Sir,
Peptic ulcers are one of the major health problems observed by people all around the globe. Ulcers occur due to the imbalance in acid secretion and mucosal defense. Excess secretion of acid from the parietal cells is due to the hyperactivity of the gastric proton pump.[1] Recent studies on polyphenolic compounds, including flavonoids and phenolic acids, have shown diverse functions, including antioxidant, antiulcer, antihyperglycemic, and antihypertensive properties.[2] Flavonoids are one of the important groups of polyphenolic substances that are abundantly distributed in the plant kingdom and have shown their potential to act in the gastrointestinal tract as antiulcer, antispasmodic, antisecretory or antidiarrheal agents.[3] Flavonoids in different studies have been seen to inhibit the enzyme activity of histidine decarboxylase, and thus, decrease the formation of histamine in the gastric mucosa.[4] They have also shown cytoprotective effects by stimulating the mucosal content of prostaglandins and mucus in the gastric mucosa. A number of them prevent and protect the gastric mucosa against various ulcers produced in experimental models. Rutin (Vitamin P), one of the widely occurring flavonoids, is known for a plethora of pharmacological effects. Studies have shown that rutin scavenges free radicals, suppresses cellular immunity, has an anti-inflammatory effect, as well as anti-carcinogenic and antimicrobial[5,6,7,8] potential. Rutin has also demonstrated an antiulcer effect, yet its effect on the gastric proton pump is still unknown.[9] Based on the above observations, the aim of the present study was to determine the activity of rutin on the gastric enzyme H+-K+ATPase.
Tris-HCL was purchased from Sigma, Germany. Magnesium chloride (MgCl2), Potassium chloride (KCl), methanol, and adenosine triphosphate (ATP) were purchased from Loba Chemie, India. Rutin was purchased from the Central Drug House, India
Proton potassium ATPase was prepared from the mucosal scraping of goats, by the method reported by Cheon et al., with the necessary modifications. The stomach from a freshly slaughtered goat was washed gently with tap water. The mucosal layer of the fundus was scrapped and homogenized in an ice cold phosphate buffer, pH 7.4. The homogenate was centrifuged for 20 minutes at 18,000 rpm. The supernatant so obtained was recentrifuged for 60 minutes at 100,000 rpm. The pellet was re-suspended in the homogenization buffer.
Different concentrations of the extract, 10 to 50 μg / ml, were incubated in the reaction mixture (40 mM Tris-HCl buffer, pH 7.4, containing 2 mM MgCl2 and 10 μg membrane protein) to make a volume of 1 ml. Next, 2 mM ATP Tris salt was utilized to start the reaction. This preparation was incubated for 20 minutes at 37°C. The reaction was terminated by adding 1 ml of ice-cold trichloroacetic acid (10% v / v). The H+-K+ ATPase activity was assayed in the presence and the absence of different doses of the extract and omeprazole. The amount of inorganic phosphate released from ATP was determined spectrophotometrically at 400 nm.[9,10] The results were expressed as mean ± standard error of the mean. The experiments were always performed in triplicate. Statistical comparison was performed using the analysis of variance (ANOVA) followed by the Bonferroni's test (*p < 0.05).
Rutin showed significant (*p < 0.05) proton pump inhibitory activity in the goat gastric mucosal homogenate [Figure 1]. The inhibitory activity was concentration-dependent, and the results were comparable with the standard drug omeprazole. Rutin reduced the hydrolysis of ATP by the goat gastric ATPase, with IC50 of 36 μg / mL. Omeprazole (10 – 50μg / mL) used as a positive control reduced H+-K+ ATPase activity with an IC50 of 22 μg / mL.
Figure 1.
