Abstract
Researchers analyse hormones to draw conclusions from changes in hormone concentrations observed under specific physiological conditions and to elucidate mechanisms underlying their biological variability. It is, however, frequently overlooked that also circumstances occurring after collection of biological samples can significantly affect the hormone concentrations measured, owing to analytical and pre-analytical variability. Whereas the awareness for such potential confounders is increasing in human laboratory medicine, there is sometimes limited consensus about the control of these factors in rodent studies. In this guide, we demonstrate how such factors can affect reliability and consequent interpretation of the data from immunoassay measurements of circulating metabolic hormones in rodent studies. We also compare the knowledge about such factors in rodent studies to recent recommendations established for biomarker studies in humans and give specific practical recommendations for the control of pre-analytical conditions in metabolic studies in rodents.
Keywords: Pre-analytical variability, Sample processing, Immunoassay, Hormone measurement, Rat, Mouse
1. Introduction
The concentration of any analyte in biological samples – regardless of how it is measured – is determined by three factors: the biological, the analytical and the pre-analytical variability. In the context of this guide, “pre-analytical variability” refers to changes in the apparent concentration of an analyte which can be attributed to the period between taking a sample and subjecting the sample to the analysis (i.e., to changes occurring ex vivo). Obviously, investigators are usually most interested in the biological variability when measuring analytes. In this respect, focus of the analysis is to compare quantitative results from a measurement of an analyte to the concentrations seen in a reference population, in other individuals or during different experimental conditions. Besides their interest in biological variability – which in most cases is part of the physiological mechanisms to be investigated – many researches are also aware of the fact that the analytical variability impacts upon the results. Measurement differences due to different methods, different operators or different labs are recognized, although often regarded as an inevitable, but overall less important source of variability. Typically, pre-analytical variability is considered to be of even minor importance, and to a certain extent the awareness for potential problems deriving from this source of variability is lacking (Fig. 1), especially outside the context of human laboratory medicine. This is of concern, because problems during the pre-analytic phase of laboratory testing have been demonstrated to account for up to 70% of the total error occurring during the biochemical analysis of human samples [1–3]. Over the past decade, the awareness for analytical and pre-analytical variability has increased in human laboratory medicine, and its importance for laboratory testing has been not only recognized in clinical routine, but also in clinical studies [4,5]. It is very unlikely that pre-analytical variability does have less impact in basic science involving animals. Most likely, the same sources of variability are present and relevant when measuring circulating metabolic factors in animal studies, but the awareness for potential confounders is much less developed.
Fig. 1.
Variables determining the measurement of circulating analytes in biological fluids.
Accordingly, this guide is intended to raise the awareness for the factor “pre-analytical variability” especially in rodent studies. We will provide examples how pre-analytical conditions can influence measurement results of several circulating metabolic hormones and give practical recommendations how to standardize and control pre-analytical conditions.
2. Biological, analytical and pre-analytical variability
2.1. Biological variability
In most biomedical studies, one or more components of the biological variability are the “variable of interest” to be addressed. In metabolic studies, the investigation of the impact of age, gender and genetic modifications, but also the impact of food intake or pharmacological interventions on circulating concentrations of hormones represent good examples for biological variability. In order to obtain meaningful experimental results for the biological variability of interest, most researchers know about the importance to control as many components of unintended biological variability. Appropriate control of study subjects and environmental conditions is difficult in humans, especially with longer observation periods. In this respect, animal studies provide the advantage of easier control of biological variability: appropriate control of factors like genetic background, but also housing conditions, environmental temperature, humidity or adherence to a diet and scheduling of sampling time points with respect to circadian or long-term biological rhythms are possible and therefore considered standards of good scientific practice. As examples, one might think about the many rodent studies describing significant effects of e.g., age and gender on metabolic hormone concentrations [6–9]. It is important to keep in mind that these factors may be variables influencing the results although they were not necessarily planned to be part of the experiment.
2.2. Analytical variability
The analytical method chosen for determination of circulating metabolic factors can greatly affect the readout of hormone analyses. Apart from differences seen between results from different analytical methods (e.g., mass spectrometry vs. immunoassay), also different types of the same method (competitive vs. sandwich type immunoassays, immunoassays involving different antibodies, etc.) can lead to different results. Furthermore, any method itself has inherent analytical variability caused by systematic and random errors.
Immunoassays still are the most frequently used method to determine circulating hormone concentrations in scientific studies. As an example to illustrate differences between results from different immunoassays available to measure the same hormone in rodent samples, Fig. 2 shows concentrations of IGF-I in different mouse strains measured by two commercially available mouse IGF-I assays (serum samples, identical pre-analytical processing) [10]. Although the IGF-I concentrations reported by both assays correlated significantly (p<0.001), the overall correlation co-efficient was only moderate (Pearson-r=0.4, r2=0.162). Passing–Bablok regression for IGF-I concentrations obtained from the two different assays confirmed the large variability (slope: 0.831; intercept: 162.4; Fig. 2). In this context it is important to realize that caution is also warranted when hormones are measured using immunoassays from the same manufacturer, but from different production batches. Larger series of samples obtained from the same experiment should be measured using only immunoassays from the same batch and samples should be randomly distributed among different plates.
Fig. 2.
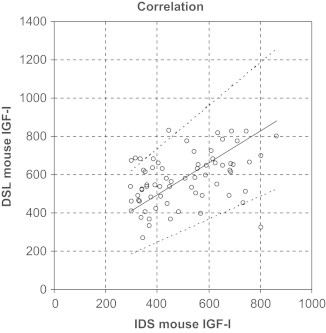
Example illustrating inter-assay variability of rodent immunoassays. Serum IGF-I was measured in serum of mice with 2 commercially available immunoassays (IDS and DSL). Passing–Bablok regression; slope: 0.831; intercept: 162.4).
The “inherent variability” with each analytical method is commonly described through the coefficients of variability within and between assays (with- and between-assay CV%). The knowledge about and control of this aspect of the analytical variability is of outmost importance for meaningful interpretation of measurement results. Since a detailed discussion of this important topic is beyond the scope of this guide focusing on pre-analytical variability, we may refer the reader to comprehensive literature for further deepening of this area [3,11–14].
Pitfall: variability between methods/inter- and intra-assay variability
Recommendations:
-
–
measure samples at least in duplicate
-
–
repeat sample measurement if the CV exceeds 15%
For larger series of samples:
-
–
maintain a single analytical method
-
–
only use immunoassays from the same manufacturer
-
–
only use assays from the same batch
-
–
distribute samples randomly between different assay plates
-
–
retain technical equipment and keep environmental conditions stable
-
–
use independent control samples covering the concentration range of interest (e.g., pools)
2.3. Pre-analytical variability
In this article, the term “pre-analytical variability” refers to changes in the apparent concentration of a metabolic hormone which occurs in the period between taking a sample and subjecting the respective sample to the hormone analysis. Per definition, the respective change of a hormone concentration in a sample takes place after the sample has “left” the organism – in other words: in vitro (as opposed to the biological variability, which describes factors modifying the circulating concentration in the sample in vivo). The main factors contributing to pre-analytical variability in vitro are differences in sample collection, handling, manipulation, processing and storage [15]. Because of their importance to hormone analyses in rodent studies, we will discuss five factors which can be a source of error and misinterpretation: matrix effects and sample type, sampling and storage conditions, dilution and contamination, blood sampling technique and finally also differences in sample pre-treatment .
