Abstract
Ghrelin, a circulating gut-hormone, has emerged as an important regulator of growth hormone release and appetite. Ghrelin-immunopositive neurons have also been identified in the hypothalamus with a unique anatomical distribution. Here, we report that ghrelin-labeled neurons receive direct synaptic input from the suprachiasmatic nucleus, the central circadian timekeeper of the brain, and lateral geniculate nucleus, a visual center, and project synaptically to the lateral hypothalamic orexin/hypocretin system, a region of the brain critical for arousal. Hypothalamic ghrelin mRNA oscillates in a circadian pattern peaking in the dark phase prior to the switch from arousal to sleep. Ghrelin inhibits the electrophysiological activity of identified orexin/hypocretin neurons in hypothalamic slices. These observations indicate that the hypothalamic neurons identified by ghrelin immunolabeling may be a key mediator of circadian and visual cues for the hypothalamic arousal system.
Keywords: Hypothalamus, Ghrelin, Circadian rhythm, Arousal, Lateral hypothalamus
Introduction
Ghrelin is an endogenous ligand of the growth hormone secretagogue receptor (GHS-R; [15]) that affects growth hormone release as well as appetite and energy expenditure [15,28,32,22]. Ghrelin is produced in the stomach and was also reported in the hypothalamus [15,28,32,22,6], sites that are consistent for the involvement of this peptide in the central regulation of both growth hormone release and energy homeostasis [15,12,13,6]. While it remains controversial, the hypothalamic distribution of ghrelin-immunolabeled neurons. Cowley et al. [6] argued for a diverse function of this population of distinct neurons. In particular, the observation that ghrelin-immunopositive neurons occupy a cell-sparse zone between distinct hypothalamic areas, including the paraventricular-, ventromedial, arcuate, and dorsomedial nuclei and the lateral hypothalamus, suggests that these cells may play a role in synchronizing the activity of these key brain sites in homeostatic, endocrine and autonomic regulation.
Synchronization of brain functions occurs at different temporal scales. One of the key entrainments of central and peripheral mechanisms is that coordinated to the daily or circadian rhythm. The master clock that generates circadian rhythms is located in the hypothalamic suprachiasmatic nucleus (SCN; [20]). While this hypothalamic structure generates circadian rhythms, it is affected by light, which serves as a dominant zeitgeber entraining diverse neuronal clocks to geophysical time. The SCN has an intrinsic rhythm approximately 24 h in length and receives direct retinal projections and indirect visual input from the intergeniculate leaflet (IGL) of the lateral geniculate nucleus (LGN) of the thalamus ([25,19,20,24,4,17]. It is thought that the SCN conveys circadian information to the rest of the brain via neuronal projections ([25,19,20,24,4,17]. The main target site of direct SCN efferents is the hypothalamic sub-paraventricular zone (Sub-PVN; [29,9]) that also receives visual input from the thalamus [10]. Lesions of the SubPVN eliminate many circadian rhythms that originate in the SCN indicating that the Sub-PVN is a key relay station for the integration of circadian/visual signals to brain function [30,18,21]. Strikingly, the Sub-PVN overlaps the location of the brain where ghrelin-producing neurons are found, thus, raising the possibility that the ghrelin producing neurons integrate circadian-visual signals for the regulation of other hypothalamic regions [6,7]. The present study investigated this proposition by determining afferent and efferent projections of the ghrelin-immunopositive neurons and their effect on electrophysiological properties of postsynaptic targets.
Materials and methods
The Institutional Animal Care and Use Committee at Yale University approved all of the procedures on animals described here.
Track-tracing and light and electron microscopic immunolabeling
SCN and LGN efferents were traced with Phaseolus vulgaris leucoagglutinin (PHA-L) in rats (250 g male Sprague-Dawley rats, Charles River; n=8). This anterograde tracer was injected iontophoretically into the SCN and LGN as described by Horvath [9,10]. Multiple labeling light and electron microscopic visualizations of tissue antigens were done using either different color and electro dense immunoperoxidase reactions, double immunofluorescence, or the use of the combination of immunoperoxidase and immunogold. Immunogold labeling was done either before embedding for electron microscopic analysis [11] or post-embedding on ultrathin sections as described earlier [6]. The antisera against PHA-L, ghrelin and orexin/hypocretin and their controls are described elsewhere [9–11, 16].
