Abstract
Objective:
To study analgesic activity and to evaluate the involvement of opioid and monoamines in the antinociceptive activity of methanol extract of leaves of Aegle marmelos.
Materials and Methods:
Analgesic activity of methanol extract (ME) of A. marmelos alone (75,150 and 300mg/kg orally) and in combination with morphine or venlafaxine (subanalgesic) were studied using tail flick test and acetic acid-induced writhing in mice. The effect of pre-treatment with opioid antagonist naltrexone 1mg/kg was also studied on antinociception induced due to ME.
Result:
ME produced a dose-dependent significant antinociceptive activity in the tail flick test and acetic acid-induced writhing in mice. (P<0.05) Administration of subanalgesic dose of ME with morphine or venlafaxine also resulted in significant (P<0.05) antinociceptive activity in both the pain models. Pre-treatment with naltrexone inhibited analgesic activity induced by ME alone and combination with morphine or venlafaxine.
Conclusion:
A.marmelos in induced antinociception is mediated through both opioid and monoaminergic pain pathways, suggest its possible use in chronic pain.
KEY WORDS: Aegle marmelos, analgesia, monoamines, opioid, tail flick latency
Introduction
Pain is a warning signal and primarily protective in nature that causes discomfort with a large subjective component.[1] Currently available analgesic drugs such as opiates and NSAIDs are known to cause adverse effects. Opiate analgesic such as morphine has strong addictive potential and other side effects including respiratory depression, drowsiness, decreased gastrointestinal motility, nausea and several alterations of endocrine and autonomic nervous system[2] whereas NSAIDs are well known for their ability to produce gastrointestinal bleeding, ulceration etc.[3] Therefore, need to develop new better drugs for relief of chronic pain. A growing body of evidence suggests that in neuropathic and chronic pain antidepressants are found effective due to inhibition of nor adrenaline (NA) and 5 hydroxy tryptamine (5HT) uptake.[4,5] There is ample evidence to suggest that descending pain inhibiting pathway involves monoamines such as NA and 5HT.[6] Plants present promise of cure as they have been the raw material for the synthesis of drugs and as an important source of new therapeutic agents. Aegle marmelos is highly reputed medicinal plant possess antidiarrheal,[7] antihyperglycemic,[8] anticancer,[9] radioprotective,[10] antidepressant[11] and analgesic activities.[12,13] Till date there are no reports available regarding involvement of opiate and monoamine system in A. marmelos induced antinociception therefore, present study is undertaken to confirm its antinociceptive activity and to explorer influence of opioid and descending pain inhibiting pathways involving monoamines for this effect.
Materials and Methods
Plant Materials
The leaves of A. marmelos were collected from their natural habitat from Gwalior city, Madhya Pradesh, India in the month of April 2008. The plant leaves were identified by Dr. K.K.Koul Professor and Head, Department of the Botany, Jiwaji University, Gwalior, India and a voucher specimen (skam/08) has been retained in our laboratory for further reference.
Preparation of Extract
The shade dried leaves were powdered using a mechanical grinder and passed through 40-mesh sieve. Powder (300 g) was successively extracted with1.5 L of petroleum ether, chloroform and methanol, in a soxhlet apparatus at 60-70°C each for 10-12 h consecutively. Solvents used were of analytical grade. Methanol was removed from the extract under vaccum and a semisolid mass was obtained. The yield of methanol extract (ME) was 11.50% (w/w). It was stored in sterile amber colored storage vials in refrigerator until used for experimentation.
Selection of Animals
Albino mice weighing 20-25 g of either sex were used for the study. They were maintained under standard laboratory condition at 24 ± 2°C and relative humidity 50 ± 5% on 12h-day/night cycle with free access to food (Pranav Agro Industries, Delhi, INDIA) and water. The animals were acclimatized to the laboratory conditions prior to experimentation. All the experiments were carried out between 10.00 h and 16.00 h at ambient temperature. The animals were drawn at random for test and control groups. The study was approved by Institutional Animal Ethics Committee.