2.3.1. Matrix effects and sample type
The matrix can be defined as the “environment” of an analyte in a biological sample, in most cases the liquid the analyte is dissolved in. Obviously, physical properties (e.g., viscosity) are different between matrices (e.g., urine vs. blood vs. cell culture supernatants). However, also the molecular composition varies with the matrix type, with differences in total protein content being one of the most important factors when it comes to standardization of pre-analytical conditions. Such differences in the matrix can affect the measurements in different ways: it either can directly affect the detection of the analyte during the measurement process, or it can affect the stability or conformation of the analyte in the sample.
Blood samples are usually collected and processed to yield either serum or plasma. The choice of the sample type can be influenced by known requirements to allow analysis of a certain analyte (e.g., cellular components cannot be analysed in serum), or simply by practical considerations (e.g., the yield of volume available for analysis is usually better when collecting plasma). In any case it is important to be aware that both, serum and plasma, also represent different sample types associated to differences in the matrix: for obtaining plasma, anticoagulants such as EDTA or heparin are immediately added to the whole blood sample. During centrifugation, the cellular components of the blood sample are separated, but coagulation factors like fibrinogen remain in the sample. In contrast, if plain serum is collected, the occurring coagulation leads to clot formation (consisting of fibrin net and cellular components). Therefore, after centrifugation serum samples lack coagulation factors and have a lower protein content compared to plasma. Such differences in protein content of samples have been shown to significantly contribute to differences in immunoassay results.
Fibrin clots may cause difficulties when handling serum samples as they can prevent aspiration of the correct volumes to a pipette tip. This can be especially problematic if the sample volume is low. The phenomenon is not only well known for automated assay systems, but also can affect manual procedures if not performed carefully. The most efficient way to significantly reduce formation of fibrin clots is to allow the blood samples to coagulate for a sufficient period of time (∼20–30 min) before centrifugation. In case fibrin clots are still present in the centrifuged sample, one might consider breaking these clots apart by devices like a toothpick and then re-spin the samples.
Serum and plasma samples do not only differ in protein content, but also in the biological activity of proteins, especially enzymes. This is in part explained by the fact that anticoagulants inhibit coagulation by forming chelate complexes with divalent cations (e.g., Ca2+), and the activity of several enzymes is dependent on the availability of divalent cations. Consequently, enzyme activity is typically lower in plasma compared to serum [16–18]. Therefore, stability of proteo-hormones susceptible to enzymatic degradation might be higher in plasma as opposed to plain serum samples. Although many hormones can be measured in both, serum and plasma samples, it is important to know that the absolute concentrations reported by immunoassays (or other measurement methods) can be substantially different. For several hormones analysed in human clinical routine laboratories, different reference ranges have to be applied to serum as opposed to plasma samples. For certain analytes (e.g., adrenocorticotropic hormone (ACTH) or renin) the use of plasma is strongly recommended since measurement results are only reliable with the increased analyte stability found in plasma [19–22]. In our own study, we found significant differences in circulating concentrations of leptin and GLP-1 when using either serum or plasma (see Fig. 4A and E).
Pitfall: matrix effects and sample type
Recommendations:
-
–
use only serum or plasma for comparison of circulating hormone concentrations between samples
-
–
allow sufficient coagulation time (∼20–30 min) for blood samples before centrifugation to reduce subsequent formation of fibrin clots in serum
-
–
use the same anticoagulant to obtain plasma within each experiment
-
–
consider analyte-specific requirements when choosing sample type
Fig. 4.
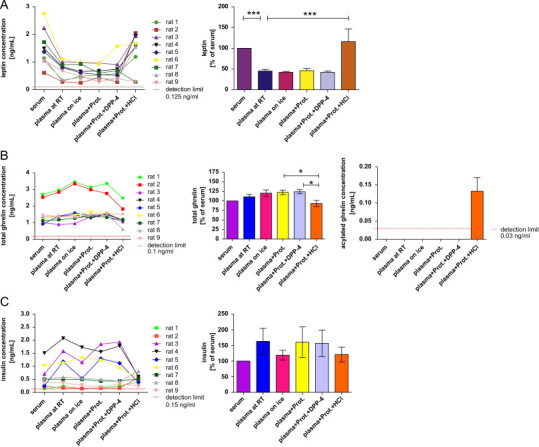
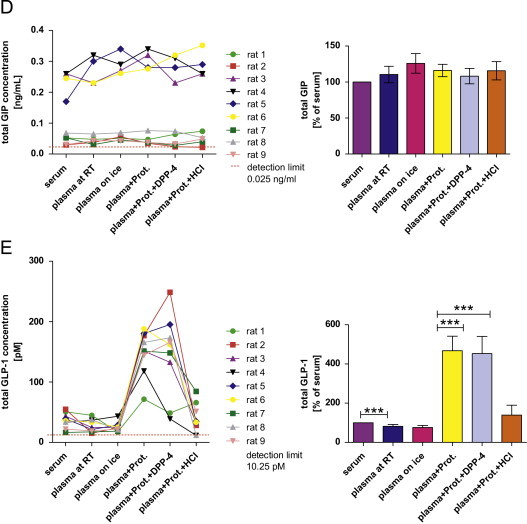
Measurement of circulating metabolic hormones ((A) leptin; (B) total and acylated ghrelin; (C) insulin; (D) total glucose-dependent insulinotropic peptide (GIP); (E) total glucagon-like peptide 1 (GLP-1)) in rats under different pre-analytical conditions. The first graph for each hormone shows absolute concentrations under different pre-analytical conditions separately for each rat. For better comparison of the different pre-analytical conditions, a second bar chart for each hormone depicts the effects in relation to the concentration obtained from serum (n=9/condition). Therefore, the serum concentration has been set to 100% and other conditions are expressed as a percentage thereof (*p<0.05, **p<0.01, ***p<0.001). Abbreviations: “RT”: room temperature; “Prot.”: addition of a general protease inhibitor; “Prot.+DPP-4”: addition of a general and a specific DPP-4 inhibitor; “Prot.+HCl”: addition of a general protease inhibitor and acidification of the sample with HCl. Data in bar charts are expressed as means±SEM.
2.3.2. Sampling and storage conditions
Further variability can be introduced by environmental conditions impacting on a sample during and after blood collection. Blood samples may either be collected in pre-chilled tubes and remain at room temperature (RT) or on ice until centrifugation. Furthermore, temperature settings and centrifugal force may differ during the centrifugation step between experiments. In our study we used serum from 5 rats and centrifuged the samples with two different frequently employed centrifugation protocols, one being “slow” and long (at 4 °C), the other protocol being “fast” and short (at RT; Fig. 3). Fig. 6 compares relative concentrations of leptin, ghrelin, insulin, GIP and GLP-1 in aliquots which underwent both centrifugation protocols. Interestingly, we did not detect significant differences between the 2 protocols for the analysed hormones. However, in the case of insulin, variability of measurement results and standard errors were higher with the “fast” centrifugation protocol (Fig. 6). After centrifugation with the fast protocol, 2 of the 5 centrifuged samples visually had a “bad quality”, cellular and liquid components were poorly separated and the serum appeared to be haemolytic. This circumstance might have affected immunoassay measurements for insulin, since the same 2 haemolytic samples showed the highest variation to the respective “slow centrifugation” counterpart samples in the insulin assay. If researchers can freely decide which centrifugation protocol should be used, we recommend using longer, but slower (i.e., lower centrifugal force) protocols, since haemolysis is less likely to occur with this procedure.