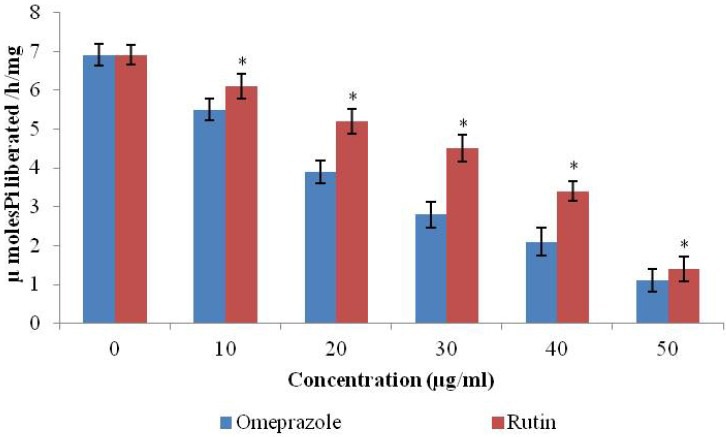
Rutin exerts antiulcer effect by inhibiting the gastric proton pump H+-K+ ATPase activity was measured with 10 – 50 μg / ml of extract and omeprazole. The experiments were always performed in triplicate. The results were expressed as the mean ± standard error of the mean. Statistical comparison was performed using the analysis of variance (ANOVA) followed by Bonferroni's test (*p < 0.05)
Various phytochemicals such as tannins, flavonoids and triterpenes from plants have earlier shown their potential to treat ulcers. Catechin and epicatechin have previously been proven as non-competitive inhibitors of H+-K+-ATPase. Gastric ulcers have been previously treated using plant polyphenols and flavonoids. It has been earlier shown in various studies that gastric damage can be defended by flavonoids. Flavonoids have proven to be excellent antioxidants; some of which are competent in enhancing prostaglandin in the mucosal content. Besides this, they safeguard the capillary integrity and reinstate the normal function of the mucus membrane. H+-K+ ATPase is a key enzyme in inducing acidity; thus in this study, the ability of rutin to inhibit H+-K+ ATPase in vitro is studied.[3,5,7,9] It is clear from the results that rutin exerts an antiulcer potential by virtue of the inhibition of the proton pump. It shows a concentrated graded response on H+-K+ ATPase against the standard drug omeprazole. It can be concluded that rutin inhibits H+-K+ ATPase, a key enzyme responsible for the secretion of acid, and thus shows antiulcer activity.
References
- 1.Konturek SJ. Gastric Cytoprotection. Scand J Gastroenterol. 1985;20:543–53. doi: 10.3109/00365528509089694. [DOI] [PubMed] [Google Scholar]
- 2.Ushida MJ, Matsui Y, Tanaka T, Matsumoto M, Hosoyama K, Mitomi H, et al. Endothelium-dependent vasorelaxation effect of 647 rutin-free tartary buckwheat extract in isolated rat thoracic aorta. J Nutr Biochem. 2008;19:700–7. doi: 10.1016/j.jnutbio.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Carlo GD, Mascolo N, lzzo AA, Capasso F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–53. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 4.Hayes NA, Foreman JC. The activity of compounds extracted from feverfew on histamine release from rat mast cells. J Pharm Pharmacol. 1987;39:466–70. doi: 10.1111/j.2042-7158.1987.tb03421.x. [DOI] [PubMed] [Google Scholar]
- 5.Kandaswami C, Middleton E. Free radical scavenging and antioxidant activity of plant flavonoids. Adv Exp Med Biol. 1994;366:351–76. doi: 10.1007/978-1-4615-1833-4_25. [DOI] [PubMed] [Google Scholar]
- 6.Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 7.Rotelli AE, Guardia T, Juárez AO, de la Rocha NE, Pelzer LE. Comparative study of flavonoids in experimental models of inflammation. Pharmacol Res. 2003;48:601–6. doi: 10.1016/s1043-6618(03)00225-1. [DOI] [PubMed] [Google Scholar]
- 8.Deschner EE, Ruperto J, Wong G, Newmark HL. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 1991;12:1193–6. doi: 10.1093/carcin/12.7.1193. [DOI] [PubMed] [Google Scholar]
- 9.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Yadav P, Ganeshpurkar A, Rai G. In vitro H+ -K+ ATPase inhibitory potential of methanolic extract of Cissus quadrangularis Linn. Pharmacogn Res. 2012;4:123–6. doi: 10.4103/0974-8490.94738. [DOI] [PMC free article] [PubMed] [Google Scholar]


