Abstract
Objectives:
This study aimed to investigate the protective effect of simvastatin (SIM) and rosuvastatin (RST) on cisplatin (CIS)-induced nephrotoxicity.
Materials and Methods:
Adult female Wistar rats were divided into six groups: Control group (Group 1) received 0.5% sodium carboxy methyl cellulose, group 2 and group 3 received SIM and RST for 10 days, respectively, and group 4 was injected single dose of CIS (7 mg/kg, i.p.). Group 5 and 6 were treated with SIM (10 mg/kg, p.o.) and RST (10 mg/kg, p.o.) for 10 days, respectively. All groups received cisplatin on the 5th day of treatment. Renal function tests like serum creatinine, urea, BUN, albumin, calcium, uric acid and magnesium, serum lipids, and markers of oxidative stress such as renal malondialdehyde (MDA) level and superoxide dismutase (SOD) and catalase (CAT) activities were measured. All tissues were investigated for histopathological changes.
Result:
CIS reduced the renal function, which was reflected with significant increase in serum urea, BUN, serum creatinine, uric acid and also significant decrease serum calcium, magnesium, albumin levels. In addition, cisplatin caused renal tubular damage with a higher MDA level, depletion of SOD and CAT activity, and elevation of serum lipids. SIM or RST ameliorate CIS induced renal damage due to improvement in renal function, oxidative stress, suppression of serum lipids, and histological alteration.
Conclusions:
This finding suggests that simvastatin and rosuvastatin may have a protective effect against cisplatin-induced kidney damage via amelioration of lipid peroxidation as well as due to improvement of renal function, and lipid-lowering effects.
KEY WORDS: Cisplatin, nephrotoxicity, rosuvastatin, simvastatin
Introduction
Many studies have assessed the effect of various substances to neutralize the nephrotoxic action of cisplatin. Statins are widely used clinically for lowering hypercholesterolemia because of their inhibitory effect on 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the rate-limiting step of the cholesterol synthesis in the liver and other tissues.[1] Statins have beneficial changes in other lipid fractions, along with significant effects on numerous measures of inflammation, immunity, oxidative stress, thrombosis, and vascular and renal function.[2,3,4,5,6,7,8] Pleiotropic effects of statins have also been implicated in reductions in heart failure.[9] Cis-dichlorodiammine platinum (II) or cisplatin (CIS) is currently among the most widely used agents in the chemotherapy of cancer.[10] Limitation to its greater efficacy is due to its nephrotoxicity. Acute and chronic nephrotoxicity of CIS occur in man and animals especially after repeated administration.[11] The pathogenesis of renal damage caused by CIS is due to an increase in lipid peroxide levels and causes generation of reactive oxygen metabolites (ROM) and inhibits the activity of antioxidant enzymes in renal tissue.[12,13] The present study was undertaken to investigate the protective effect of simvastatin (SIM) and rosuvastatin (RST) in CIS-induced nephrotoxicity through pleiotropic effect.
Materials and Methods
Drugs and Chemicals
Rosuvastatin and simvastatin were obtained from Zydus Cadila, Ahmedabad, India. Cisplatin injection was obtained from VHB limited, Mumbai, India. All other chemicals and reagents used in the study were of analytical grade.
Experimental Animals
The experimental protocol was approved by the Institutional Animal Ethics Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The experiment was carried out on healthy adult female Wistar rats weighing 200-250 g. Rats were housed in polypropylene cages, maintained under standardized condition (12-h light/dark cycle, 24°C, 35 to 60% humidity) and allowed free access to diet (Nav Maharashtra Oil Mills Pvt. Ltd., Pune) and purified drinking water ad libitium.
