Abstract
Objectives:
Oxidative stress with subsequent lipid peroxidation (LP) has been suggested as a mechanism for lead-induced toxicity. The current study was carried out to evaluate antioxidant activity of hesperetin against lead acetate-induced oxidative stress.
Materials and Methods:
The male rats were treated with hesperetin in combination with lead acetate (500 mg/L).
Results:
The results indicated that hesperetin alone did not induce any significant changes in the biochemistry of serum, liver, and kidney tissues. On the other hand, lead-induced oxidative stress as indicated by significant changes in serum biochemical parameters, including increased lipid peroxide and decreased reduced glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) levels in liver and kidney tissues. Hesperetin succeeded in improving these biochemical parameters towards the normal values of control.
Conclusions:
It suggests that hesperetin shows antioxidant activity and plays a protective role against lead-induced oxidative damage in liver and kidney of rats.
KEY WORDS: Antioxidants, hesperetin, lead, oxidative stress, rats
Introduction
Lead is a major human health hazard due to its wide distribution in the environment and in biological systems.[1] Sources of human exposure to this metal include many foods, drinking water, and dust.[2] Pregnant women, children, and city inhabitants are at risk of lead intoxication. Lead induces a broad range of physiological and biochemical dysfunctions.[3]
Several mechanisms have been proposed to explain lead-induced toxicity; however, no mechanisms have been explicitly defined. Oxidative stress with subsequent lipid peroxidation (LP) induced by production of reactive oxygen species (ROS) has been reported to be one of the important mechanisms involved in toxic effects of lead. The release of malondialdehyde (MDA) is an index of LP. Bones and blood contain 90 and 4% of the total body lead, respectively; the remaining lead residues are mainly found in liver and kidneys. The liver and kidneys play a major role in elimination of lead; therefore, they account for the toxic actions.[4]
Cell membranes are targets for oxidative damage produced by xenobiotics including heavy metals.[5] Peroxidative decomposition of membrane lipids is catastrophic for living systems. Antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) scavenge free radicals and lipid peroxides and detoxify them.[3]
Biological compounds with antioxidant properties contribute to protection of cells and tissues against deleterious effects of ROS and other free radicals.[6] Flavonoids are the most abundant antioxidants in plants and in human diet. They have attracted a great deal of attention in recent years for their antioxidative, antibacterial, and hepatoprotective activities.[7] Hesperetin (5,7,3’-trihydroxy-4-methoxyl flavanone), is one of the most abundant flavonoids found in citrus fruits.[8] Hesperetin shows a wide spectrum of pharmacological effects such as anti-inflammatory, anticarcinogenic, antihypertensive, antiatherogenic effects, and antioxidant properties.[9] Studies have shown that hesperetin is a powerful radical scavenger that promotes cellular antioxidant defense-related enzyme activity.[10]
To our knowledge, there are no other reports available on protective effect of hesperetin on lead-induced toxicity in experimental animals. In view of this, the present study was designed to elucidate whether hesperetin, when administered with lead acetate, can ameliorate oxidative stress-induced hepatic and renal dysfunction caused by lead.
Materials and Methods
Chemicals
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea, uric acid, creatinine, MDA, reduced glutathione (GSH), CAT, SOD, and GPx kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Lead acetate was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Hesperetin (purity: >98%, high performance liquid chromatography (HPLC)) was purchased from Xi’an Xiaocao Botanical Development Co., Ltd (Xi’an, China).
Animals and Treatment
Adult male Sprague-Dawley rats (8-weeks old, weighing approximately 180 g) were purchased from the Experimental Animal Center of Henan Province (Zhengzhou, China). The rats were maintained under standard laboratory conditions (temperature 24 ± 2°C, natural light-dark cycle), and had free access to drinking water and a commercial standard pellet diet. After acclimatization to laboratory conditions, the animals were randomly divided into four groups, six rats in each. Group 1, untreated control; group 2, treated orally with hesperetin (50 mg/kg/day); group 3, treated orally with lead acetate (500 mg Pb/L); and group 4 treated orally with hesperetin (50 mg/kg/day) plus lead acetate (500 mg Pb/L).[11,12] All experimental groups received test solutions in a volume of 10 ml/kg.
The experiments lasted for 8 weeks. At the end of the experimental period, blood samples were collected from all animals. The serum obtained after centrifugation (1500 rpm for 10 min at 4°C) was used for various serum biochemical assays. Then all animals were sacrificed; the liver and kidney were removed, weighed and washed using chilled saline solution. Tissue was minced and homogenized (10%, w/v) in PBS (pH7.4) and then centrifuged (3000 rpm for 10 min). The resulting clear supernatant was used for various enzymatic and non-enzymatic biochemical assays.