Fig. 3.
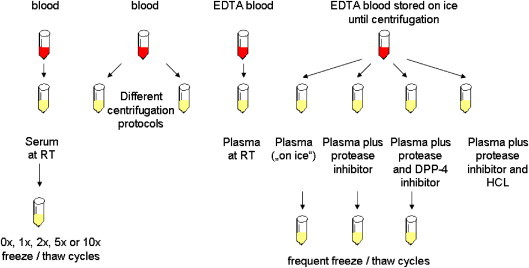
Experimental setup scheme illustrating the different handling and processing strategies for blood samples which were used for the analyses of metabolic hormones in rats.
Fig. 6.
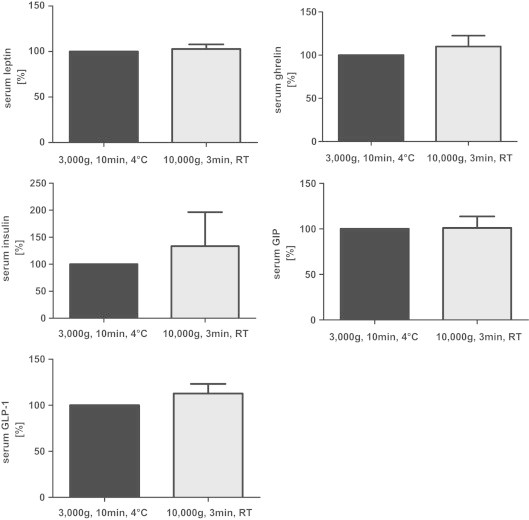
Effect of different centrifugation protocols on circulating metabolic hormones (leptin, total ghrelin, insulin, total GIP and total GLP-1) in rat serum (n=5/centrifugation protocol). Concentrations measured in aliquots centrifuged with the standard centrifugation protocol (3000g for 10 min at 4 °C) have been set to 100%, the concentrations from aliquots centrifuged with the “fast” protocol (10,000g for 3 min at room temperature) are expressed as a percentage thereof. Data are expressed as means±SEM.
Several hormones have been shown to be temperature sensitive and to potentially undergo conformational changes or even proteolysis when exposed to inappropriate temperatures or temperature changes, which can result in significant changes of concentrations measured [20,23–26]. Therefore, storage temperatures and the number of freeze and re-thawing cycles can be critical for accurate measurement of metabolic hormones. The lower the storage temperature, the unlikelier is degradation of the sample. A storage temperature of minus 70 °C or lower has been proven suitable for long-term storage and accurate measurements of most analytes [27]. In this context, however, it is important to note that even in the frozen state, evaporation of samples can occur, and adequate storage tubes with securely closing caps must be used to avoid lyophilization of samples [28]. Repeated freeze/thaw cycles should be prevented, although some hormones exhibit remarkable stability here. One might also consider to subject samples to milder thawing procedures than full thawing to room temperature. If repeated thawing of a sample becomes necessary, it is expected that gentle thawing on ice/ice-water can reduce the pre-analytical variability. However, this remains to be tested for each analyte separately. If possible, for analysis of several analytes from the same experiment in the same animal, multiple aliquots should be prepared and stored according to the respective requirements. Especially the so-called “freezer-studies” using historic samples which underwent different (and sometimes unknown) numbers of freeze/thaw cycles are at risk to produce artificial results resulting from differences in pre-analytical conditions.
Depending on the analyte in question, the addition of protease inhibitors to samples before storage can improve freeze/thaw stability of the analyte. As an example, results from the measurement of plasma insulin, ghrelin, GIP and GLP-1 after repeated freeze/thaw cycles are shown with and without addition of protease inhibitors (Fig. 5B–E ).
Pitfall: temperature/storage conditions
Recommendations:
-
–
stay consistent with the temperatures conditions during sampling, processing and centrifugation
-
–
use temperatures below −70 °C for long-term sample storage
-
–
do not save money by using cheaper, but inadequate storage tubes
-
–
avoid frequent freezing and re-thawing cycles
-
–
prepare and store samples in aliquots
Fig. 5.
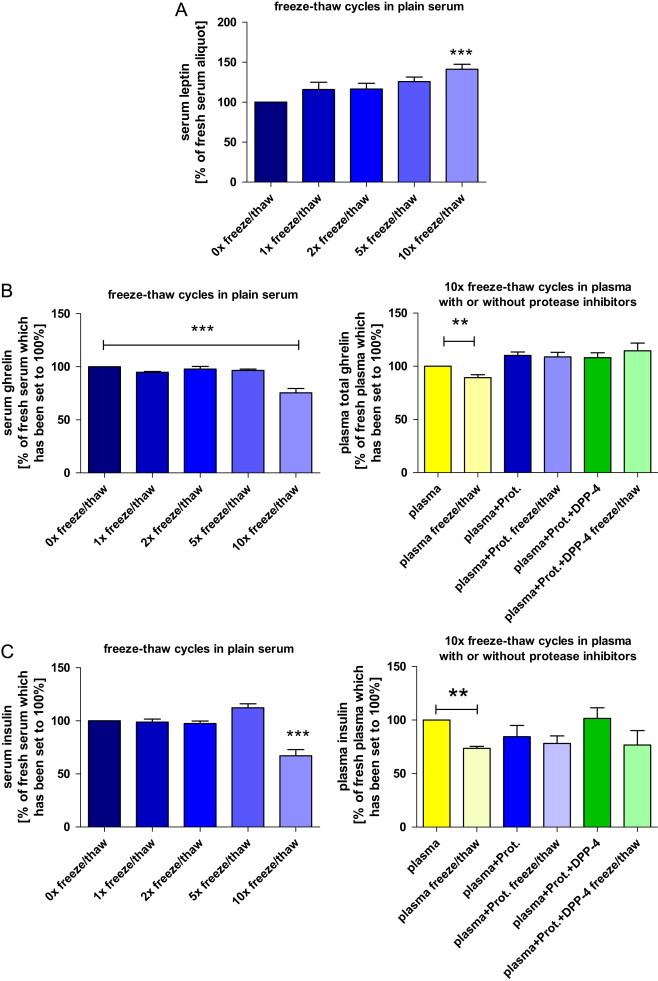
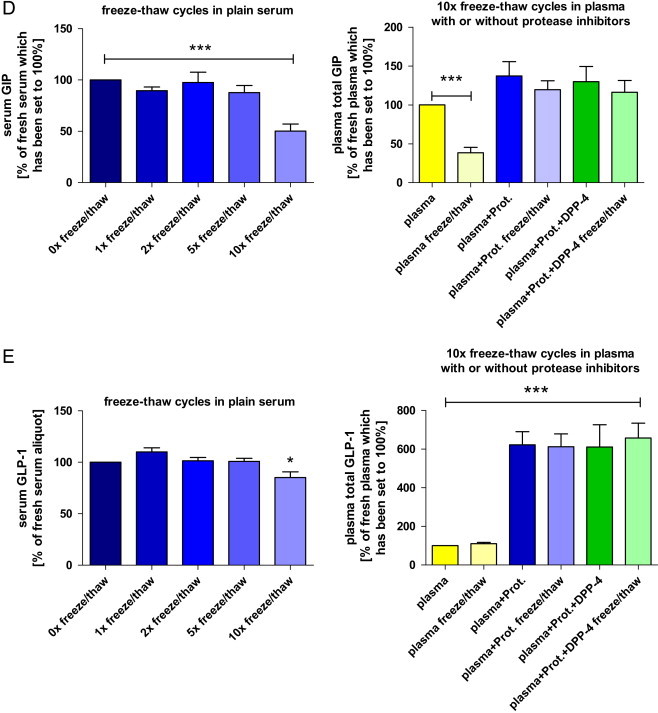
Impact of repeated freeze–thaw cycles on circulating metabolic hormones ((A) leptin; (B) total ghrelin; (C) insulin; (D) total glucose-dependent insulinotropic peptide (GIP); (E) total glucagon-like peptide 1 (GLP-1)) in rats. The first bar chart for each hormone shows hormone concentrations in plain serum after 0, 1, 2, 5 (n=5 each) and 10 (n=9) consecutive freeze–thaw cycles. Data are presented as a percentage of the fresh (untouched) serum aliquot which has been set to 100%. The second graph for each hormone (not available for leptin) depicts the effects of 10× freezing and re-thawing (“freeze/thaw”) in native EDTA plasma and in EDTA plasma samples which were pre-treated with one or two protease inhibitors. Again, values are expressed in relation to the fresh (untouched) plasma aliquot without the addition of any protease inhibitors (n=5/condition). Hormone concentrations of the fresh aliquots have been set to 100% (*p<0.05, **p<0.01, ***p<0.001). Data are expressed as means±SEM. Abbreviations: “Plasma+Prot.”: EDTA blood (plasma) including a general protease inhibitor; “Plasma+Prot.+DPP-4”: EDTA blood (plasma) including a general protease inhibitor and a specific DPP-4 inhibitor.