Analgesic Activity
Tail-flick Test
Mice (20–25g) were fasted overnight before the study. A tail-flick analgesiometer (TECHNO) was used to measure response latencies as described by Armour and Smith.[14] The distal part of the tail except 1 mm was placed over a heated nichrome wire and the time taken by the mice to withdraw the tail was recorded 1 h prior to treatment in triplicate at 15 min interval. The mean of the three readings was recorded as pre-drug latency time. The heat intensity was adjusted such that the average withdrawn latency was 1.5-4 s and a maximum cut-off time of 10 s was adopted to prevent undue tissue damage. Responses were measured at time 0, 30, 60, 90, and 120 min after treatment of the animals with vehicle and drugs. The differences between tail flick latencies (TFL) before and after drug administration among different groups were compared for statistical analysis. Tail flick responses were observed in three sets of experiments using analgesiometer.
Mice were divided into five groups of six animals each. Group I received gum acacia (GA) at the dose of 10ml/kg p.o. served as negative control, Group II received morphine sulfate 1mg/kg p.o. served as positive control while Group III, IV and V were treated with ME of A. marmelos 75,150 and 300 mg/kg p.o. respectively.
To study the effect of combination treatments mice were divided into eight groups of six animals, each. Group I received gum acacia at the dose of 10ml/kg p.o. served as negative control, Group II and Group III were treated with morphine sulfate 1mg/kg p.o. and venlafaxine 25mg/kg p. o. respectively, served as standard control. Group IV, V and VI received ME of A. marmelos 75mg/kg p.o., morphine sulfate0.25mg/kg p.o.,venlafexine 7.5mg/kg p.o. respectively served as a subeffectve dose groups, GroupVII and VIII received combination of ME of A. marmelos 75/kg p.o.either with morphine 0.25 mg/kg p.o. or venlafexine 7.5mg/kg p.o.
To study the effect of naltrexone pre-treatment mice were divided into six groups of six animals, each. Group I received gum acacia at the dose of 10ml/kg p.o. served as negative control, Group II were treated with naltrexone 1mg/kg p.o. alone, Group III and IV, received naltrexone 1 mg/kg p.o.30 min prior to ME of A. marmelos 300 mg/kg p.o. and venlafexine 25 mg/kg p.o. respectively whereas GroupV and VI received naltrexone 30 min prior to combination of subeffective dose of ME of A. marmelos with either morphine or venlafexine.
Mouse Writhing Assay
The acetic acid-induced abdominal writhing test was performed by intraperitoneal injection of acetic acid (10 ml/kg, 1% v/v in normal saline) according to the procedure described.[15] Mice received treatments 30 min before intraperitoneal injection of acetic acid and observed for constriction of the abdominal muscles together with stretching of the hind limbs. The writhes were cumulatively counted for 30 min following acetic acid injection. A significant reduction in the number of acetic acid-induced abdominal constrictions of the treated mice, compared to that of control group of mice, was taken as an indication of analgesic effect.
The mouse writhing assay was performed in two sets of experiments:
To study the effect of different doses of ME treatments groups were similar to TFL except group II received diclofenac sodium 5mg/kg p.o. and served as positive control.
To study the effect of combination treatment groups were similar to TFL.
Drugs
Morphine sulfate was procured from Cancer Hospital and Research Institute, Gwalior, India. Diclofenac (Ranbaxy) and venlafaxine (Cipla protec) were purchased and used in the study. All the drugs and ME were administered orally as 2% gum acacia suspension prepared just before administration.
Statistical Analysis
Data is represented as Mean ± SEM and analyzed using one-way ANOVA followed by Tukey multiple comparison tests. P<0.05 were considered statistically significant.