Biochemical Assays
The levels of ALT, AST, urea, uric acid, and creatinine in serum were estimated spectrophotometrically using commercial kits. Analysis of liver and kidney MDA and GSH levels were performed with commercial kits. The activities of liver and kidney SOD, CAT, and GPx were also assayed using commercial kits.
Statistical Analysis
Data were expressed as mean ± SE of a number of experiments (n = 6). The statistical significance was evaluated by one-way analysis of variance (ANOVA) using SPSS 15.0 software package (Chicago, IL, USA) and individual comparisons were obtained by Duncan's multiple range test (DMRT). Values were considered statistically significant when P < 0.05.
Results
Effect on Serum Biochemical Parameters
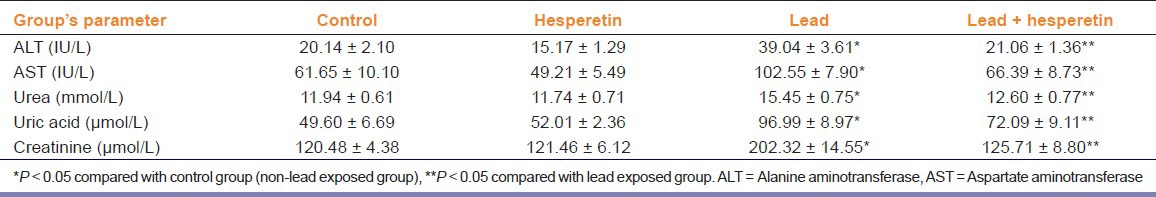
A significant increase in levels of ALT, AST, urea, uric acid, and creatinine in the serum of lead-treated rats was observed when compared with controls. Administration of hesperetin along with lead significantly restored these values to near normal when compared with lead-alone treated rats [Table 1].
Table 1.
Effect of hesperetin on lead-induced serum biochemical parameters of liver and kidney function in rats (Mean ± SE, n = 6)
Effect on Hepatic Oxidative Stress and Antioxidant Parameters
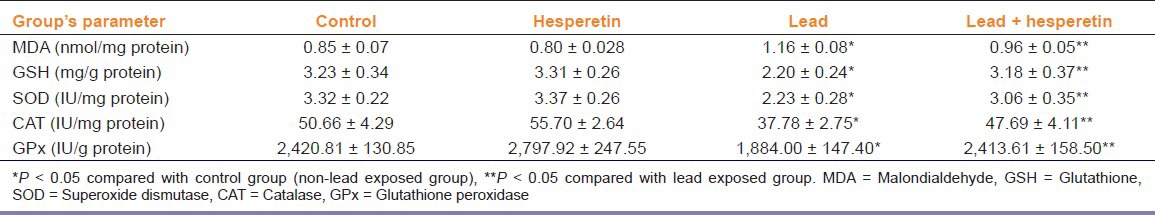
A significant decrease in level of GSH content and activities of SOD, GPx, and CAT; as well as an increase in MDA content were observed in lead-treated rats. Administration of hesperetin (50 mg/kg/day) along with lead significantly (P < 0.05) increased the levels of GSH, SOD, GPx, and CAT and decreased the liver MDA content when compared with lead-alone treated rats [Table 2].
Table 2.
Effect of hesperetin on lead-induced lipid peroxidation and the antioxidant status of rat liver (Mean ± SE, n = 6)
Effect on Renal Oxidative Stress and Antioxidant Parameters
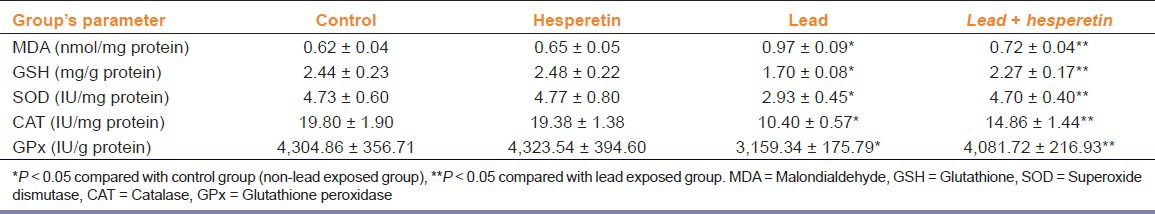
Renal GSH level and SOD, GSH-Px, and CAT activities significantly decreased; while MDA content significantly increased in lead poisoned rats. Treatment with hesperetin (50 mg/kg/day) significantly increased levels of GSH, SOD, GPx, and CAT and decreased the liver MDA content of lead intoxicated rats [Table 3].