2.3.3. Dilution and contamination
The third source of introducing pre-analytical variability is (unintended) dilution or contamination of the blood sample [29]. Dilution could for example play a role when the blood collection device has been rinsed with a clearing solution (e.g., saline) prior to or between repeated blood sampling. Another source of errors – which is frequently overlooked also in clinical practice – is the substantial impact of inappropriate filling and mixing of the blood sample with the anticoagulants [30]. In rodent studies, where it sometimes is impossible to obtain a certain quantity of blood, differences in the relative composition of the sample (volume of blood to volume of anticoagulant or additive) can be expected to be an even more important source of pre-analytical variability. Also soluble coatings of blood collection devices have been described as a source of pre-analytical errors through contamination of the samples. As a consequence, strict adherence to standardized sampling procedures has been recommended for pharmacokinetic and pharmacodynamic studies [31].
Haemolysis can be regarded as a specific case of “contamination” of the sample. Inappropriate handling, mechanical or osmotic stress lead to rupture of erythrocyte membranes, resulting in the release of intracellular components into the blood sample. For many clinical routine parameters, haemolysis has been shown to significantly affect the outcome of laboratory measurements and haemolysis may lead to an overestimation of the true concentration in certain analytes [32,33]. In children, Bellomo et al. have recently shown that haemolysis results in falsely low immunoreactive insulin concentrations. The order of magnitude of the changes observed was great enough to lead to misinterpretation of the results [34]. Ideally, the influence factor “haemolysis” should be evaluated for every specific analyte. Unless haemolysis has been shown to be uncritical for a specific analyte, it should be regarded as a potential source of pre-analytical variability also in rodent samples and – although sometimes difficult to obtain – samples without signs of haemolysis should be favoured for measurement of circulating metabolic factors. If usage of a haemolysed sample is inevitable, the obtained hormone concentration should be critically reviewed for plausibility.
Pitfall: dilution or contamination
Recommendations:
-
–
standardize type of equipment for blood sampling and processing
-
–
standardize blood sampling procedure as much as possible
-
–
avoid haemolysis/use of haemolysed samples
2.3.4. Blood sampling technique
Interestingly, the best studied factor during the pre-analytical phase in rodents is the influence of different blood sampling techniques. It has clearly been established that especially the blood sampling site (e.g., retro-orbital plexus, decapitation, tail vein or tail tip sampling) significantly affects the measurable concentrations of analytes in blood samples from mice and rats [35–39]. Although the impact of the sampling site has been investigated for only a relatively small number of different analytes, it may be assumed that the blood sampling site is a relevant variable affecting measurable concentrations of most analytes. Therefore, consistency of the blood sampling technique is of outmost importance in rodent studies. A practical example from rodent studies is the process of multiple blood samplings during stimulation or suppression tests (e.g., glucose tolerance tests). When performing such a procedure, frequent practice is to take most blood samples from easy accessible sites like the tail vein, but the terminal sample – in case the dynamic test is the endpoint of an experiment – from another site, e.g., after euthanasia by cardiac puncture or through decapitation (which might be considered less stressful for the animal). However, based on the studies mentioned above [39] and our own observations, it is important to also collect the terminal blood sample of the test from the same site in order to be consistent.
Pitfall: different blood sampling techniques
Recommendations:
-
–
stay consist with the blood sampling procedure and use only samples collected from the same site
2.3.5. Sample pre-treatment
One way to avoid changes in measured hormone concentrations induced by the pre-analytical factors described above is the application of specific sample pre-treatment protocols. As mentioned, when collecting blood to yield plasma, the researcher may choose to add one of several anticoagulants (commonly used: EDTA, heparin, citrate and hirudin, see also chapter “matrix effects”). For humans, information about most of the commonly used anticoagulants and their influence on the measurement of many analytes including several hormones exist [29,40–42]. In rodents, however, systematic studies investigating if the type of anticoagulants has an influence on the measurement of circulating metabolic factors are lacking. The situation is similarly unexplored for the addition of protease inhibitors. Immediate addition of a general protease inhibitor is frequently recommended for many peptide hormones, although publications on the topic providing detailed information are lacking. For some hormones, the addition of other specific, auxiliary enzyme inhibitors is recommended. As an example, the accurate measurement of the incretin hormones GIP and GLP-1 seems to require the addition of protease and DPP-4 inhibitors [43,44]. Immediate pre-treatment and acidification of the plasma sample with HCl is recommended before ghrelin and acylated ghrelin can be measured. In this case, acidification of the sample to achieve a very low pH is required to preserve the octanoyl group of ghrelin [45,46].
While it is unquestioned that adequate sample pre-treatments are necessary for measurement of several hormones, it remains speculative if and into which direction such pre-treatment strategies might also affect the measured concentrations of other analytes. This lack of knowledge may become a critical factor especially in rodent studies, where it is common practice that only one or two tubes of blood are collected. In many cases, sample pre-treatment is performed to meet the requirements for the primary analyte of interest. However, if at a later time point it becomes interesting or important to also measure additional circulating factors (which were not originally planned in the study), the question if the same pre-treated sample can also be used to accurately measure other analytes becomes relevant. Unfortunately, there is no general answer to this question and ideally it needs to be evaluated for every analyte and each type of sample pre-treatment . Below we present results from a study where we measured several metabolic hormones in samples which were differently pre-treated (Fig. 4A–E ). To illustrate the complexity of the issue we would like to point out that the addition of a general protease inhibitor to EDTA plasma samples – while effective in protecting some of the analytes – at the same time led to increased variability of measurement results for other hormones.
