.Kelley SP, Bratt AM, Hodge CW. Targeted gene deletion of the 5-HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol. 2003;461:19–25. doi: 10.1016/s0014-2999(02)02960-6. [DOI] [PubMed] [Google Scholar]


