Abstract
We are presenting a case of Methotrexate treated stable plaque psoriasis, in whom inflamed psoriatic plaques of drug toxicity were misdiagnosed as disease exacerbation. Erosive psoriatic plaques were present in the absence of biochemical or hematological derangements. Ulceration of psoriatic plaques in the presence of disturbed hematological profile is well described as a harbinger of methotrexate toxicity, but this kind of erosions in the absence of any systemic involvement is the first report of its kind.
KEY WORDS: Methotrexate toxicity, myelosuppression, psoriasis exacerbation
Introduction
Psoriasis is characterized by red, scaly, sharply demarcated, indurate plaques, characteristically affecting the extensors and scalp.[1] Methotrexate is the commonly prescribed oral immunosuppressive drug. It is a double-edged sword, as it is safe and effective drug if continuously monitored, but can lead to deleterious side effects on unmonitored intake. Methotrexate is an FDA approved drug for treating severe psoriasis and may be administered intravenously, intramuscularly, subcutaneously, or orally.[2] Its side effects range from trivial nonspecific to life-threatening toxic epidermal necrolysis. Rarely, ulceration within psoriatic plaques has been reported, which also could be an early harbinger of methotrexate toxicity.[2]
Case Report
A 50-year-old HIV negative male with plaque psoriasis involving 45% of the patient's total body surface area with a Psoriasis Area Severity Index (PASI) score of 17 was administered 7.5 mg methotrexate weekly orally after the recommended baseline investigations were carried out. A week later, there was increased activity of the lesions, with signs of increased inflammation. Dose of methotrexate was increased to 10 mg per week after repeating routine recommended investigations. Two weeks later patient came back with extremely painful superficial erosions of the pre-existing psoriatic plaques [Figures 1a, 2a] along with history of headache, blurring of vision, and diarrhea.
Figure 1.
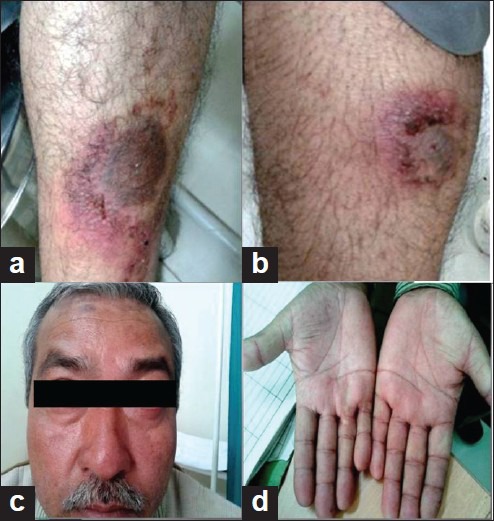
(a) Inflamed psoriatic plaques; (b) erosive psoriatic plaques; (c) facial erythema; (d) palmar erythema
Figure 2.
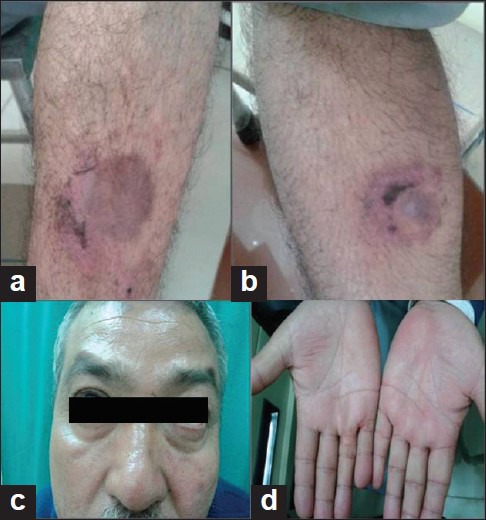
(a) Decreased inflammation 2 weeks later; (b) healed erosions; (c) resolved facial erythema; (d) disappearing palmar erythema
On examination, the patient was conscious and normotensive. Cutaneous examination revealed intense erythema and erosions over the pre-existing psoriatic plaques [Figures 1a, b], facial and palmar erythema [Figures 1c, d], and mucositis. Systemic examination was otherwise normal. Laboratory studies revealed a hemoglobin of 11.2 g/dL, red blood cell count of 4.45 lakhs/μL, mean corpuscular volume of 96 fL, white blood cell count (WBC) of 5500/μL, and platelet count of 2,60,000/μL. Serum biochemistry showed a serum creatinine level of 0.8 mg/dL, blood urea nitrogen 35.0 mg/dL, total bilirubin 0.4 mg/dL, serum glutamate oxaloacetate transaminase (SGOT) 27 IU/L, serum glutamate pyruvate transaminase (SGPT) 25 IU/L, and alkaline phosphatase 113 IU/L. Prothrombin time and partial thromboplastin time were within normal limits. Urine and stool analysis, chest X-ray, and electrocardiogram were normal. Blood culture was sterile and there was no other evidence of systemic infection. Serum concentrations of methotrexate and metabolites were not measured due to limited resources.