Pitfall: sample pre-treatment
Recommendations:
-
–
adhere to established analyte-specific pre-treatment procedures
-
–
be consistent in blood sampling procedures and sample processing
3. Awareness for pre-analytical variability
The impact of pre-analytical variability upon the results of immunoassays is often underestimated. Even in clinical practice reliable, evidence-based guidelines are scarce. If existing, they are not uniformly adopted, representing a major problem for the standardization of measurements [47] in clinical routine diagnostics. There are also concerns with respect to pre-analytical variability in research studies in humans. Only recently, a meta-analysis compared pre-analytical blood processing techniques reported in 87 articles describing studies which investigated the analyte amyloid-β peptide (supposed to be a promising biomarker for Alzheimer's disease). While it became apparent that the discrepancies between pre-analytical methodologies of several studies were likely to have contributed to the significant variability in amyloid-β levels observed between studies, the authors also reported that for many of the studies analysed, a precise description of the pre-analytical methods was lacking in the respective publications [48]. Thus, Watt and colleagues emphasized the need for a consensus on pre-analytical processing of blood samples [48]. Sticking to Alzheimer's disease, Vanderstichele et al. very recently published a consensus paper on different pre-analytical issues like tube types, centrifugation, time and temperature before and during storage, repeated freeze/thaw cycles, and length of storage on concentrations of biomarkers in cerebrospinal fluid [49]. This example from Alzheimer's research illustrates that in studies involving humans, the situation is not ideal, but the awareness has tremendously increased over the past years. This is also documented by an increasing number of published studies, reviews and guidelines providing support how to minimize pre-analytical variability [50–55] and giving practical recommendations concerning biobanking and long-term storage [28,56–58]. Although many issues regarding pre-analytical variability in humans are still a matter of debate or uncertainty, there is no doubt that the situation is much better than the situation in rodent studies. As mentioned above, the majority of rodent studies dealing with pre-analytical factors investigated the impact of the blood collection site on the concentration of circulating analytes [38,39,59]. Very few animal studies exist about the many other pre-analytical factors described in this article. If existing, most of the studies were performed in large animals like dogs [60], minipigs [61], bovine [62] or sheep [63], in which sampling of larger blood volumes is not critical. In rodents, only a few respective studies exist for only a few selected analytes. One example is a study in mice investigating the effects of the anticoagulant and the temperature on measurement of circulating tumour necrosis factor [64]. Another example is the elaborated study by Stengel and colleagues, who clearly have shown that standard blood processing (EDTA-blood on ice) can hinder reliable measurements of several circulating metabolic parameters in rats [46]. The authors demonstrated that their newly developed blood processing method (“RAPID” method) eliminated the breakdown of several hormones and significantly improved the recovery for most peptides. These very practical recommendations not only facilitate the accurate measurement of the investigated analytes, but also highlight that poor control of pre-analytical conditions may severely influence immunoassay measurements in rodent studies.
A major factor explaining why pre-analytical variability is rarely studied in rodents is certainly the limited blood volume of mice and rats. Comparing the influence of different sampling procedures or other pre-analytical conditions on hormone measurements requires the concomitant withdrawal of several aliquots of blood, with even more blood being required when the study is performed for different analytes. This problem was also the major reason why we used adult rats instead of mice for our study described below.
Another reason explaining why studies on pre-analytical variables are sometimes considered evitable for rodent studies is the fact that in animal experiments researchers often only are interested to compare hormone concentrations between experimental groups of a single experiment. As long as all blood samples from such an experimental run have been processed identically, the assumption is that a potential pre-analytical bias would affect all samples from the experiment in the same way, thus still allowing scientific comparisons of individuals or groups. However, as stated above, pre-analytical variability becomes a pitfall when absolute concentrations from different studies are compared or when studies require comparison of current analyte concentrations with concentrations from previous experiments (“freezer studies”).
Finally, neither commercial assay manufacturers nor health authorities seem to apply the same degree of care and surveillance to assays performed with animal samples as they do for assays used in human studies. The market for rodent assays is comparably small (when compared to the much large demands deriving from human research and diagnostics), but rapidly moving with new analytes being frequently put on the agenda. This in part might explain the limited interest of commercial manufacturers to conduct systematic evaluations assessing the pre-analytic variability for rodent circulating factors.
4. Analysis of pre-analytical variability in circulating metabolic factors of rodents
In order to specifically address pre-analytical variables for some of the hormones more frequently measured in our animal experiments, we set up a systematic study for a small set of metabolic hormones in rats. Fig. 3 summarizes the different sample processing and pre-treatment conditions which we compared. Six pre-analytical conditions were tested (a detailed description for sampling and processing of samples can be found in the respective methods section): (1) serum collected and stored at room temperature (RT) and (2) EDTA plasma collected and stored at RT, (3) EDTA plasma collected in pre-chilled tubes and immediately stored on ice (“plasma on ice”), (4) “plasma on ice” with a general protease inhibitor (plasma+prot.), (5) “plasma on ice” with a general protease inhibitor plus a specific DPP-4 inhibitor (plasma+prot.+DPP-4), and (6) plasma on ice” with a general protease inhibitor plus subsequent acidification with HCl (plasma+prot.+HCl). Furthermore, serum samples were subjected to 1, 2, 5 and 10 consecutive freezing and re-thawing cycles before analysis. Finally, using EDTA plasma samples subjected to ten successive freeze and thaw cycles, we also investigated the potential protective effect of adding protease inhibitors (Fig. 5B–E ).
All immunoassay results have been corrected for the respective dilution factors which were introduced through the sample pre-treatment . The impact of different pre-analytical conditions upon several circulating metabolic hormones is depicted in Fig. 4. The figure shows two graphs for each hormone: first, absolute hormone concentrations separately for each rat under each pre-analytical condition. Second, hormone concentrations presented in relation to plain serum. The latter has been done to allow better comparison between the different pre-analytical conditions. Therefore, hormone concentrations of serum at RT have been set to 100% and the other pre-analytical conditions are expressed as a percentage thereof.
As expected, absolute hormone concentrations varied considerably between the 9 rats. Of note, we intentionally had introduced a high degree of biological variability (i.e., fasting/non-fasting, males/females) to obtain samples covering a wide range of hormone concentrations. This is important if assessing pre-analytical variability as the impact of pre-analytical conditions on assay results can be more pronounced at low or high hormone concentrations. The results for the different analytes – especially when presented as a percentage of the “standard condition” – clearly demonstrate that the order of magnitude and the direction of a potential change in measured concentration introduced by a specific pre-analytical treatment cannot be predicted in advance. Measurement of leptin and total GLP-1 yielded considerably different results depending on the matrix (serum or plasma). Addition of HCl or protease inhibitors showed a significant effect on measurements of leptin, ghrelin and total GLP-1 . Interestingly, the addition of protease inhibitors highly increased the detected concentrations of total GLP-1, while measurement of all other hormones was not affected by this pre-treatment (Fig. 4). Acylated ghrelin was only detectable in plasma samples which have previously been acidified with HCl (Fig. 4B). Sampling and storage of the EDTA plasma tubes on ice immediately after blood collection had no detectable effect on the measured hormone concentrations when compared to EDTA blood collected and stored at room temperature. In contrast, 10 times freeze and thawing cycles of serum affected the concentrations of all investigated analytes. Interestingly, even the effect of repeated freeze–thaw cycles on measured hormone concentrations was not unidirectional. While resulting in significantly lower measured concentrations of most analytes (after 10×), the measured concentration of serum leptin was increased by about 25% after 5 times of repeated freezing and re-thawing (not significant) and by more than 40% after 10× freezing and thawing (p<0.001; Fig. 5A). For all investigated analytes, we did not detect significant (and meaningful) differences for up to 2 repeated freeze–thaw cycles.