Results
Effect of A. marmelos on Tail Flick Response (TFL)
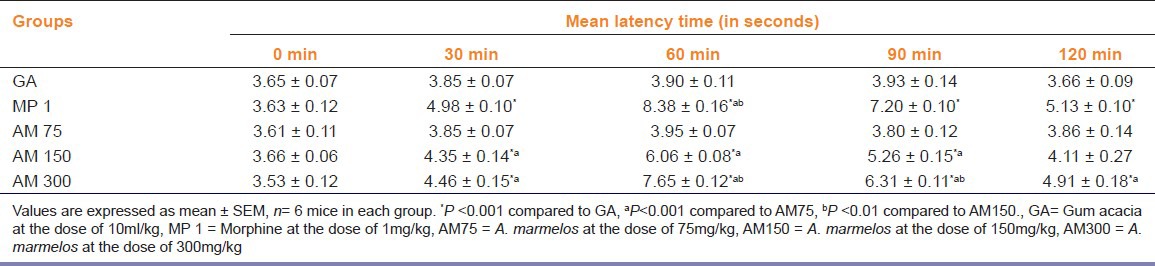
A. marmelos significantly increase the mean latency elicited by TFL (P<0.05) at 150 and 300 mg/kg as compared to the gum acacia-treated mice [Table 1]. Morphine (1mg/kg) elicited significant (P<0.05) antinociceptive activity as compared to GA, ME at the dose of 75 and 150 mg/kg treated mice.
Table 1.
Effect of methanol extract of Aegle marmelos leaves on tail flick latency in mice
Effect of A. marmelos on Writhing
A significant (P<0.001) inhibition of the writhes were elicited by the ME at 150 and 300mg/kg as compared to the gum acacia [Figure 1]. Standard drug diclofenac (5 mg/kg) elicited significant (P<0.001) inhibition of the writhes as compared to GA, ME (75 and 150mg/kg) treated mice. The percentage inhibitions of writhes were 28, 58 and 61% for the ME150, 300mg/kg and standard diclofenac 5mg/kg treated animals respectively as compared with control group. The effect of the extract of the plant at 300 mg/kg was comparable with the reference drug diclofenac sodium [Figure 1].
Figure 1.
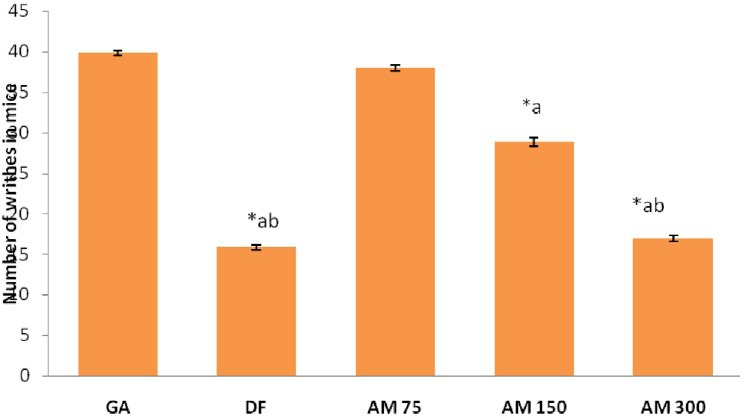
Effect of different doses of Aegle marmelos on number of writhes in mice. Values are expressed as mean ± SEM, n = 6 *P < 0.001 compared to GA, aP < 0.001 compared to AM 75, bP < 0.01 compared to AM 150, DF = diclofenac 5 mg/kg, AM 75 = A marmelos 75 mg/kg, AM 150 = A marmelos 150 mg/kg, AM 300 = A. marmelos 300 mg/kg
Effects of Combination Treatments on TFL and Writhing
Standard drug morphine at 1mg/kg and venlafaxine at 25mg/kg produced significant (P<0.001) inhibition of pain perception as compared to control, morphine 0.25mg/kg and venlafaxine 7.5 mg/kg treated mice respectively in both the pain models. Combination of subeffective dose of A. marmelos with morphine showed significant pain inhibition (P<0.001) as compared with control, ME (75mg/kg) and morphine (0.25mg/kg) treated mice. Combination of subeffective dose of A. marmelos with venlafaxine also caused significant pain inhibition as compared to control, ME (75mg/kg) and venlafaxine (7.5mg/kg) treated mice respectively. The pain inhibiton was comparable with venlafaxine 25mg/kg in both the pain models: TFL test [Table 2] and writhing test [Figure 2].
