Sir,
Second-generation antipsychotics are known to be associated with higher incidence of metabolic syndrome (MS).[1] Withania somnifera (WS) is a herb in the Ayurvedic system of medicine and has been found to decrease blood glucose comparable to an oral hypoglycemic.[2] A significant increase in urine sodium and urine volume, and a significant decrease in serum cholesterol, triglycerides, low-density lipoproteins, and very low density lipoproteins have been reported with use of WS, indicating its potential use as a diuretic and hypocholesterolemic agent.[3] Hence, a trial was planned with an objective to study its metabolic effects in schizophrenia patients receiving antipsychotic therapy and suffering from MS.
A randomized, double blind, placebo controlled, clinical trial was conducted in psychiatry OPD of a tertiary care teaching hospital after approval of Institutional Ethics Committee. Schizophrenia patients receiving second-generation antipsychotics for 6 months or more, having serum triglycerides more than 150 mg/dl, high-density lipoprotein (HDL) cholesterol less than 40 mg/dl in men and less than 50 mg/dl in women, fasting blood glucose(FBG) level more than 100 mg/dl, aged above 18 years were included. Patients suffering from other psychiatric/systemic illnesses, receiving concurrent medicines, pregnant/lactating women were excluded. Patients were briefed about the study and written informed consent was obtained from those willing to participate. Baseline investigations (routine hematological, liver and kidney function tests, FBG, serum triglycerides, HDL cholesterol) were performed. Baseline blood pressure and weight were recorded.
Selected patients were randomly allocated by SAS systems of Windows to two groups of 15 each, to receive either Withania somnifera extract (WSE) [Cap Strelaxin, manufactured by M/s Pharmanza Herbal Pvt. Ltd. Gujarat, India] containing 400 mg of WSE per capsule or matching placebo given in a dose of one capsule thrice daily for 1 month. Patients were informed to report in the case of any adverse effect. After 1 month of treatment, FBG, serum triglycerides, and HDL cholesterol were repeated. Comparison of baseline and post-treatment values was done by paired ‘t’ test and unpaired ‘t’ test was used to compare results of the two groups. Data was analyzed by using Graph pad Prism version 4.03 software. P<0.05 was considered statistically significant.
Out of 30 patients, 3 from WS and 2 from the placebo group were lost to follow-up. No change in body weight and blood pressure was observed after 1 month of treatment in both groups.
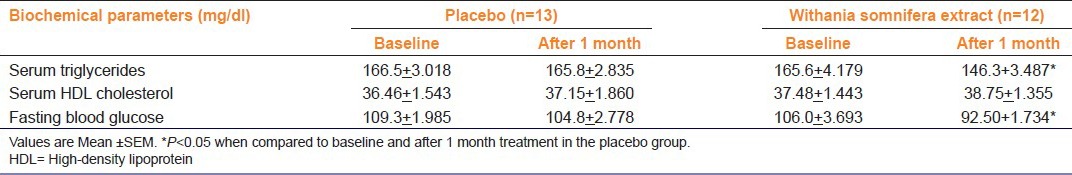
Demographic characteristics were comparable in the two treatment groups. No change in all three biochemical parameters was found after 1 month of treatment in the placebo group [Table 1]. However, a statistically significant (P<0.05) reduction in serum triglycerides and FBG was observed after 1 month of WS treatment compared to the placebo group. Patients of both groups reported feeling of isolation and depression.
Table 1.
Comparison of serum triglycerides, HDL cholesterol, and fasting blood glucose in patients treated with Withania somnifera
Hypolipidemic and hypoglycemic actions of WS have been reported in experimental studies[3] and in clinical trials after 1 month of treatment.[2] A clinical trial in patients of noninsulin-dependent diabetes mellitus with hypercholesterolemia reported a decrease in blood glucose with WS being comparable to that of an oral hypoglycemic.[2] Bhattacharya et al. have suggested that WS has an antioxidant effect which may be responsible for its diverse pharmacological properties.[4]
Hypoglycemic and hypolipidaemic effects of WS observed in this trial are significant and deserve to be explored further. The results of this trial, if confirmed through large clinical trials, will certainly aid the therapeutics not only for patients having drug-induced MS but also for those having MS due to other causes. Symptoms like feeling of isolation and depression reported in this trial are known to occur during the natural course of schizophrenia. No ADR attributable to WSE was reported. Further studies are required to establish the efficacy and safety of WS for patients suffering from MS.
Acknowledgments
We thank the Indian Council of Medical Research for providing financial support for this trial. We are grateful to Dr. Lal Hingorani Pharmanza, Gujrat (India) for providing trial medicines.
References
- 1.Jacob R, Chowdhury AN. Metabolic comorbidity in schizophrenia. Indian J Med Sci. 2008;62:23–31. [PubMed] [Google Scholar]
- 2.Andallu B, Radhika B. Hypoglycemic, diuretic and hypocholesterolemic effect of Winter cherry (Withania somnifera, Dunal) root. Indian J Exp Biol. 2000;38:607–9. [PubMed] [Google Scholar]
- 3.Visavadiya NP, Narasimhacharya AV. Hypocholesteromic and antioxidant effects of Withania somnifera (Dunal) in hypercholesteremic rats. Phytomedicine. 2007;14:136–42. doi: 10.1016/j.phymed.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya SK, Satyan KS, Chakrabarti A. Effect of Trasina, an Ayurvedic herbal formulation on pancreatic islet superoxide dismutase activity in hyperglycemic rats. Indian J Exp Biol. 1999;35:297–9. [PubMed] [Google Scholar]