We next asked if the pre-analytical variability introduced by frequent sample freeze and thaw cycles is also present when using plasma instead of serum and if this variability can be reduced by addition of protease inhibitors (for details see Fig. 3 and the respective methods section). We therefore measured and compared concentrations of insulin, ghrelin, GIP and GLP-1 and found that – as expected – repeated freezing and re-thawing of plasma samples also led to significantly lower concentrations of these hormones (except GLP-1, which generally showed very low concentrations without the addition of protease inhibitors). This effect was most obvious for measurement of total GIP, since frequent freeze and thaw cycles resulted in GIP concentrations which were more than 60% lower when compared to the fresh aliquots. The immediate addition of protease inhibitors to EDTA-blood abolished “freeze/thaw susceptibility” of all analytes investigated. Similar to the experiments using fresh plasma aliquots (Fig. 4), also in this frequent freeze and thaw experiment the addition of protease inhibitors to EDTA plasma resulted in higher readings for both incretin hormones measured (Fig. 5D and E). Interestingly and in contrast to some recommendations, in our experiments there was no significant difference between the results obtained with addition of a general protease inhibitor alone or in combination with the (much more expensive) specific DPP-4 inhibitor (Fig. 5).
5. Recommendations and conclusion
Literature data and our own experiments clearly demonstrate that pre-analytical variability also impacts upon metabolic hormone measurements in rodent studies. The first and may be most important recommendation is to have an increased awareness for a potential variability introduced by pre-analytical conditions. This for example applies when researchers decide to measure a newly discovered parameter in an ongoing experiment and compare the results to older samples which either underwent different sample pre-treatments or were stored in the freezer for longer periods. Without the awareness for a potential influence of pre-analytical variability, measured concentrations of such an analyte may lead to conclude that a specific treatment had an effect, but the differences can actually be attributed to pre-analytical variability.
For analysis of metabolic factors – especially by immunoassays – adherence to constant conditions during the pre-analytical phase is of outmost importance. All samples analysed during a series of experiments should undergo the same, uniform pre-analytical and analytical procedures. The results from our study have shown that the addition of a general protease inhibitor to a plasma samples – as recommended and required for measurement of GLP-1– did not influence measured concentrations of other hormones like leptin, total ghrelin, GIP and insulin. We conclude that samples pre-treated with protease inhibitors to measure GLP-1 can also be used for unbiased analysis of other analytes. Not unexpectedly, however, in plain sera or plasma samples subjected to frequent (10×) freeze and thawing cycles, hormone concentrations were considerably altered (some increased, some decreased). Therefore, frequent freezing and thawing should be avoided. In our experiments, we did not detect significant differences between hormone measurements after 0 or 2 repeated freezing and thawing cycles. Importantly, however, this cannot be taken as a general recommendation but needs to be verified specifically for each analyte. In laboratory routine, we encourage preparation of multiple aliquots immediately after centrifugation since this is the best option to prevent pre-analytical influence through freeze and thaw cycles. Furthermore, addition of protease inhibitors immediately after sample collection turned out to be an effective measure to protect the analyte and allowed unbiased measurement of the above mentioned hormones even after several thawing cycles.
Caution should be taken when treating samples with very specific pre-treatment protocols like in the case of acylated ghrelin. Of course, this pre-treatment allows accurate measurement of acylated ghrelin, but can severely interact with measurement of other analytes by either increasing detected levels (e.g., leptin) or completely hamper measurement of an analyte (e.g., growth hormone, data not shown).
Also when publishing animal studies, researchers should precisely detail sample processing and storage procedures used in their experiments. Implementation of this request also is a task for journals and reviewers. In order to allow others the judgement if comparisons are adequate or not, and if they can compare hormone concentrations from their own studies to those published from others, a precise description of pre-analytical conditions used is mandatory.
As a practical consequence from the evaluations shown here, in our laboratory rodent blood is routinely sampled to yield both, serum and EDTA plasma, and aliquots are stored. Plasma samples are routinely treated with a general protease inhibitor. In this way, multiple analyses of circulating metabolic factors may be performed even in the future with a reduced risk of introducing a pre-analytical bias.
6. Methods
6.1. Animals, sample processing and sample pre-treatments
In this study, 14 weeks old Wistar rats (n=9; Charles River, Sulzfeld, Germany) were sacrificed by decapitation under isoflurane anaesthesia and whole trunk blood was collected. In order to obtain samples covering a wide range of hormone concentrations, we deliberately chose to use 7 male and 2 female rats, 5 of them being fasted for 6 h and 4 not being fasted before sacrifice. The broad range of concentrations was used for measurements since pre-analytical variability and analytical issues might differentially affect the measurements of hormones at low and high concentrations. Fig. 3 summarizes the different blood processing steps and sample pre-treatments . Blood collection from the same rat was performed using a serum tube and 5 EDTA plasma tubes (S-Monovette®, Sarstedt, Nuembrecht, Germany, 1.6 mg EDTA/ml blood). The serum tube and one plasma tube remained at room temperature and the other 4 plasma tubes were pre-chilled on ice until centrifugation (approx. 25 min). In 2 of the pre-chilled EDTA plasma tubes, respective amounts of a general protease inhibitor (Complete®, Roche, Basel, Switzerland) were immediately added. To the last pre-chilled EDTA tube, the general protease inhibitor plus a specific DPP-4 inhibitor was added (DPP-IV inhibitor, Millipore, Schwalbach, Germany) (Complete: 150 μl/ml blood; DPP-4 inhibitor: 10 μl/ml blood). Samples were centrifuged at 3000g for 10 min at 4 °C (Multifuge X3R, Thermo Scientific, Langenselbold, Germany). After centrifugation, one of the plasma samples including the general protease inhibitor was further processed by acidification with 1 N HCl (for each 100 μl of plasma, 20 μl HCl were added) according to previous recommendations [65]. Samples were then separated in at least 8 aliquots (Nunc™ cryotubes, Thermo Scientific) and stored at −80 °C until further analysis. Plain Serum was divided into 16 aliquots. 8 of them were immediately stored at −80 °C and the remaining 8 aliquots underwent 10 subsequent freeze–thaw cycles before storage at −80 °C.