Induction of cisplatin-induced kidney damage
The rats were divided into six groups of six animals each. Group 1 animal received 0.5% sodium CMC orally for 10 days. Group 2 and 3 received SIM (10 mg/kg, p.o.) and RST (10 mg/kg, p.o.) for 10 days, respectively. Group 4 was injected single dose of CIS (7 mg/kg, i.p.). Group 5 and 6 were treated with SIM (10 mg/kg, p.o) and RST (10 mg/kg, p.o) for 10 days, respectively. All groups received cisplatin on the 5th day of treatment.[14]
At the end of treatment on 10th day, blood samples were withdrawn from retro-orbital plexus under light ether anesthesia without any anti-coagulant and allowed for 10 minutes to clot at room temperature. It was centrifuged at 2500 rpm for 20 minutes for serum separation. The serum obtained was kept at 4°C until used. Studies have been carried out to determine kidney function markers such as urea, BUN, creatinine, albumin, calcium and magnesium and uric acid and serum lipid levels like total cholesterol, LDL-cholesterol and triglycerides were estimated from serum sample using standard diagnostic kit (SPAN Diagnostics and Crest Biosystems, India).
Estimation of biomarkers of Oxidative stress
Kidney was removed and kept in cold conditions (precooked in inverted Petridis on ice). The tissues were cross chopped with surgical scalpel into fine slices in chilled 0.25 M sucrose, quickly blotted on a filter paper. The tissues were minced and homogenized in 10 mM Tris-HCl buffer, pH 7.4 (10 %w/v) with 25 strokes of tight Teflon pestle of glass homogenizer at a speed of 2500 rpm. The clear supernatant was used for oxidative stress markers assays. The levels of malondialdehyde (MDA) formation and the activities of endogenous antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) were estimated by the method of Slater and Sawyer[15] Hugo Aebi as given by Hugo[16] and Mishra and Fridovich,[17] respectively.
Histopathology
For light microscopic evaluation, kidney tissues of each group were fixed in 10% phosphate buffered formalin. Paraffin-embedded specimens were cut into 6 mm-thick sections and stained with hematoxylin and eosin (H&E). The kidney tissues were examined under a light microscope (Olympus Bioxl) for the presence of tubular changes by an observer blinded to the animal treatment group.
Statistical Analysis
All of the data are expressed as mean ± SEM. Statistical significance between more than two groups was tested using one-way ANOVA followed by the Bonferroni multiple comparisons test. Calculations were done using Graphpad Prism software. The significance level was set at P < 0.05 for all tests.
Results
Effects of statins on CIS-induced kidney dysfunction
Administration of single injection of CIS (7 mg/kg, i.p.) caused a marked reduction in renal function, as characterized by significant (P < 0.001) increase in serum urea, BUN, creatinine, uric acid and a significant (P < 0.001) decrease serum calcium, magnesium, albumin levels when compared to control group [Table 1]. Thus, these data indicate that a single intraperitoneally injection of 7 mg/kg CIS impairs kidney functions.
Table 1.
Effect of simvastatin and rosuvastatin treatment (10 mg/kg/day p.o. for 10 days) on kidney function tests of control and cisplatin-injected rats
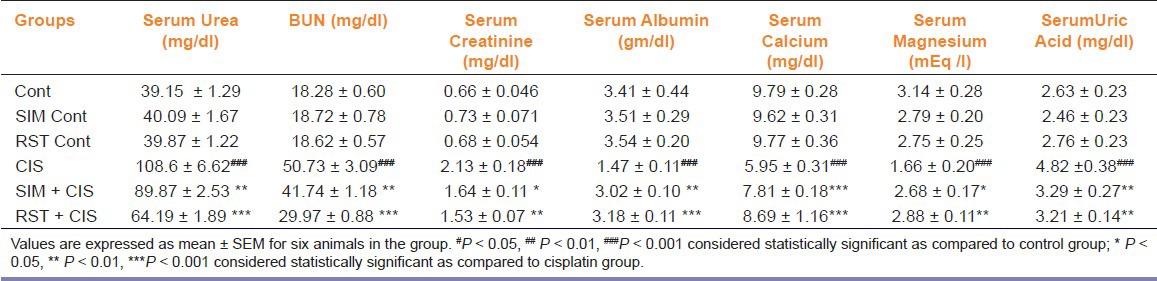
The treatment with SIM showed significant decrease in BUN (P < 0.01), urea (P < 0.01), and creatinine (P < 0.05) and a significant increase in albumin (P < 0.01) as compared to CIS-treated group. The treatment with RST showed highly significant decrease in BUN (P < 0.001), urea (P < 0.001), creatinine (P < 0.01) and significant increase in albumin (P < 0.001) as compared to CIS-treated group.