Real time PCR analysis of ghrelin mRNA in the hypothalamus
Adult male C57BL/6J mice were maintained on light:dark (LD) 12 h:12 h cycle for 2 weeks and then sacrificed every 6 h beginning at ZT 0 (defined as lights on) for 24 h. The medial basal hypothalamus was dissected rapidly and frozen on dry ice (n=3 at each time point). Total RNA was extracted from the frozen sample with Tri reagent diluted to 0.1 mg/ml, and used in TaqMan real-time EZ RT-PCR (Applied Biosystems, Foster City, CA). Transcript analysis was determined using the comparative amplification detection threshold of target gene expression (CT) on an ABI 7700 Sequence Detector and mRNA relative abundance (2−ΔΔCT) was determined by normalization to GAPDH mRNA levels (ΔCT) and comparison to the average normalized level (ΔΔCT). Probe and primer sets were designed with Primer Express (Applied Biosystems) with forward and reverse primers located in different exons.
Electrophysiology
Whole cell recordings were made from hypocretin/orexin neurons identified by GFP expression under control of the hypocretin promoter ([16]; from Dr. T. Sakurai). The bath solution consisted of (in mM): NaCl, 124; KCl, 3; CaCl2, 2; MgCl2, 2; NaH2PO4, 1.23; NaHCO3, 26; glucose, 10; pH, 7.4 with NaOH, and was continuously bubbled with 5% CO2 and 95% O2. The pipette solution contained (in mM): KMeSO4, 145; MgCl2, 1; Hepes, 10; EGTA, 1.1, Mg-ATP, 2; Na2-GTP, 0.5, pH 7.3 with KOH. In current clamp, neurons were held at resting membrane potential, generally near −60 mV. A Heka EPC9 amplifier was used on an upright Olympus BX51WI microscope with IR-DIC optics and a FITC filter cube to detect GFP-expressing hypocretin/orexin neurons.
Results
SCN and IGL innervation of hypothalamic ghrelin-immunopositive cells
To determine whether circadian and secondary visual projections innervate ghrelin-immunopositive neurons, the anterograde tracer, P. vulgaris leucoagglutinin (PHA-L), was injected into either the SCN or the IGL of the LGN (Fig. 1a and Fig. 2b). Both of these regions gave rise to hypothalamic projections corresponding to earlier descriptions [29,10]. As we reported previously [10], SCN and IGL efferents project with an overlapping distribution to the hypothalamus, with the most extensive SCN and IGL terminals present in the Sub-PVN (Fig. 1a and c). The same area contains the majority of ghrelin-immunoreactive neurons (Fig. 3a). Axon terminals originating in both the SCN and LGN were in close proximity to ghrelin perikarya (Figs. 1 and 2). Electron microscopic analysis revealed that SCN and IGL efferents established symmetrical synaptic contacts on ghrelin-immunoreactive cell bodies and dendrites (Figs. 1 and 2).
Fig. 1.
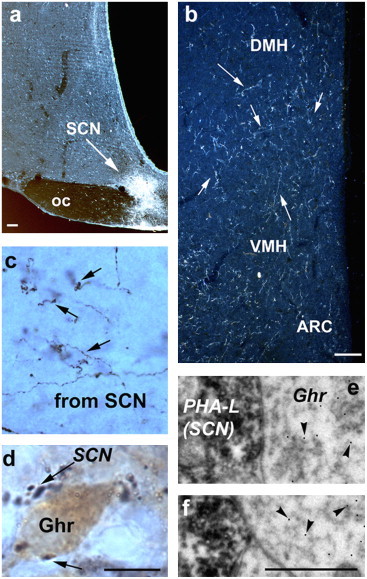
SCN innervation of ghrelin neurons. (a) The anterograde tracer, PHA-L, was injected into the SCN (bar scale represents 100 μm). (b) PHA-L-labeled SCN efferents were abundant in the cell-sparse area of the hypothalamus an area that also contained ghrelin-immunopositive neurons (see Fig. 3a). Bar scale represents 100 μm. (c–d) SCN efferents in this area arborized into putative axon terminals (arrows on c) that were frequently in direct apposition (arrows on d) to ghrelin-immunoreactive cell bodies (bar scale represents 10 μm). (e–f) Electron microscopic analyses of putative contacts showed symmetrical synaptic contacts between PHA-L-labeled, SCN efferents and immunogold labeled (arrowheads) ghrelin perikarya (bar scale on f represents 1 μm for both e and f). Ghrelin-immunopositive neurons are distributed in a continuum between the PVN, ARC, DMH and LH. These are targeted by both SCN and LGN efferents as revealed by anterograde tracing (see panels of a and c). III: third ventricle; bar scale on b represents 100 μm. oc: optic chiasm; VMH: ventromedial hypothalamic nucleus; DMH: dorsomedial hypothalamic nucleus; ARC: arcuate nucleus.