For the freeze and thaw study in plasma, EDTA blood from 5 additional, not-fasted male Wistar rats (Charles River, Sulzfeld, Germany) was collected. This time, freshly collected blood from each rat was partitioned in 3 in pre-chilled EDTA tubes. The blood in the first EDTA tube was left native and stored on ice, while a general protease inhibitor (Complete) was added to the second blood sample. The third blood sample was mixed with the same general protease inhibitor (Complete) and a specific DPP-4 inhibitor (DPP-IV inhibitor, Millipore; Complete: 150 μl/ml blood; DPP-4 inhibitor: 10 μl/ml blood). After centrifugation at 3000g at 4 °C for 10 min, the 3 plasma samples were split into 10 aliquots. 5 of the aliquots were immediately stored at −80 °C until further analyses, while the other 5 aliquots underwent ten consecutive freeze and thaw cycles from −80 °C to full thawing to room temperature (22 °C). Insulin, total GIP, total GLP-1 and total ghrelin were measured by immunoassays in fresh aliquots and aliquots which underwent 10 freeze/thaw cycles. All measurement results have been normalized for the respective dilution factors. The respective hormone concentration measured in the fresh (untouched) aliquot from each rat was set to 100%. The result obtained from the “counterpart” freeze/thaw cycle sample is expressed as a percentage thereof.
Because of the differences we observed between hormone concentrations measured in untouched samples and the same samples after 10× freeze/thaw cycles we performed an additional study, in which blood was taken (by decapitation under isoflurane anaesthesia) from 5 not fasted male Wistar rats (10-weeks old). After standard centrifugation at 3000g the serum samples were split into multiple aliquots and subjected to 0, 1, 2 or 5 consecutive freeze and thaw cycles, respectively. In the same experiment, we also investigated if differences in centrifugation protocols have an impact on measured hormone concentrations. For this purpose, whole blood was collected in several Eppendorf™ cups and remained at room temperature for 25 min. Samples were then centrifuged (Centrifuge 5415R, Eppendorf, Germany) either with a standard centrifugation protocol (3000g at 4 °C for 10 min) or with a rapid, “high-g” protocol at room temperature (10,000g at room temperature for 3 min). After the respective pre-analytical procedures, all samples were stored at −80 °C until measurement of leptin, insulin, GIP, GLP-1 and ghrelin.
6.2. Immunoassays
Leptin (Mediagnost, Reutlingen, Germany), insulin (Alpco, Salem, NH, USA), total ghrelin, acylated ghrelin, total GIP and total GLP-1 (all assays from Millipore) were measured by immunoassay through experienced technical staff as per manufacturers’ instructions. For the correlation analysis of IGF-I levels in fresh serum samples from 69 mice (Fig. 2), mouse IGF-I assays from IDS (Boldon, UK) and DSL (Webster, TX, USA) were used. After measurement, all immunoassay results have been corrected for the respective dilution factors deriving from sample pre-treatments.
6.3. Statistics
Statistical analysis was performed using the SPSS software package (SPSS Inc., version 15.0, Chicago, USA), Microsoft Excel and graph pad prism (Graph pad Software, Version 5, La Jolla, USA). Statistical comparison between plain serum and plasma at RT, as well as the comparison of different centrifugation protocols was done by Students t-test. Multiple comparisons of pre-analytical conditions and freeze–thaw stability data were performed with one-way ANOVA and Dunnet post-hoc tests (⁎p<0.05, ⁎⁎p<0.01, ⁎⁎⁎p<0.001). Correlation analysis was performed by parametric Pearson analysis and by Passing–Bablok regression. For better comparison between different sample processing protocols and pre-treatments, hormone concentrations measured in aliquots obtained by “standard conditions” (plain serum, fresh aliquots and aliquots centrifuged with the standard protocol, respectively) were set to 100%. This was done by first assigning the respective hormone concentration measured in each rat under the “standard condition” the numerical value 100%. Second, the measured hormone concentrations from the other conditions in the same rat were calculated as a percentage thereof. Therefore, no standard error of the mean (SEM) is depicted in graphs for the respective “standard conditions”. All other data are presented as means±SEM.
Conflict of interest
None declared.
Acknowledgements
We would like to thank Ms. Sarina Meurer and Mr. Amon Horngacher (Medizinische Klinik und Poliklinik IV, Munich) for excellent technical support. Furthermore, we want to express our gratitude to Dr. Jenny Manolopoulou (IDS, Boldon, UK) for expert help with the statistical analysis and the Passing–Bablok regression.
This study has been funded in parts by a grant from the FöFoLe programme of the Medical Faculty of the LMU Munich (Grant no. 744).
References
- 1.Becan-McBride K. Laboratory sampling. Does the process affect the outcome? J Intraven Nurs. 1999;22:137–142. [PubMed] [Google Scholar]
- 2.Lippi G., Chance J.J., Church S., Dazzi P., Fontana R., Giavarina D. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49:1113–1126. doi: 10.1515/CCLM.2011.600. [DOI] [PubMed] [Google Scholar]
- 3.Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006;44:750–759. doi: 10.1515/CCLM.2006.123. [DOI] [PubMed] [Google Scholar]
- 4.Bonini P., Plebani M., Ceriotti F., Rubboli F. Errors in laboratory medicine. Clin Chem. 2002;48:691–698. [PubMed] [Google Scholar]
- 5.Banks R.E. Preanalytical influences in clinical proteomic studies: raising awareness of fundamental issues in sample banking. Clin Chem. 2008;54:6–7. doi: 10.1373/clinchem.2007.097667. [DOI] [PubMed] [Google Scholar]
- 6.Palmer A.J., Chung M.Y., List E.O., Walker J., Okada S., Kopchick J.J. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150:1353–1360. doi: 10.1210/en.2008-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielohuby M., Bidlingmaier M. Highlighting the importance of age and gender in obesity research. Obes Metab—Milan. 2008;4:221–223. [Google Scholar]
- 8.Catalano K.J., Bergman R.N., Ader M. Increased susceptibility to insulin resistance associated with abdominal obesity in aging rats. Obes Res. 2005;13:11–20. doi: 10.1038/oby.2005.4. [DOI] [PubMed] [Google Scholar]
- 9.Reed J.A., Benoit S.C., Pfluger P.T., Tschop M.H., D'Alessio D.A., Seeley R.J. Mice with chronically increased circulating ghrelin develop age-related glucose intolerance. Am J Physiol Endocrinol Metab. 2008;294:E752–760. doi: 10.1152/ajpendo.00463.2007. [DOI] [PubMed] [Google Scholar]
- 10.Bielohuby M., Schaab M., Kummann M., Sawitzky M., Gebhardt R., Binder G. Serum IGF-I is not a reliable pharmacodynamic marker of exogenous growth hormone activity in mice. Endocrinology. 2011;152:4764–4776. doi: 10.1210/en.2011-1432. [DOI] [PubMed] [Google Scholar]