Treatment with SIM showed a significant increase in calcium (P < 0.001) as well as magnesium (P < 0.05) level and a significant decrease in uric acid (P < 0.01) level as compared to CIS-treated group, and the treatment with RST showed a significant increase in calcium (P < 0.001) as well as magnesium (P < 0.01) level and a significant decrease in uric acid (P < 0.01) level as compared to CIS-treated group.
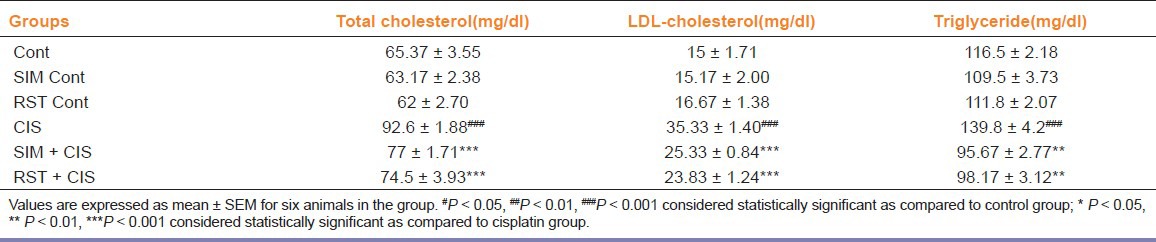
CIS treatment significantly increased serum total cholesterol, LDL-cholesterol, and TG levels compared with control group (P < 0.001). RST or SIM alone did not affect serum lipids levels, but co-administration of SIM and RST in cisplatin-treated rats significantly suppressed serum total cholesterol (P < 0.001), LDL-cholesterol (P < 0.001), and TG (P < 0.01) levels as compared to CIS-treated alone [Table 2].
Table 2.
Effect of simvastatin and rosuvastatin treatment (10 mg/kg/day p.o. for 10 days) on serum levels of lipid on control and cisplatininjected rats
Effect statins on markers of oxidative stress
Oxidative stress increases in CIS-treated group as compared to control group. The treatment with SIM and RST showed a significantly reduced oxidative stress level.
The treatment with SIM showed a significant increase in SOD (P < 0.001), CAT (P < 0.001) activities and a significant decrease in MDA (P < 0.01) levels as compared to CIS-treated group [Figure 1]. The treatment with RST showed a significant increase in SOD (P < 0.01), CAT (P < 0.001) activities and significant decrease in MDA (P < 0.001) level as compared to CIS-treated group [Figure 1].
Figure 1.
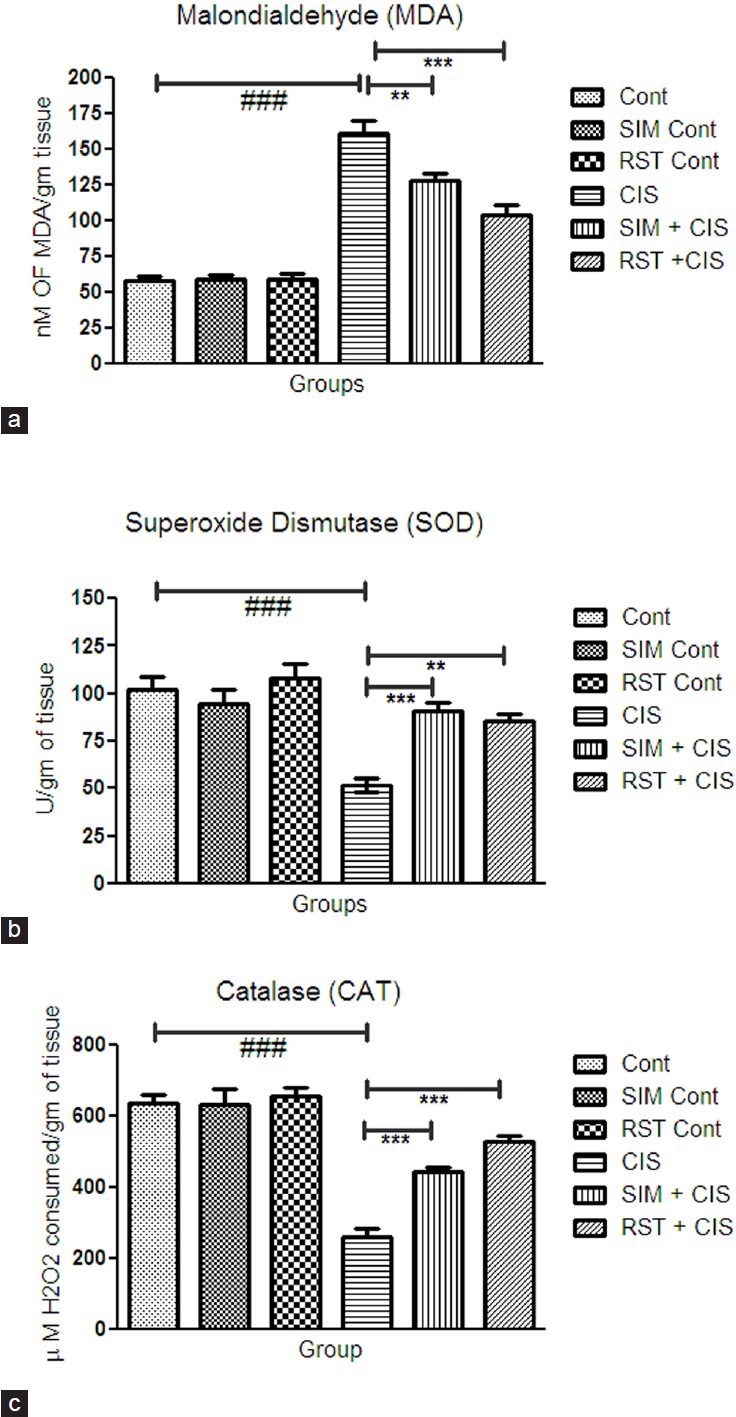
Effect of simvastatin and rosuvastatin on biomarkers of oxidative stress: (a) MDA, (b) SOD, and (c) CAT activity, #P< 0.05, ##P< 0.01, ###P< 0.001 vs. control; *P< 0.05, **P< 0.01, ***P< 0.001 vs. cisplatin
Histopathological changes