Fig. 2.
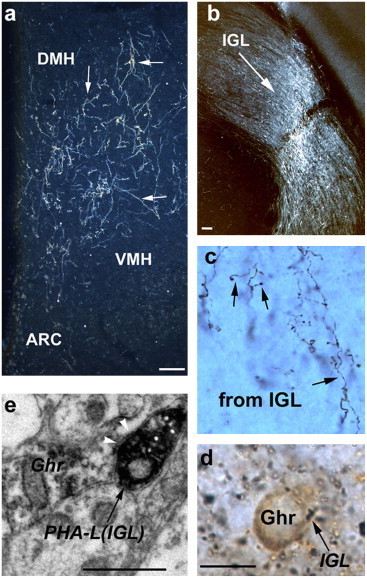
IGL innervation of ghrelin neurons. a–b. The anterograde tracer, PHA-L, was also injected into the IGL (b; bar scale represents 100 μm) of the thalamic LGN. PHA-L-labeled IGL efferents were equally represented in the cell-sparse area of the hypothalamus as were SCN fibers (a; bar scale represents 100 μm). c–d. IGL efferents in this area arborized into putative axon terminals (arrows on c) that were frequently in direct apposition (arrows point to dark boutons on d) to ghrelin-immunoreactive (light brown labeling) cell bodies (bar scale represents 10 μm). e. Electron microscopic analyzes of putative contacts showed symmetrical synaptic contacts between PHA-L-labeled, IGL efferents and immunogold labeled (arrowheads) ghrelin perikarya (bar scale represents 1 μm). oc: optic chiasm; VMH: ventromedial hypothalamic nucleus; DMH: dorsomedial hypothalamic nucleus; ARC: arcuate nucleus.
Fig. 3.
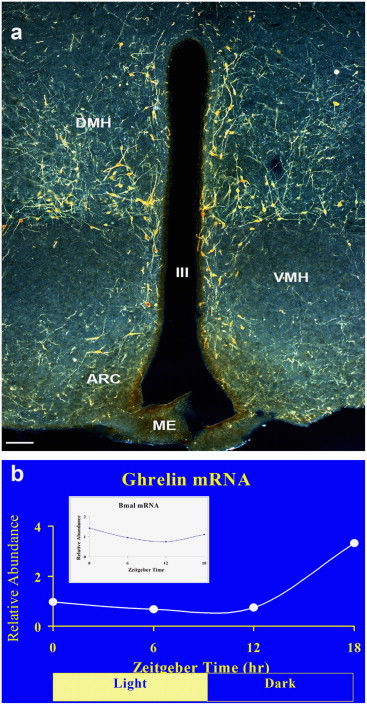
Circadian oscillation of hypothalamic ghrelin mRNA. (a) Distribution of ghrelin-immunoreactive neurons in the hypothalamus. (b) Measurement of the relative abundance of ghrelin mRNA in the hypothalamus shows circadian rhytmicity in parallel with the circadian expression pattern of the mRNA of BMAL.
Circadian oscillation of ghrelin mRNA in the hypothalamus
We analyzed ghrelin mRNA expression in the hypothalamus throughout the 24-h day using real time RT-PCR . This analysis confirmed that ghrelin mRNA is expressed within the hypothalamus ([15,28,32]; Nakasato et al., 2001; [6]). Its expression increased greater than 2.5-fold during the dark period at ZT 18, in parallel with an increase in the expression of Bmal1 (Fig. 3b), a basic helix loop helix transcription factor that is a core component of the intrinsic circadian clock [3].
Ghrelin-immunolabelled axons innervate orexin neurons
One of the areas of the brain that is most heavily targeted by ghrelin-immunoreactive projections is the lateral hypothalamus-perifornical region (Fig. 4a and b). Thus, we sought to determine whether ghrelin-labeled projections innervate lateral hypothalamic orexin/hypocretin neurons. Ghrelin-immunopositive fibers were frequently found to be in close apposition to orexin cell bodies and dendrites (Fig. 4c and d). Electron microscopy revealed that the synaptic membrane specializations of these contacts were symmetrical (Fig. 4e and f), typical of inhibitory synapses.