Table 3.
Effect of hesperetin on lead-induced lipid peroxidation, and the antioxidant status of rat kidney (Mean ± SE., n = 6)
Discussion
Redox disturbances are known to negatively impact body systems through the generation of ROS, which can modify proteins, lipids, and DNA.[5] Liver is one of the targets for lead accumulation and responds to toxic insult caused by lead by increasing activities of transaminases.[2,13] Liver is the major site of metabolism, thus these enzymes are regarded as markers of liver injury.[14] Several researchers have reported increased ALT and AST activities in liver during lead poisoning, which corroborates our findings.[13] Administration of hesperetin (50 mg/kg) attenuated lead-induced hepatotoxicity as shown by decreased levels of ALT and AST, thus offering protection against lead toxicity in rats. Furthermore, it has been reported that hesperetin decreases liver marker enzymes during 7,12-dimethylbenz(a)anthracene-[15] and cadmium[16] -induced hepatotoxicity via its antilipoperoxidative activity. The above effects clearly indicate that hesperetin may offer protection by stabilizing the cell membrane in hepatic damage induced by lead.
The kidney is vulnerable to damage due to larger perfusion and increased concentration of excreted compounds which occur in renal tubular cells.[17] Urea is the major nitrogen-containing metabolic product of protein metabolism. Uric acid is the major product of purine nucleotides, adenosine and guanosine. The levels of urea and creatinine in serum were used as indicators of renal function. Elevated blood urea is known to be correlated with increased protein catabolism in mammals and/or the conversion of ammonia to urea as a result of increased synthesis of arginase involved in urea production.[17] Hyperuricemia is a renal prognostic factor. Therefore, elevated serum uric acid concentrations can reflect the bodily response to increased production of endogenous oxygen species because uric acid is a potent scavenger of peroxynitrite. In previous study, increased serum creatinine reflected the diagnosis of renal failure.[18] The administration of hesperetin protects kidney function from lead intoxication as indicated by significant restoration of serum urea, uric acid, and creatinine.
Lead is known to produce oxidative damage in liver and kidney by enhancing LP.[19] MDA is a marker of oxidative stress. GSH can act as a nonenzymatic antioxidant by direct interaction of sulfhydryl (SH) groups with ROS, or it can be involved in enzymatic detoxification reaction for ROS, as a cofactor or a coenzyme, because it is a tripeptide containing cysteine which has a reactive SH group with reductive potency.[3] In this study, we observed a significant increase in MDA level and a decrease in GSH level in lead-treated rat liver and kidney. These findings may be due to high ability of lead to bind with SH group of GSH and lead-induced oxidative stress. Interestingly, we found that hesperetin markedly increased GSH level and decreased MDA level in lead-treated rat liver and kidney. This suggested that hesperetin could, at least partly, attenuate oxidative stress by increasing GSH level and decreasing lipid peroxide level in lead-treated rat liver and kidney.
Lead has high affinity for SH groups or metal cofactors in antioxidant enzymes and molecules, which results in a reduction in antioxidant enzyme activities, such as SOD, CAT, and GPx. SOD, CAT, and GPx form the first line of defense against ROS and decrease in their activities contribute to oxidative stress in the tissues.[20] In lead-exposed animals, the activity of SOD was inhibited to some degree. CAT is a major antioxidant enzyme having heme as the prosthetic group. Lead is known to reduce absorption of iron in gastrointestinal tract and to inhibit heme biosynthesis. GPx is a hydroperoxide-degrading enzyme, which requires selenium for activity and was decreased in lead-poisoned rats. Loss of GPx activity can be correlated to the antagonistic effects between lead and selenium as suggested by Schrouzer.[3] In this study, treatment with hesperetin resulted in a significant improvement in all antioxidant enzyme activities. The effective protective effects of hesperetin may be due to radical scavenging activity of its components.
Conclusion
In conclusion, this study demonstrated that lead acetate was capable of causing marked oxidative damage and inhibited activities of antioxidant enzymes. Treatment with hesperetin could minimize these hazards, and may be useful and reasonable in the treatment of lead toxicity.
Acknowledgments
The present study was financially supported by the Research Fund for Doctor of Henan University of Science and Technology (09001489).