The architecture of the kidney was disturbed with CIS administration as compared to normal structural features of control animal. CIS administration caused a severe tubular necrosis, vaculation, and tubular dilation. SIM and RST treatment showed amelioration of tubular damage induced by CIS while SIM and RST alone did not have any effect on morphology of kidney [Figure 2]. These results indicated that treatment with SIM and RST afforded protection against CIS-induced nephrotoxicity.
Figure 2.
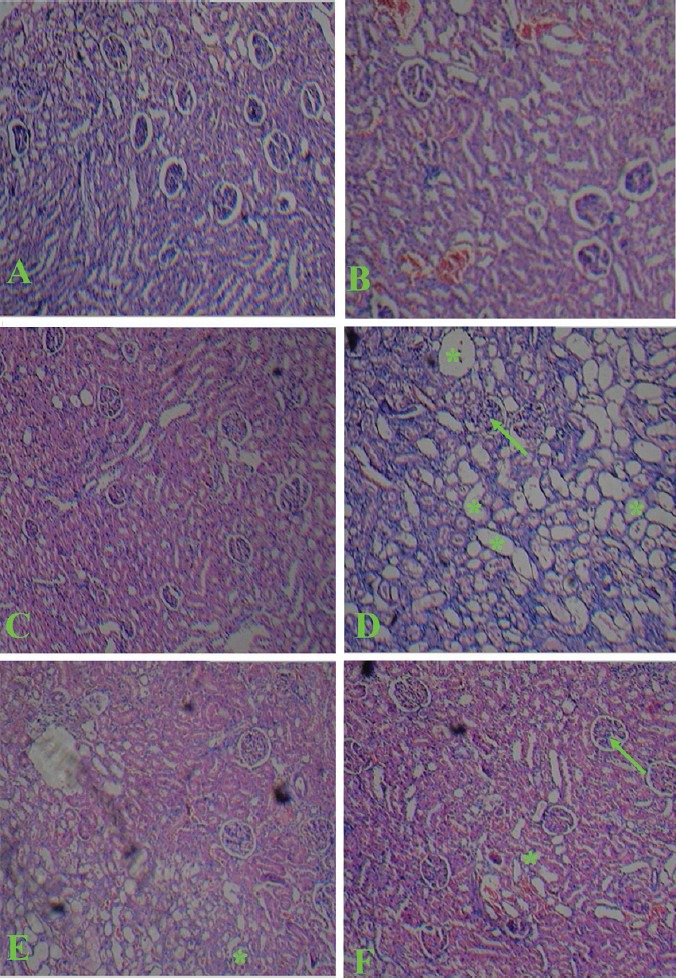
Light microscopy of kidney tissues from rats (HE stained kidney sections). (A) Control group, (B) Simvastatin group, (C) Rosuvastatin, (D) Cisplatin. Shows severe glomerular degeneration (arrow) and degeneration in tubular cells (asterisks), (E) Cisplatin + Simvastatin, (F) Cisplatin + Rosuvastatin
Discussion
The results demonstrate that daily SIM and RST treatment markedly ameliorate CIS-induced kidney damage as shown in microscopic examination and biochemical parameters. CIS is one of the widely used cytotoxic agents in the treatment of several forms of cancers. In spite of its clinical usefulness, there are constraints in using this drug as it causes nephrotoxicity and neurotoxicity. Other less frequent toxic effects like hepatotoxicity, which are generally observed after administration of high doses of CIS, can also alter the clinical situation in patients.[18,19,20,21] The mechanism of nephrotoxicity due to cisplatin might be due to the release of free radicals.[22] In the present study, CIS injection produced severe degeneration in glomeruli, proximal, and distal tubules. Tubular swelling and necrosis are in line with earlier studies, which show similar findings.[23,24] The renal function tests such as BUN, serum urea, serum creatinine, and serum uric acid were elevated and serum albumin, calcium, and magnesium levels were lowered in CIS-injected animals as compared to control group. It is probable that increased reactive oxygen species (ROS) production in the renal tissue may be responsible for this damage of the organ as reflected by the change in the levels of MDA and activities of SOD and