Fig. 4.
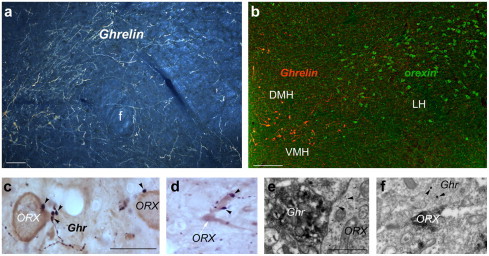
(a) Ghrelin-immunolabeled efferents are abundant in the perifornical region. Bar scale represents 100 μm. (b) Ghrelin-immunoreactive neurons (red fluorescence) are distinct from the lateral hypothalamic orexin/hypocretin neurons (green fluorescence). Bar scale represents 100 μm. (c) In the lateral hypothalamus-perifornical region, ghrelin-immunolabeled axon terminals (arrowheads) are in close proximity to orexin/hypocretin-producing perikarya. Bar scale represents 10 μm. (d) Ghrelin-immunopositive boutons (arrowheads) were also associated with orexin/hypocretin dendrites. (e) Electron micrograph showing direct apposition between a ghrelin-immunoreactive axon terminal and an orexin/hypocretin-labeled (arrowheads point to immunogold) perikaryon. Bar scale represents 1 μm. (f) Electron micrograph showing direct apposition between a ghrelin-immunoreactive axon terminal (arrowheads point to large immunogold particles representing ghrelin immunoreactivity with postembedding labeling) and an orexin/hypocretin-labeled (immunoperoxidase) perikaryon. Bar scale represents 1 μm.
Ghrelin inhibits the activity of orexin neurons
We analyzed the effect of ghrelin on the electrophysiological properties of orexin/hypocretin neurons (Fig. 5). This analysis was performed on slices of transgenic mice in which orexin/hypocretin neurons are selectively labeled with green fluorescence protein (GFP; [16]). Ghrelin was applied by flow pipette to mouse hypothalamic slices. In current clamp, the frequency of action potentials was substantially decreased by ghrelin (0.5 μM) by 72±8% (from mean 2.3±0.2 Hz SEM; range 1.3 Hz–3 Hz) to 0.7 Hz±0.2 Hz; (range from 0 to 1.7 Hz; n=11, P<0.01) (Fig. 5a). After washout, spike frequency recovered to 1.9±0.1 Hz (range 1–2 .7 Hz). Ghrelin (0.5 μM) hyperpolarized the membrane potential from −61±0.7 mV (range −57 mV to 65 mV) to −66±1.3 mV (range from −59 to −75 mV, n=11, P<0.05), and this recovered to −62±1 mV after washout. Lower concentrations of ghrelin (100 nM) evoked a more modest inhibition of spikes (from 2.6±0.3 Hz to 1.9±0.1 Hz, mean decrease 25±5%, P<0.05) and hyperpolarized the membrane potential from −62±2.4 mV to −65±2.1 mV (n=6). To test whether ghrelin had a direct effect on hypocretin neurons, input resistance, membrane potential, and current voltage relations were tested in the presence of 0.5 μM tetrodotoxin to block spike-dependent synaptic activity; no detectable effect on these parameters was identified (n=6) (Fig. 5c). We then tested the hypothesis that ghrelin might exert an inhibitory effect by attenuating excitatory synaptic input to hypocretin cells. Ghrelin 0.5 μM decreased the frequency of spontaneous EPSCs (mean decrease to 72±9.4%, range 41–95 %, n=5, P<0.05) and recovered to 86±5.2% (Fig. 5d). To test whether ghrelin might act presynaptically, we studied miniature EPSCs recorded from hypocretin neurons. Ghrelin decreased the frequency of mEPSCs (mean decrease to 66±11%, range 26–91 , n=5, P<0.05), which recovered to 85±6.9% after wash out, but had no effect on the cumulative probability distribution (Fig. 5e), suggesting ghrelin induced a presynaptic inhibition of glutamate release onto hypocretin neurons.
Fig. 5.