Footnotes
Source of Support: The research fund for doctor of Henan University of Science and Technology (09001489)
Conflict of Interest: None declared
References
- 1.Xia D, Yu X, Liao S, Shao Q, Mou H, Ma W. Protective effect of Smilax glabra extract against lead-induced oxidative stress in rats. J Ethnopharmacol. 2010;130:414–20. doi: 10.1016/j.jep.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 2.El-Nekeety AA, El-Kady AA, Soliman MS, Hassan NS, Abdel-Wahhab MA. Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem Toxicol. 2009;47:2209–15. doi: 10.1016/j.fct.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Sivaprasad R, Nagaraj M, Varalakshmi P. Combined efficacies of lipoic acid and 2, 3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem. 2004;15:18–23. doi: 10.1016/j.jnutbio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Lockitch G. Perspectives on lead toxicity. Clin Biochem. 1993;26:371–81. doi: 10.1016/0009-9120(93)90113-k. [DOI] [PubMed] [Google Scholar]
- 5.Halliwell B, Gutteridge J. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 6.Ciaccio M, Valenza M, Tesoriere L, Bongiorno A, Albiero R, Livrea MA. Vitamin A inhibits doxorubicin-induced membrane lipid peroxidation in rat tissues in vivo. Arch Biochem Biophys. 1993;302:103–8. doi: 10.1006/abbi.1993.1186. [DOI] [PubMed] [Google Scholar]
- 7.Zheleva-Dimitrova D, Nedialkov P, Girreser U, Kitanov G. Benzophenones and flavonoids from Hypericum maculatum and their antioxidant activities. Nat Prod Res. 2011;26:1576–83. doi: 10.1080/14786419.2011.582468. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Izquierdo A, Gil MI, Ferreres F, Tomás-Barberán FA. In vitro availability of flavonoids and other phenolics in orange juice. J Agric Food Chem. 2001;49:1035–41. doi: 10.1021/jf0000528. [DOI] [PubMed] [Google Scholar]
- 9.Yang HL, Chen SC, Senthil Kumar KJ, Yu KN, Lee Chao PD, Tsai SY, et al. Anti-oxidant and Anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J Agric Food Chem. 2012;60:522–32. doi: 10.1021/jf2040675. [DOI] [PubMed] [Google Scholar]
- 10.Pollard SE, Whiteman M, Spencer JP. Modulation of peroxynitrite-induced fibroblast injury by hesperetin: A role for intracellular scavenging and modulation of ERK signalling. Biochem Biophys Res Commun. 2006;347:916–23. doi: 10.1016/j.bbrc.2006.06.153. [DOI] [PubMed] [Google Scholar]
- 11.Liu CM, Zheng YL, Lu J, Zhang ZF, Fan SH, Wu DM, et al. Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol. 2010;29:158–66. doi: 10.1016/j.etap.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res. 2012;146:354–9. doi: 10.1007/s12011-011-9268-6. [DOI] [PubMed] [Google Scholar]
- 13.Mehana EE, Meki AR, Fazili KM. Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp Toxicol Pathol. 2012;64:291–5. doi: 10.1016/j.etp.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Liss GM, Greenberg RA, Tamburro CH. Use of serum bile acids in the identification of vinyl chloride hepatotoxicity. Am J Med. 1985;78:68–76. doi: 10.1016/0002-9343(85)90464-4. [DOI] [PubMed] [Google Scholar]
- 15.Choi EJ. Antioxidative effects of hesperetin against 7, 12-dimethylbenz (a) anthracene-induced oxidative stress in mice. Life Sci. 2008;82:1059–64. doi: 10.1016/j.lfs.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Pari L, Shagirtha K. Hesperetin protects against oxidative stress related hepatic dysfunction by cadmium in rats. Exp Toxicol Pathol. 2012;64:513–20. doi: 10.1016/j.etp.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Renugadevi J, Prabu SM. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol. 2010;62:171–81. doi: 10.1016/j.etp.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Donadio C, Lucchesi A, Tramonti G, Bianchi C. Creatinine clearance predicted from body cell mass is a good indicator of renal function. Kidney Int Suppl. 1997;63:166–8. [PubMed] [Google Scholar]
- 19.Yin ST, Tang ML, Su L, Chen L, Hu P, Wang HL, et al. Effects of epigallocatechin-3-gallate on lead-induced oxidative damage. Toxicology. 2008;249:45–54. doi: 10.1016/j.tox.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Antonio-Garcia MT, Massó-Gonzalez EL. Toxic effects of perinatal lead exposure on the brain of rats: Involvement of oxidative stress and the beneficial role of antioxidants. Food Chem Toxicol. 2008;46:2089–95. doi: 10.1016/j.fct.2008.01.053. [DOI] [PubMed] [Google Scholar]