catalase in the study. Another player in the induction of these changes is the depletion of thiol groups in kidney. Both these effects, in concert, led to the development of CIS-induced nephrotoxicity. DNA fragmentation, tubular necrosis as well as tubular apoptosis have also been implicated in nephrotoxicity induced by CIS.[25] It is evident from prior clinical data that CIS administration results in elevation in serum urea and creatinine levels as a result of nephrotoxicity.[26,27] Statins represent a class of anti-hyperlipidemic drugs that have a lot of pleiotropic effects. In the present study, pre-treatment with RST and SIM was able to counteract CIS-mediated renal damage. Treatment with these statins improved functional nephrotoxicity indices. Several studies have shown a renoprotective effect of statins in different animal models.[28] Renal tissue lesions induced by CIS were significantly improved by SIM and RST. This is evident from histopathological findings and by an improvement in serum creatinine, urea, and BUN values. Since ROS generation mediates CIS nephrotoxicity, it may be presumed that antioxidant effect of statins may be protecting these conditions. SIM, in particular, reverses oxidant-antioxidant imbalance and has good hydroxyl radical scavenging activity.[29] RST also shows a dose-dependent antioxidant effect. These effects are primarily mediated by up-regulation of antioxidant defense protein heme oxygenase-1(HO-1).[30] The antioxidant effect of SIM and RST plays a significant role in amelioration of CIS-induced renal damage, but the lipid-lowering effect of statins may also be involved in this mechanism. RST and SIM treatment decreased serum cholesterol, LDL-cholesterol, and TG level in CIS-treated rats as compared to CIS treated alone. Therefore, it is possible that lipid accumulation on the renal tubule might be prevented, thereby showing renoprotective effects. The major renoprotective effect of both, RST and SIM is, therefore, mediated by normalization of ROS production.