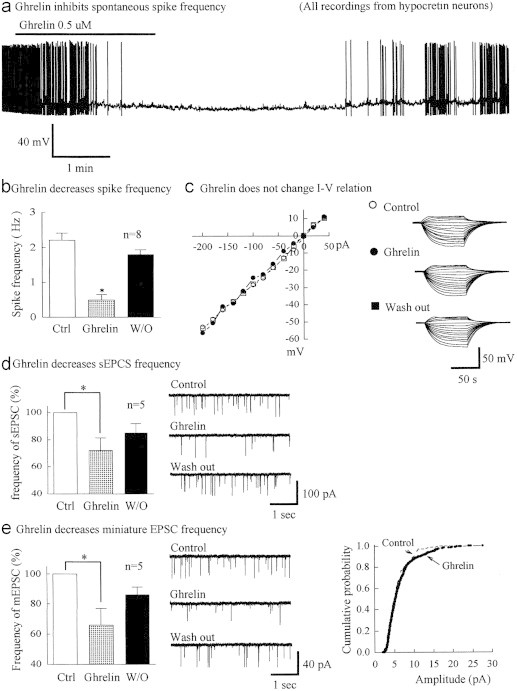
(a) Application of ghrelin (0.5 μM) blocks spikes in this hypocretin/orexin neuron. (b) Bar graph showing ghrelin reduces spike frequency (⁎p<0.05). (c) No detectable effect of ghrelin (0.5 μM) on current voltage relation, with representative traces on right. (d) Ghrelin reduced spontaneous sEPSC frequency in the presence of the GABA-A receptor antagonist bicuculline (30 μM). (e) In tetrodotoxin (0.5 μM+30 μM bicuculline), ghrelin (0.5 μM) reduced frequency of miniature EPSCs, with little effect on cumulative probability distribution.
Discussion
The current anatomical observations established the hypothalamic ghrelin-immunolabeled neuronal population as a main target of circadian (SCN) efferents and secondary visual afferents (IGL). The convergence of SCN and IGL efferents onto hypothalamic ghrelin-immunoreactive neurons and circadian oscillation of hypothalamic ghrelin mRNA suggest that these neurons may couple (by their projections) light-dark cycle-entrained circadian rhythms with hypothalamic processes, including the regulation of sleep/wake cycles. Indeed, we and others [27] found that a major efferent projection of hypothalamic ghrelin-immunoreactive neurons is onto lateral hypothalamic orexin cells. While the region including the subparaventricular zone and dorsomedial nucleus, where ghrelin-labeled neurons are located, are the main hypothalamic projection fields of both the SCN and the thalamic ventral lateral geniculate body [2,29,10], the SCN was shown to send direct projections to lateral hypothalamic orexin neurons as well as in Ref. [1] suggesting a complex anatomical circuitry for circadian regulation for arousal.
The orexin/hypocretin system is a dominant regulator of sleep/wake cycles [5,14] of which activation is critical for arousal. Our physiological observations showed a robust suppression of orexin neuronal activity by ghrelin with a presynaptic mode of action. This latter observation is consistent with our previous ghrelin binding results that showed predominant association of labeled ghrelin with presynaptic terminals rather than postsynaptic membranes in the lateral hypothalamus [6]. Based primarily on isolated neurons, Yamanaka et al. [33] suggested that ghrelin have excitatory actions on isolated hypocretin cells. As the 27 hypocretin neurons tested in the present study in hypothalamic slices showed a consistent ghrelin mediated inhibition, as evidenced by a decrease in spike frequency, membrane hyperpolarization, and decrease in excitatory synaptic input, it us unclear whether the difference between the present work and the other work on ghrelin is based on acutely dissociated cells vs. slices, differences in ghrelin synthesis, neuronal state, or some other as yet undetermined factor. Other transmitters or modulators appeared to have similar action in slices and cultured hypocretin neurons [33,16].
The observed inhibitory tone of ghrelin on orexins neurons corresponds to ghrelin's suppression of locomotor activity [26], and, it is also in line with the observation that ghrelin promotes slow-wave sleep [31]. In this regard, it was intriguing to note that the suppression of locomotor activity and the induction of feeding by ghrelin does not occur in parallel: feeding was increased at the beginning of the dark-phase while the decrease in locomotor activity only became apparent 4 h into the dark-phase, which then remained suppressed throughout the 48 h observation period [26]. Thus, it may be that the induction of food intake and suppression of locomotor activity by ghrelin take place concurrently but by independent hypothalamic signaling pathways. This notion is further supported by the work of Funato et al. [8], which showed orexin/hypocretin signaling via orexin receptor 2 promotes suppression of feeding.