Table 2.
Effect of combination treatment on tail flick latency in mice
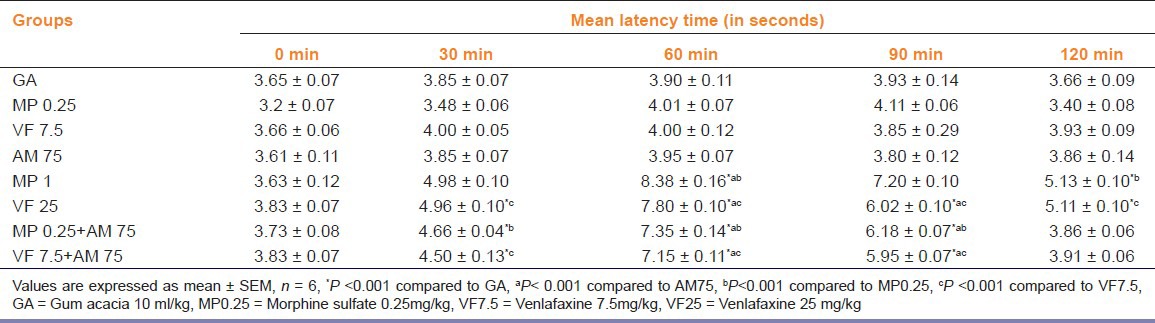
Figure 2.
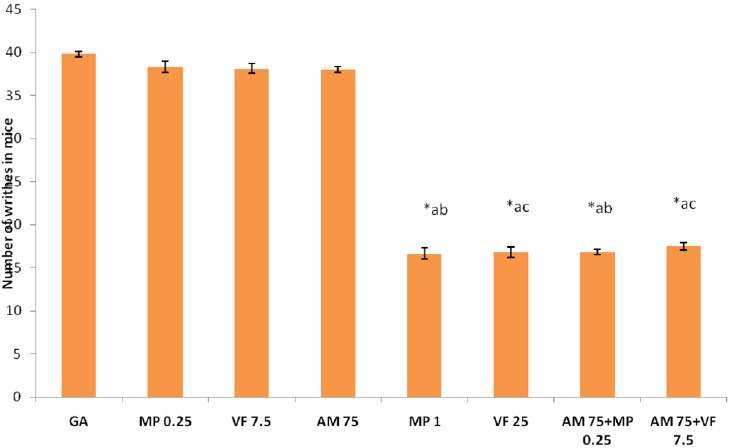
Effect of combined treatments on number of writhes in mice. Values are expressed as mean ± SEM, n = 6, *P < 0.001 compared to GA, aP < 0.001 compared to AM 75, bP < 0.001 compared to MP 0.25, cP < 0.001 compared to VF 7.5. GA = gum acacia at the dose of 10 ml/kg, DF = Diclofenac 5 mg/kg, MP 0.25 = Morphine 0.25 mg/kg, VF 7.5 = Venlafaxine 7.5 mg/kg, VF 25 = Venlafaxine 25 mg/kg, AM 75 = A. marmelos 75 mg/kg
Effects of Naltrexone Pre-treatment on Antinociception Induced by A. marmelos when Administered Alone or in Combinations
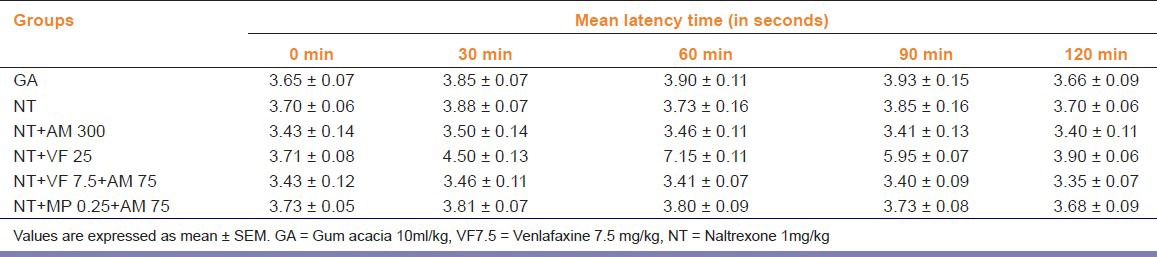
Administration of 1mg/kg naltrexone did not increase TFL period in mice as compared to control (P>0.05). Pre-treatment of naltrexone before administration of A. marmelos at the dose of 300 mg/kg and venlafaxine at the dose of 25mg/kg to mice respectively caused inhibition of antinociceptive effect. Naltrexone pre-treatment also resulted in inhibition of antinociceptive effect in mice treated with combination of subanalgesic dose of A. marmelos with venlafaxine and morphine respectively [Table 3].
Table 3.
Effect of Naltrexone pre-treatment on Aegle marmelos-induced tail flick latency in mice used alone or in combination with either venlafaxine or morphine
Discussion
The thermal stimulation-induced pain model is considered to be selective to screen out centrally acting analgesic activity.[16] In the present study radiant heat induced tail flick test was used as a model of nociception which is selective for opioid-like compounds. The effectiveness of analgesic agents in tail flick model is highly correlated with relief of human pain perception.[17] The results obtained in present study indicate that ME of A. marmelos possess dose-dependent analgesic effect on the TFL test hence it is suggestive of central action. In pain transmission numerous neurotransmitters are involved.