Conclusion
These results indicate that RST and SIM improve biochemical and histological alterations induced by CIS. However, RST shows more significant renoprotective effect than SIM. These renoprotective effects are beyond the lipid-lowering effects of statins. Mechanisms of this renoprotective effect mainly include amelioration of lipid peroxidation induced by CIS as well as activation of defense mechanisms.
Acknowledgement
We acknowledge our Head of the Department Dr. A. K. Seth for his co-operation and encouragement for publishing this research paper.
Footnotes
Source of Support: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Conflict Interest: No
References
- 1.Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 1995;31:9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- 2.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109:39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 3.Nissen SE. High-dose statins in acute coronary syndromes: Not just lipid levels. JAMA. 2004;292:1365–7. doi: 10.1001/jama.292.11.1365. [DOI] [PubMed] [Google Scholar]
- 4.Ray KK, Cannon CP. The potential relevance of the multiple lipid independent (pleiotropic) effects of statins in the management of acute coronary syndromes. J Am Coll Cardiol. 2005;46:1425–33. doi: 10.1016/j.jacc.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 5.Douglas K, O’Malley PG, Jackson JL. Meta-analysis: The effect of statins on albuminuria. Ann Intern Med. 2006;145:117–24. doi: 10.7326/0003-4819-145-2-200607180-00009. [DOI] [PubMed] [Google Scholar]
- 6.Balk EM, Karas RH, Jordan HS, Kupelnick B, Chew P, Lau J. Effects of statins on vascular structure and function: a systematic review. Am J Med. 2004;117:775–90. doi: 10.1016/j.amjmed.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 7.Rosenson RS, Tangney CC. Antiatherothrombotic properties of statins: Implications for cardiovascular event reduction. JAMA. 1998;279:1643–50. doi: 10.1001/jama.279.20.1643. [DOI] [PubMed] [Google Scholar]
- 8.Mason RP, Walter MF, Jacob RF. Effects of HMG-CoA reductase inhibitors on endothelial function: role of microdomains and oxidative stress. Circulation. 2004;109:II34–41. doi: 10.1161/01.CIR.0000129503.62747.03. [DOI] [PubMed] [Google Scholar]
- 9.Khush KK, Waters DD, Bittner V, Deedwania PC, Kastelein JJ, Lewis SJ, et al. Effect of high-dose atorvastatin on hospitalizations for heart failure. Subgroup analysis of the Treating to New Targets (TNT) study. Circulation. 2007;115:576–83. doi: 10.1161/CIRCULATIONAHA.106.625574. [DOI] [PubMed] [Google Scholar]
- 10.Einhorn LH. Curing metastatic testicular cancer. Proc Natl Acad Sci U S A. 2002;99:4592–5. doi: 10.1073/pnas.072067999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshida M, Iizuka K, Hara M, Nishijima H, Shimada A, Nakada K, et al. Prevention of nephrotoxicity of cisplatin by repeated oral administration of ebselen in rats. Tohoku J Exp Med. 2000;191:209–20. doi: 10.1620/tjem.191.209. [DOI] [PubMed] [Google Scholar]
- 12.Kim YK, Jung JS, Lee SH, Kim YW. Effects of antioxidants and Ca2+ in cisplatin-induced cell injury in rabbit renal cortical slices. Toxicol Appl Pharmacol. 1997;146:261–9. doi: 10.1006/taap.1997.8252. [DOI] [PubMed] [Google Scholar]
- 13.Mora Lde O, Antunes LM, Francescato HD, Bianchi Mde L. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2003;47:517–22. doi: 10.1016/s1043-6618(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 14.Osman AM, El-Sayed EM, El-Demerdash E, Al-Hyder A, El-Didi M, Attia AS, et al. Prevention of cisplatin-induced nephrotoxicity by methimazole. Pharmacol Res. 2000;41:115–21. doi: 10.1006/phrs.1999.0569. [DOI] [PubMed] [Google Scholar]