Orexin/hypocretin neurons project heavily to a number of other regions of the brain that also promote arousal, including the locus coeruleus, dorsal raphe, dorsal tegmentum, where orexin/hypocretin enhances neuronal activity [11,16]. An elegant study by Aston-Jones et al. [2] revealed that the dorsomedial nucleus of the hypothalamus, both anatomically and physiologically is critical for the circadian regulation of sleep-wake cycles giving further support for ghrelin's mediatory role in this process.
In conclusion, the data presented here establishes a hypothalamic neuronal population immunolabelled for ghrelin as a major recipient of circadian and secondary visual projections in the subparaventricular zone of the hypothalamus, the expression of which shows circadian rhythmicity. This group of cells, in turn, are linked to the lateral hypothalamic orexin/hypocretin system (Fig. 6). The activity of this critical arousal promoting neuronal population is suppressed by ghrelin in slices and this is likely to be the mechanism of action by which central ghrelin suppresses locomotor activity. Because ghrelin mRNA production was found to peak in the second part of the dark phase in nocturnal mice, central ghrelin may be key in transitioning from arousal to sleep. This is in line with the observed effect of ghrelin in promoting slow-wave sleep in humans [31] and establishes a novel function of brain ghrelin that may be independent from the previously described influences of this peptide on food intake and growth hormone release.
Fig. 6.
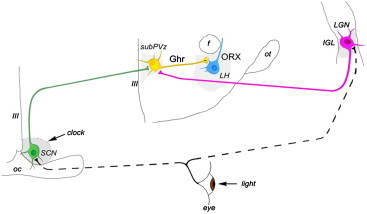
Schematic illustration of the observations of the present study: efferents of the master clock (clock) located in the hypothalamic SCN (green) and of the LGN (purple), both of which receive direct visual input from the retina, target ghrelin-immunolabeled neurons (yellow) in the subparaventricular zone (subPVz). Ghrelin neurons, in turn, project onto the lateral hypothalamic orexin/hypocretin neurons of which activity is suppressed by ghrelin. Because ghrelin mRNA shows circadian oscillation and peaks in the second part of the dark phase, and ghrelin suppresses orexin/hypocretin neuronal activity and locomotor behavior, hypothalamic ghrelin-immunopositive neurons or circulating ghrelin may be critical in transitioning from awake state to sleep.
Conflict of interest
None declared.
Acknowledgments
This work was supported NIH grants DP1 DK006850 to TLH and R01 DK 090625 to JB.
References
- 1.Abrahamson E.E., Leak R.K., Moore R.Y. The suprachiasmatic nucleus projects to posterior hypothalamic arousal systems. Neuroreport. 2001;12:435–440. doi: 10.1097/00001756-200102120-00048. [DOI] [PubMed] [Google Scholar]
- 2.Aston-Jones G., Chen S., Zhu Y., Oshinsky M.L. A neural circuit for circadian regulation of arousal. Nature Neuroscience. 2001;4:732–738. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- 3.Bunger M.K., Wilsbacher L.D., Moran S.M., Clendenin C., Radcliffe L.A., Hogenesch J.B. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009–1017. doi: 10.1016/s0092-8674(00)00205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Card J.P., Moore R.Y. Organization of lateral geniculate-hypothalamic connections in the rat. Journal of Comparative Neurology. 1989;284:135–147. doi: 10.1002/cne.902840110. [DOI] [PubMed] [Google Scholar]
- 5.Chemelli R.M., Willie J.T., Sinton C.M., Elmquist J.K., Scammell T., Lee C. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 6.Cowley M.A., Smith R.G., Diano S., Tschop M., Pronchuk N., Grove K.L. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 7.Dallman M.F. Filling the interstices: ghrelin neurons plug several holes in regulation of energy balance. Neuron. 2003;37:550–553. doi: 10.1016/s0896-6273(03)00083-7. [DOI] [PubMed] [Google Scholar]
- 8.Funato H., Tsai A.L., Willie J.T., Kisanuki Y., Williams S.C., Sakurai T. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metabolism. 2009;9(1):64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvath T.L. Suprachiasmatic efferents avoid phenestrated capillaries but innervate neuroendocrine cells including those producing dopamine. Endocrinology. 1997;138:1312–1320. doi: 10.1210/endo.138.3.4976. [DOI] [PubMed] [Google Scholar]