[6] In the present study we investigated the role of endogenous opioids, NA and 5 HT in analgesia. Subanalgesic dose of A. marmelos showed synergism with subanalgesic dose of morphine suggesting involvement of opiate system in A. marmelos-induced analgesic activity. An extensive body of evidence shows that tricyclic antidepressants, particularly those that modulate both NA and 5-HT signaling, like venlafaxine and mirtazapine reliably relieve pain, while analgesia with selective serotonin reuptake inhibitors is of lesser magnitude or are less reliably produced.[18] The development and continued investigation of the analgesic effects of these newer antidepressant agents is likely not only to enhance treatment of depression but also pain.[19] These drugs cause spinal inhibition of pain by increasing NA and 5HT level which inhibit the release of transmitters carrying pain sensation from nerve endings.[20] In the present study the subanalgesic dose of A. marmelos showed synergies with a subanalgesic dose of venlafaxine in tail flick model suggesting involvement of NA and 5HT in analgesic activity of A. marmelos. The serotonergic and noradrenergic systems have been implicated in the descending inhibitory control of pain in humans and animals.[21] Thus the present study revealed involvement of opioid as well as monoaminergic pain pathways in A. marmelos induced analgesic activity and is in accordance with earlier reports that several antidepressants produce analgesia which appears to be mediated by both noradrenergic and serotonergic signaling as well as opioid mechanisms.[22]
Naltrexone pre-treatment inhibited the antinociceptive effect of A. marmelos when used alone confirmed that A. marmelos in part acts by blocking opioid receptors. Naltrexone also blocked antinociception induced by combination of A. marmelos with venlafaxine suggesting inhibition of descending pain inhibiting pathway by A. marmelos that involves monoamines. Such response of A. marmelos is in accordance with earlier reports on the drugs which prevent reuptake of NA and 5HT and their action is blocked by naloxone.[23] It seems that A. marmelos exerts complex modulation of the descending noradrenergic and serotonergic systems that exert inhibitory influences on the transmission of nociceptive information, probably in addition to effects on opioid receptors in the primary neurons of the spinal cord.