- 15.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride and other halogeno alkanes or peroxidative reactions in rat liver fractions in vitro. Biochem J. 1971;123:805–14. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hugo EB. Oxidoreductases acting on groups other than CHOH: catalase. In: Colowick SP, Kaplan NO, Packer L, editors. Methods in Enzymology. Vol. 105. London: Academic Press; 1984. pp. 121–5. [Google Scholar]
- 17.Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 18.Hesketh MA, Twaddell T, Finn A. A possible role for cisplatin (DDP) in the transient hepatic enzyme elevation noted after ondansetron administration. Proc Am Assoc Clin Oncol. 1990;9:323. [Google Scholar]
- 19.Vermorken JB, Pinedo HM. Gastrointestinal toxicity of cisdiamminedichloroplatinum (II) Neth J Med. 1982;25:270–4. [PubMed] [Google Scholar]
- 20.Cavalli F, Tschopp L, Sonntag RW, Zimmermann A. Cisplatin-induced hepatic toxicity. Cancer Treat Rep. 1978;62:2125–6. [PubMed] [Google Scholar]
- 21.Koc A, Duru M, Ciralik H, Akcan R, Sogut S. Protective agent, erdosteine, against cisplatin-induced hepatic oxidant injury in rats. Mol Cell Biochem. 2005;278:79–84. doi: 10.1007/s11010-005-6630-z. [DOI] [PubMed] [Google Scholar]
- 22.Ali BH, Al Moundhri MS. Agents ameliorating or augmenting the nephrotoxicity of cisplatin and other platinum compounds: a review of some recent research. Food Chem Toxicol. 2006;44:1173–83. doi: 10.1016/j.fct.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Fujieda M, Morita T, Naruse K, Hayashi Y, Ishihara M, Yokoyama T, et al. Effect of pravastatin on cisplatin-induced nephrotoxicity in rats. Hum Exp Toxicol. 2011;30:603–15. doi: 10.1177/0960327110376551. [DOI] [PubMed] [Google Scholar]
- 24.Teixeira RB, Kelley J, Alpert H, Pardo V, Vaamonde CA. Complete protection from gentamicin-induced acute renal failure in the diabetes mellitus. Kidney Int. 1982;21:600–12. doi: 10.1038/ki.1982.67. [DOI] [PubMed] [Google Scholar]
- 25.Victor MG, Miguel AF, Carlos A, Jose MP. Is cisplatin-induced cell death always produced by apoptosis? Mol Pharmacol. 2001;59:657–63. doi: 10.1124/mol.59.4.657. [DOI] [PubMed] [Google Scholar]
- 26.Santoso JT, Lucci JA, 3rd, Coleman RL, Schafer I, Hannigan EV. Saline, mannitol, and furosemide hydration in acute cisplatin nephrotoxicity: A randomized trial. Cancer Chemother Pharmacol. 2003;52:13–8. doi: 10.1007/s00280-003-0620-1. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi T, Nazneen A, Abid MR, Razzaque MS. Cisplatin-associated nephrotoxicity and pathological events. Contrib Nephrol. 2005;148:107–21. doi: 10.1159/000086055. [DOI] [PubMed] [Google Scholar]
- 28.Gianella A, Nobili E, Abbate M, Zoja C, Gelosa P, Mussoni L, et al. Rosuvastatin treatment prevents progressive kidney-inflammation and fibrosis in stroke-prone rats. Am J Pathol. 2007;170:1165–77. doi: 10.2353/ajpath.2007.060882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franzoni F, Quiñones-Galvan A, Regoli F, Ferrannini E, Galetta F. A comparative study of the in vitro antioxidant activity of statins. Int J Cardiol. 2003;90:317–21. doi: 10.1016/s0167-5273(02)00577-6. [DOI] [PubMed] [Google Scholar]
- 30.Grosser N, Erdmann K, Hemmerle A, Berndt G, Hinkelmann U, Smith G, et al. Rosuvastatin upregulates the antioxidant defense protein heme oxygenase-1. Biochem Biophys Res Commun. 2004;325:871–6. doi: 10.1016/j.bbrc.2004.10.123. [DOI] [PubMed] [Google Scholar]