- 10.Horvath T.L. An alternate pathway for visual signal integration into the hypothalamo-pituitary axis: retinorecipient intergeniculate neurons project to various regions of the hypothalamus and innervate neuroendocrine cells including those producing dopamine. The Journal of Neuroscience. 1998;18:1546–1558. doi: 10.1523/JNEUROSCI.18-04-01546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath T.L., Peyron C., Diano S., Ivanov A., Aston-Jones G., Kilduff T.S. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. Journal of Comparative Neurology. 1999;415:145–159. [PubMed] [Google Scholar]
- 12.Horvath T.L., Diano S., Sotonyi P., Heiman M., Tschop M. Minireview: ghrelin and the regulation of energy balance—a hypothalamic perspective. Endocrinology. 2001;142:4163–4169. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 13.Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nature Reviews Neuroscience. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- 14.Kiyashchenko L.I., Mileykovskiy B.Y., Maidment N., Lam H.A., Wu M.F., John J. Release of hypocretin (orexin) during waking and sleep states. Journal of Neuroscience. 2002;22:5282–5286. doi: 10.1523/JNEUROSCI.22-13-05282.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojima M., Hosoda H., Date Y., Nakazato M., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 16.Li Y., Gao X.B., Sakurai T., van den Pol A.N. Hypocretin/orexin excites hypocretin neurons via a local glutamate neuron a potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 17.Low-Zeddies S.S., Takahashi J.S. Chimera analysis of the clock mutation in mice shows that complex cellular integration determines circadian behavior. Cell. 2001;105:25–42. doi: 10.1016/s0092-8674(01)00294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J., Zhang Y.H., Chou T.C., Gaus S.E., Elmquist J.K., Shiromani P. Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. Journal of Neuroscience. 2001;21:4864–4874. doi: 10.1523/JNEUROSCI.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore R.Y., Lenn N.J. A retinohypothalamic projection in the rat. Journal of Comparative Neurology. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 20.Moore R.Y. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Federation Proceedings. 1983;42:2783–2789. [PubMed] [Google Scholar]
- 21.Moore R.Y., Danchenko R.L. Paraventricular-subparaventricular hypothalamic lesions selectively affect circadian function. Chronobiology International. 2002;19:345–360. doi: 10.1081/cbi-120002876. [DOI] [PubMed] [Google Scholar]
- 22.Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kanga K. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 24.Pickard G.E. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neuroscience Letters. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- 25.Sousa-Pinto A. Electron microscopic observations on the possible retinohypothalamic projection in the rat. Experimental Brain Research. 1970;11:528–538. doi: 10.1007/BF00233973. [DOI] [PubMed] [Google Scholar]
- 26.Tang-Christensen M., Vrang N., Ortmann S., Bidlingmaier M., Horvath T.L., Tschöp M. Central administration of ghrelin and agouti-related protein (83–132) increases food intake and decreases spontaneous locomotor activity in rats. Endocrinology. 2004;145(10):4645–4652. doi: 10.1210/en.2004-0529. [DOI] [PubMed] [Google Scholar]
- 27.Toshinai K., Date Y., Murakami N., Shimada M., Mondal M.S., Shimbara T. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinology. 2003;144:1506–1512. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- 28.Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 29.Watts A.G., Swanson L.W., Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. Journal of Comparative Neurology. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 30.Watts A.G., Sheward W.J., Whale D., Fink G. The effects of knife cuts in the sub-paraventricular zone of the female rat hypothalamus on oestrogen-induced diurnal surges of plasma prolactin and LH, and circadian wheel-running activity. Journal of Endocrinology. 1989;122:593–604. doi: 10.1677/joe.0.1220593. [DOI] [PubMed] [Google Scholar]
- 31.Weikel J.C., Wichniak A., Ising M., Brunner H., Friess E., Held K. Ghrelin promotes slow-wave sleep in humans. American Journal of Physiology Endocrinology and Metabolism. 2003;284:E407–E415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- 32.Wren A.M., Small C.J., Ward H.L., Murphy K.G., Dakin C.L., Taheri S. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 33.Yamanaka A., Beuckmann C.T., Willie J.T., Hara J., Tsujino N., Mieda M. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]