Analgesics can also act on the peripheral nervous system to block pain perception due to acetic acid-induced writhing in experimental animals.[24] In the present study, ME of A. marmelos inhibited acetic acid-induced writhing in mice when used at the dose of 150 and 300mg/kg alone as well as in combination of subanalgesic dose with that of morphine. This result is in confirmation with the earlier reports that acetic acid-induced writhing is readily reversed by nonsteroidal anti-inflammatory drugs as well as by opioid agonists such as morphine.[20] Further, our study showed that combination of subanalgesic dose of ME with that of venlafaxine also inhibited acetic acid-induced writhing response which is in agreement with previous reports[20,25] that the selective serotonergic and noradrenergic reuptake inhibitor produced a dose-dependent antinociceptive effect in the acetic acid-induced writhing test in mice. Thus, ME of A. marmelos was efficacious in reducing inflammation-related pain and central sensitization in the present studies.
The results of the present study demonstrated that the A. marmelos posses analgesic activity which is evident in the central and peripheral experimental pain models. A. marmelos induced antinociception involves both opioid and the monoaminergic pain pathways, suggesting a potential use of A. marmelos in neuropathic and chronic pain. Further investigations are required to find active principles responsible for these effects.
Acknowledgments
We are thankful to Dr. B. R. Shrivastva, Professor and head, Department of Surgery, Gajara Raja Medical College Gwalior and Director, Cancer Hospital and Research Institute, Gwalior INDIA for providing tablet Morphine sulfate for the study.
Footnotes
Source of Support: Nil
Conflict Interest: None declared
References
- 1.Merskey H, Albe-Fessard DG, Bonica JJ, Carmon A, Dubner R, Kerr FW, et al. Pain terms: A lists with definitions and notes on usage. Pain. 1979;6:249–52. [Google Scholar]
- 2.Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, et al. Opioid complications and side effects. Pain Physician. 2008;11(suppl 2):S105–20. [PubMed] [Google Scholar]
- 3.Straube S, Tramèr MR, Moore RA, Derry S, McQuay HJ. Mortality with upper gastrointestinal bleeding and perforation: Effects of time and NSAID use. BMC Gastroenterol. 2009;9:41. doi: 10.1186/1471-230X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mc Quay HJ, Moore RA. Antidepressants and chronic pain. BMJ. 1997;314:763–4. doi: 10.1136/bmj.314.7083.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sierralta F, Pinardi G, Miranda HF. Effect of p-chlorphenylalanine and alpha-2-methyltyrosine on the antinociceptive effect of antidepressant drugs. Pharmacol Toxicol. 1995;77:276–80. doi: 10.1111/j.1600-0773.1995.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 6.Jha PK, Mazumdar B, Bhatt JD. Analgesic activity of venlafaxine and its interaction with tramadol, celecoxib and amlodipine in mice. Indian J Pharmacol. 2006;38:181–4. [Google Scholar]
- 7.Shobha FG, Thomas M. Study of antidiarrheal activity of four medicinal plants in castor oil induced diarrhea. J Ethnopharmacol. 2001;76:73–6. doi: 10.1016/s0378-8741(00)00379-2. [DOI] [PubMed] [Google Scholar]
- 8.Kamalakkannan N, Prince PS. Hypoglycemic effect of water extract of Aegle marmelos fruits in streptozotocin diabetic rats. J Ethnopharmacol. 2003;87:207–10. doi: 10.1016/s0378-8741(03)00148-x. [DOI] [PubMed] [Google Scholar]
