Abstract
Terlipressin, an analog of the natural hormone arginine-vasopressin, is a splanchnic constrictor that is used to control variceal bleeding in portal hypertension. It has a very good safety profile compared to vasopressin. Although rare, adverse effects such as hyponatremia and seizure can occur. We describe a 7-year-old male child who developed hyponatremia induced by infusion of terlipressin which resulted in a generalized seizure. After withdrawal of terlipressin, the serum sodium level became normal. Through this case, we emphasize the importance of monitoring patient's electrolyte levels during the course of terlipressin therapy.
KEY WORDS: Adverse drug reaction, children, hyponatremia, terlipressin
Introduction
Within the last few decades, mortality due to variceal bleeding has decreased significantly due to the introduction of new treatment modalities including vasoactive drugs such as terlipressin.[1] Terlipressin, an arginine-vasopressin analog, is a potent vasoconstrictor of splanchnic circulation.[2] It is commonly used in the management of patients with bleeding esophageal varices and portal hypertension. It has a better safety profile and longer duration of action as compared to vasopressin. Terlipressin may cause adverse effects like: paleness, hypertension, arrhythmia, headache, and gastrointestinal manifestations.[3] We herein present a case of terlipressin-induced hyponatremic seizures, a rare adverse effect of this medication.
Case Report
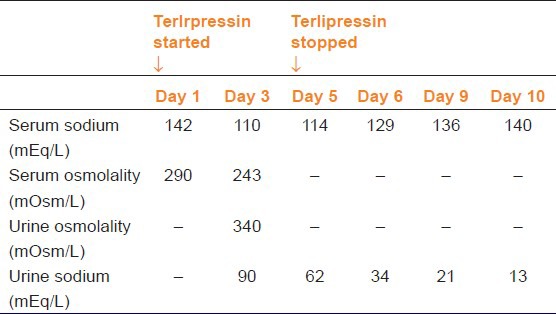
A 7-year-old male child presented with two episodes of hematemesis (around 100 ml of fresh blood). There was no history of abdominal pain, malena, fever, drug intake, and bladder/bowel complaints. There was no history of blood transfusion or jaundice in the past. His family history and birth history was normal. On admission, blood pressure was 78/58 mmHg, heart rate was 110 beats/min, and respiratory rate was 24 breaths/min. Pallor was present. There was no icterus, edema, cyanosis, or jaundice. Spleen was palpable 3 cm below left costal margin. Liver span was 8 cm with soft consistency and smooth margins. After initial fluid resuscitation, patient was given 15 cc/kg of packed cell transfusion. His hemoglobin was 5.7 g/dl; white blood cell count 7200/cumm; platelet count 1.6 lac/cumm. His liver function tests, serum electrolytes, renal function tests, prothrombin time, plasma thromboplastin time, and bleeding time were normal. A provisional diagnosis of extra-hepatic portal vein obstruction with variceal bleeding was made. An esophagogastroduodenoscopy (EGD) showed two large esophageal varices with active bleeding. Endoscopic variceal ligation was planned in the patient. However, as the patient was not hemodynamically stable, a bolus of 2 mg of terlipressin was initiated, followed by 1 mg every 6 hourly. Color Doppler sonography revealed multiple collateral channels replacing the portal vein (portal cavernoma) and splenomegaly. The patient was stable until the third hospital day when he had a generalized tonic seizure and became drowsy. His sodium level had dropped from 142 mEq/L on admission to 110 mEq/L and serum osmolality had decreased to 240 mOsm/kg. Intravenous correction for hyponatremia was started. Computed tomography scan of brain, cerebrospinal fluid examination, and electroencephalography were normal. After 2 days of starting sodium correction, the sensorium had slightly improved but hyponatremia was persistent. On the fifth hospital day, terlipressin was stopped and serum electrolytes measured the next day showed a dramatic increase in sodium level, although not to a normal level. After 4 days of stopping terlipressin, the serum sodium was normal. This can be considered as a ‘probable’ adverse drug reaction as per causality assessment with Naranjo's Scale.[4] Table 1 shows the serial serum sodium values during the hospital stay. The patient underwent endoscopic ligation of the esophageal varices and was discharged on the tenth day of admission.
Table 1.