Though the baseline investigations at first and subsequent visits (7 and 14 days) were within normal limits, based on the clinical features, a possibility of methotrexate toxicity was considered. The patient was inappropriately self-administering 10 mg methotrexate daily orally. He was given injection leucovorin calcium 20 mg IV stat at the time of presentation. In view of normal hematological and biochemical parameters, further doses of leucovorin were not given. Further doses of methotrexate were withheld and simultaneously topical steroids (fluticasone propionate cream 0.05%) and oral antihistaminics (cetrizine) were administered, which resulted in dramatic improvement of the cutaneous lesions within 7 days and complete healing in 14 days [Figures 2a-d].
Discussion
In dermatology practice, methotrexate is mainly used for its immunomodulatory action (<30 mg/week) rather than being administered for its cytotoxic oncological doses[2] (100–250 mg/m2/week). Adverse cutaneous reactions to MTX are usually dose related and have mainly been reported either in patients receiving extremely large doses (chemotherapy) or with associated risk factors or due to inappropriate self-medication.[3]
The patient in reference was receiving only methotrexate for the treatment of psoriasis and there was no history of any other drug intake. The causality assessment by Naranjo algorithm[4] showed that this adverse drug reaction (ADR)had probable association with methotrexate. In previous studies, it was shown that ulceration of the psoriatic plaques is a widely reported sign of methotrexate toxicity but is always associated with myelosuppression.[5,6] Cutaneous signs, to start with, may present as pain and erythema of the psoriatic plaques to superficial erosions. These changes may be misdiagnosed as a flare of psoriasis or an acute episode of pustular psoriasis, both of which can lead to an increment of methotrexate dosage.
The case presented here developed painful erosions of psoriatic plaques as a result of inappropriate self-medication. Though the laboratory parameters were within normal limits, unexpectedly painful superficial erosions and other presenting symptoms suggested methotrexate toxicity. The case with psoriatic plaque erosions with normal hematological parameters is the first of its kind, as the previously reported cases were due to high methotrexate doses or had laboratory evidence of myelosuppression.
In conclusion, increased erythema of psoriatic plaques in the presence of normal laboratory profile should not always be looked upon as exacerbation of disease as it could be an indicator of methotrexate toxicity. Better communication and education of the patients about the proper method and frequency of drug intake, the adverse effect of the drug, the importance of compliance, and regular follow-up is necessary. Patients should also be cautioned that self-administration of medicine can be harmful and should be avoided. An awareness of the fact that psoriasis per se is not curable and inappropriate self-administration will not provide any additional benefits, but are likely to cause harm.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Griffiths CE, Barker JN. Psoriasis. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook's Textbook of Dermatology. 8th ed. UK: Wiley-Blackwell Publications; 2010. pp. 20.1–60. [Google Scholar]
- 2.High WA, Fitzpatrick JE. Cytotoxic and Antimetabolite agents. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology in General Medicine. 7th ed. NY: McGraw-Hill; 2008. pp. 2163–6. [Google Scholar]
- 3.Truchuelo T, Alcántara J, Moreno C, Vano-Galván S, Jaén P. Focal skin toxicity related to methotrexate sparing psoriatic plaques. Dermatol Online J. 2010;16:16. [PubMed] [Google Scholar]
- 4.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 5.Fridlington JL, Tripple JW, Reichenberg JS, Hall CS, Diven DG. Acute methotrexate toxicity seen as plaque psoriasis ulceration and necrosis: A diagnostic clue. Dermatol Online J. 2011;17:2. [PubMed] [Google Scholar]
- 6.Agarwal KK, Nath AK, Thappa DM. Methotrexate toxicity presenting as ulceration of psoriatic plaques: a report of two cases. Indian J Dermatol Venereol Leprol. 2008;74:481–4. doi: 10.4103/0378-6323.44305. [DOI] [PubMed] [Google Scholar]