- 9.Lotufu LV, Khan MT, Ather A, Wilke DV, Jiminez PC, Pessoa C, et al. Studies of the anticancer potential of plants used in Bangladesh folk medicine. J Ethnopharmacol. 2005;99:21–3. doi: 10.1016/j.jep.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Jagetia GC, Venktesh P, Balinga MS. Evaluation of radioprotective effect of bael leaf (Aegle marmelos) extract in mice. Int J Radiat Biol. 2004;80:281–90. doi: 10.1080/09553000410001679776. [DOI] [PubMed] [Google Scholar]
- 11.Kothari S, Minda M, Tonpay SD. Anxiolytic and antidepressant activities of methanol extract of Aegle marmelos leaves in mice. Indian J Physiol Pharmacol. 2010;54:318–28. [PubMed] [Google Scholar]
- 12.Veerappan A, Shigeru M, Rengnathan D. Studies on the anti-inflammatory, antipyretic and analgesic properties of the leaves of Aegle marmelos Corr. J Ethnopharmacol. 2005;96:159–63. doi: 10.1016/j.jep.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Shankarananth V, Balkrishnan ND, Suresh G, Sureshpandian E, Edwin E, Sheeja E. Analgesic activity of methanol extract of Aegle marmelos leaves. Fitoterapia. 2007;78:258–9. doi: 10.1016/j.fitote.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 14.D’Armour FE, Smith DL. A method for determining loss of pain sensation. J Pharmacol Exp Ther. 1941;72:74–9. [Google Scholar]
- 15.Koster R, Anderson M, De Beer EJ. Acetic acid test for analgesic screening. Proc Soc Exp Biol. 1959;18:412–5. [Google Scholar]
- 16.Wong CH, Day P, Yarmush JG, Wu WH, Zbuzek VK. Nifedipine induced analgesia after epidural injections in rats. Anesth Analg. 1994;79:303–6. doi: 10.1213/00000539-199408000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Knighton RS, Dumke PR. The production of analgesic activity in man by animal testing. In: Knighton RS, Dumke PR, editors. Pain. Boston: Little Brown and Co; 1966. pp. 163–82. [Google Scholar]
- 18.Tura B, Tura SM. The analgesic effect of tricyclic antidepressants. Brain Res. 1990;518:19–22. doi: 10.1016/0006-8993(90)90948-b. [DOI] [PubMed] [Google Scholar]
- 19.Valverde O, Mico JA, Maldonado R, Mellado M, Gibert RJ. Participation of opioid and monoaminergic mechanisms on the antinociceptive effect induced by tricyclic antidepressants in two behavioral pain tests in mice. Prog Neuropsychopharmacol Bio Psychiatry. 1994;18:1073–92. doi: 10.1016/0278-5846(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 20.Laura MB, Fei X, Raul RG, Marc GC. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci. 2000;20:9040–5. doi: 10.1523/JNEUROSCI.20-24-09040.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones CK, Petre SC, Shanon HE. Efficacy of duoloxetine a potent and balanced serotonergic and noradrenergic reuptake inhibitor in inflammatory and acute pain models. J Pharmacol Exp Ther. 2005;2:726–32. doi: 10.1124/jpet.104.075960. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber S, Backer MM, Pick CG. The antinociceptive effect of venlafaxine in mice is mediated through opioid and adrenergic mechanisms. Neurosci Lett. 1999;273:85–8. doi: 10.1016/s0304-3940(99)00627-8. [DOI] [PubMed] [Google Scholar]
- 23.Ardid D, Guilboud G. Antinociceptive effect of acute and chronic injections of tricyclic antidepressant drugs in a new model of mononeuropathy in rats. Pain. 1992;49:279–87. doi: 10.1016/0304-3959(92)90152-2. [DOI] [PubMed] [Google Scholar]
- 24.Turner RA. New York: Academic Press; 2009. Screening Methods in Pharmacology; pp. 152–63. [Google Scholar]
- 25.Singh VP, Jain NK, Kulkarni SK. On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res. 2001;915:218–26. doi: 10.1016/s0006-8993(01)02854-2. [DOI] [PubMed] [Google Scholar]