Laboratory investigations of the pateint
Discussion
Vasopressin exerts its major physiologic effects via two receptor subtypes (V1A and V2).[2] Activation of the V1A receptors causes vasoconstriction in the splanchnic vessels, which redistributes blood flow and maintains blood pressure during hypotensive states, and in the portal vein, which helps lower portal pressure. Activation of the V2 receptor inserts vasopressin-sensitive water channels in the cell membrane, promoting the reabsorption of water and formation of concentrated urine.[2] Excessive reuptake of electrolyte-free water into the blood circulation may lead to hyponatremia. Terlipressin is a vasopressin analog in which the V1A-receptor affinity has been increased and the V2-receptor affinity has been decreased. Thus, terlipressin has stronger vasoconstrictive effects than vasopressin and lesser effects on water reabsorption in the kidney collecting ducts. The vasoconstrictive effects of terlipressin on splanchnic vessels and post-glomerular blood vesels increases blood flow to the kidneys and leads to an increased glomerular filtration pressure and diuresis. Since terlipressin exerts lesseer effect on the V2 receptor, having only 3% of the antidiuretic effects of vasopressin, hyponatremia should not be a problem. However, terlipressin is slowly metabolized in the liver to vasopressin.[5] Since vasopressin has quite strong agonistic effects on V2-receptors there is a slight possibility that terlipressin may stimulate water reabsorption indirectly via its conversion to vasopressin and, thus cause hyponatremia. There have been several reports of hyponatremia caused by terlipressin in adult patients. Table 2 reveals some of the cases of hyponatremia due to terlipressin therapy seen in adult patients.[2,3,5,6,7,8] A PUBMED search done in children (0-18 years) with keywords ‘terlipressin’ and ‘hyponatremia’ did not reveal any case and hence ours is the first documented case of hyponatremic seizure due to terlipressin therapy in a pediatric patient.
Table 2.
Terlipressin therapy caused hyponatremia - Evidences in literature

It has been observed that terlipressin does not cause significant decrease in serum sodium in patients with advanced liver disease. The development of acute hyponatremia is related to the degree of occupancy of renal V2 vasopressin receptors before the initiation of terlipressin therapy.[9] In patients with advanced liver disease and low serum sodium levels, the renal V2 receptors are likely to be occupied by endogenous vasopressin. Therefore, terlipressin therapy may not have significant antidiuretic effect because the V2 receptors would be occupied already by the endogenous ligand. By contrast, in patients with less severe liver dysfunction and normal serum sodium levels, terlipressin would cause solute-free water retention because V2 vasopressin receptors are not occupied by endogenous vasopressin. Sola et al., found that patients who developed hyponatremia in their study had a better prognosis in terms of survival compared to those who did not develop hyponatremia.[9] Thus development of hyponatremia during terlipressin therapy indirectly signifies that the patient has less severe liver dysfunction and a better chance of survival.
Conclusions
In conclusion, we report the first case of hyponatremic seizure due to terlipresin therapy in a pediatric patient. Although rarely described in literature, clinicians should be aware of this association as timely diagnosis and early intervention can significantly affect the outome. Also, we highlight the need for frequent monitoring of serum electrolytes during the course of terlipressin use.
Acknowledgment
We would like to thank the Dean of our institute for permitting us to publish this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None
References
- 1.D’Amico G, De Franchis R. Upper digestive bleeding in cirrhosis. Post-therapeutic outcome and prognostic indicators. Hepatology. 2003;38:599–612. doi: 10.1053/jhep.2003.50385. [DOI] [PubMed] [Google Scholar]
- 2.Hyun JJ, Seo YS, Lee KG, Keum B, Yim HJ, Jeen YT, et al. Terlipressin-induced hyponatremic seizure. Scand J Gastroenterol. 2010;45:501–4. doi: 10.3109/00365520903477355. [DOI] [PubMed] [Google Scholar]
- 3.Krag A, Borup T, Moller S, Bendtsen F. Efficacy and safety of terlipressin in cirrhotic patients with variceal bleeding or hepatorenal syndrome. Adv Ther. 2008;25:1105–40. doi: 10.1007/s12325-008-0118-7. [DOI] [PubMed] [Google Scholar]
- 4.Zaki SA. Adverse drug reaction and causality assessment scales. Lung India. 2011;28:152–3. doi: 10.4103/0970-2113.80343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunwoodie E, Jowett S. Terlipressin causing a hyponatraemic seizure. Scand J Gastroenterol. 2007;42:665. doi: 10.1080/00365520701191849. [DOI] [PubMed] [Google Scholar]
- 6.Douriez E, Mollard P, Laval C, Albengres E, Laporte JP, Tillement JP. Severe hyponatremia after repeated administration of terlipressin. Therapie. 1993;48:518–9. [PubMed] [Google Scholar]
- 7.Feu F, Ruiz del Arbol L, Bañares R, Planas R, Bosch J Variceal Bleeding Study Group. Double-blind randomized controlled trial comparing terlipressin and somatostatin for acute variceal hemorrhage. Gastroenterology. 1996;111:1291–9. doi: 10.1053/gast.1996.v111.pm8898643. [DOI] [PubMed] [Google Scholar]
- 8.Escorsell A, Ruiz del Arbol L, Planas R, Albillos A, Ban˜ares R, Cale's P, et al. Multicenter randomized controlled trial of terlipressin versus sclerotherapy in the treatment of acute variceal bleeding: The TEST study. Hepatology. 2000;32:471–6. doi: 10.1053/jhep.2000.16601. [DOI] [PubMed] [Google Scholar]
- 9.Solà E, Lens S, Guevara M, Martín-Llahí M, Fagundes C, Pereira G, et al. Hyponatremia in patients treated with terlipressin for severe gastrointestinal bleeding due to portal hypertension. Hepatology. 2010;52:1783–90. doi: 10.1002/hep.23893. [DOI] [PubMed] [Google Scholar]