- 11.Sonntag O. Analytical interferences and analytical quality. Clin Chim Acta. 2009;404:37–40. doi: 10.1016/j.cca.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Laron Z., Bidlingmaier M., Strasburger C.J. Indications, limitations and pitfalls in the determination of human growth hormone, IGF-I and their binding proteins. Pediatr Endocrinol Rev. 2007;5(Suppl. 1):555–569. [PubMed] [Google Scholar]
- 13.Strasburger C.J., Bidlingmaier M. How robust are laboratory measures of growth hormone status? Horm Res. 2005;64(Suppl. 2):1–5. doi: 10.1159/000087745. [DOI] [PubMed] [Google Scholar]
- 14.Petersen P.H., Ricos C., Stockl D., Libeer J.C., Baadenhuijsen H., Fraser C. Proposed guidelines for the internal quality control of analytical results in the medical laboratory. Eur J Clin Chem Clin Biochem. 1996;34:983–999. [PubMed] [Google Scholar]
- 15.Tammen H. Specimen collection and handling: standardization of blood sample collection. Methods Mol Biol. 2008;428:35–42. doi: 10.1007/978-1-59745-117-8_2. [DOI] [PubMed] [Google Scholar]
- 16.Wojcik M., Stec W.J. The effect of divalent cations on the catalytic activity of the human plasma 3′-exonuclease. Biometals. 2010;23:1113–1121. doi: 10.1007/s10534-010-9358-5. [DOI] [PubMed] [Google Scholar]
- 17.Jung K. Consideration of preanalytical conditions to use circulating matrix metalloproteinases as diagnostic markers. Transplantation. 2005;79:744. doi: 10.1097/01.tp.0000147342.04121.d5. [DOI] [PubMed] [Google Scholar]
- 18.Verspaget H.W., Kuyvenhoven J.P., van Hoek B. Preanalytical conditions and circulating matrix metalloproteinases. Transplantation. 2005;79:745–746. doi: 10.1097/01.tp.0000147343.33801.35. [DOI] [PubMed] [Google Scholar]
- 19.Meakin J.W., Tingey W.H., Jr., Nelson D.H. The catabolism of adrenocorticotropic hormone: the stability of adrenocorticotropic hormone: the stability of adrenocorticotropic hormone in blood, plasma, serum, and saline. Endocrinology. 1960;66:59–72. doi: 10.1210/endo-66-1-59. [DOI] [PubMed] [Google Scholar]
- 20.Reisch N., Reincke M., Bidlingmaier M. Preanalytical stability of adrenocorticotropic hormone depends on time to centrifugation rather than temperature. Clin Chem. 2007;53:358–359. doi: 10.1373/clinchem.2006.080622. [DOI] [PubMed] [Google Scholar]
- 21.Campbell D.J., Nussberger J., Stowasser M., Danser A.H., Morganti A., Frandsen E. Activity assays and immunoassays for plasma Renin and prorenin: information provided and precautions necessary for accurate measurement. Clin Chem. 2009;55:867–877. doi: 10.1373/clinchem.2008.118000. [DOI] [PubMed] [Google Scholar]
- 22.Locsei Z., Racz K., Patocs A., Kovacs G.L., Toldy E. Influence of sampling and storage conditions on plasma renin activity and plasma renin concentration. Clin Chim Acta. 2009;402:203–205. doi: 10.1016/j.cca.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Jane Ellis M., Livesey J.H., Evans M.J. Hormone stability in human whole blood. Clin Biochem. 2003;36:109–112. doi: 10.1016/s0009-9120(02)00440-x. [DOI] [PubMed] [Google Scholar]
- 24.Schneider S., Brummer V., Carnahan H., Dubrowski A., Askew C.D., Struder H.K. Stress hormone stability: processing of blood samples collected during parabolic flight. A pre-flight comparison of different protocols. Clin Biochem. 2007;40:1332–1335. doi: 10.1016/j.clinbiochem.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 25.Livesey J.H., Ellis M.J., Evans M.J. Pre-analytical requirements. Clin Biochem Rev. 2008;29(Suppl. 1):S11–15. [PMC free article] [PubMed] [Google Scholar]
- 26.Reimers T.J., McCann J.P., Cowan R.G. Effects of storage times and temperatures on T3, T4, LH, prolactin, insulin, cortisol and progesterone concentrations in blood samples from cows. J Anim Sci. 1983;57:683–691. doi: 10.2527/jas1983.573683x. [DOI] [PubMed] [Google Scholar]
- 27.Lombardi G., Lanteri P., Colombini A., Banfi G. Blood biochemical markers of bone turnover: pre-analytical and technical aspects of sample collection and handling. Clin Chem Lab Med. 2012;50:771–789. doi: 10.1515/cclm-2011-0614. [DOI] [PubMed] [Google Scholar]
- 28.Schrohl A.S., Wurtz S., Kohn E., Banks R.E., Nielsen H.J., Sweep F.C. Banking of biological fluids for studies of disease-associated protein biomarkers. Mol Cell Proteomics. 2008;7:2061–2066. doi: 10.1074/mcp.R800010-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowen R.A., Hortin G.L., Csako G., Otanez O.H., Remaley A.T. Impact of blood collection devices on clinical chemistry assays. Clin Biochem. 2010;43:4–25. doi: 10.1016/j.clinbiochem.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Lippi G., Franchini M., Montagnana M., Salvagno G.L., Poli G., Guidi G.C. Quality and reliability of routine coagulation testing: can we trust that sample? Blood Coagul Fibrinolysis. 2006;17:513–519. doi: 10.1097/01.mbc.0000245290.57021.46. [DOI] [PubMed] [Google Scholar]
- 31.Kontny N.E., Hempel G., Boos J., Boddy A.V., Krischke M. Minimization of the preanalytical error in plasma samples for pharmacokinetic analyses and therapeutic drug monitoring—using doxorubicin as an example. Ther Drug Monit. 2011;33:766–771. doi: 10.1097/FTD.0b013e31823aa8ab. [DOI] [PubMed] [Google Scholar]
- 32.Lippi G., Salvagno G.L., Montagnana M., Brocco G., Guidi G.C. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44:311–316. doi: 10.1515/CCLM.2006.054. [DOI] [PubMed] [Google Scholar]
- 33.Koseoglu M., Hur A., Atay A., Cuhadar S. Effects of hemolysis interferences on routine biochemistry parameters. Biochem Med (Zagreb) 2011;21:79–85. doi: 10.11613/bm.2011.015. [DOI] [PubMed] [Google Scholar]
- 34.Bellomo G., Sulas M.G., Mairate E., Bardone M.B., Rolla R. Hemolysis is a major cause of variability in insulin measurement during oral glucose tolerance test in children. Clin Lab. 2012;58:67–74. [PubMed] [Google Scholar]
- 35.Arola L., Palou A., Remesar X., Herrera E., Alemany M. Effect of stress and sampling site on metabolite concentration in rat plasma. Arch Int Physiol Biochim. 1980;88:99–105. doi: 10.3109/13813458009075674. [DOI] [PubMed] [Google Scholar]
- 36.Fitzner Toft M., Petersen M.H., Dragsted N., Hansen A.K. The impact of different blood sampling methods on laboratory rats under different types of anaesthesia. Lab Anim. 2006;40:261–274. doi: 10.1258/002367706777611433. [DOI] [PubMed] [Google Scholar]
- 37.Aasland K.E., Skjerve E., Smith A.J. Quality of blood samples from the saphenous vein compared with the tail vein during multiple blood sampling of mice. Lab Anim. 2010;44:25–29. doi: 10.1258/la.2009.009017. [DOI] [PubMed] [Google Scholar]
- 38.Christensen S.D., Mikkelsen L.F., Fels J.J., Bodvarsdottir T.B., Hansen A.K. Quality of plasma sampled by different methods for multiple blood sampling in mice. Lab Anim. 2009;43:65–71. doi: 10.1258/la.2008.007075. [DOI] [PubMed] [Google Scholar]
- 39.Vahl T.P., Ulrich-Lai Y.M., Ostrander M.M., Dolgas C.M., Elfers E.E., Seeley R.J. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- 40.Evans M.J., Livesey J.H., Ellis M.J., Yandle T.G. Effect of anticoagulants and storage temperatures on stability of plasma and serum hormones. Clin Biochem. 2001;34:107–112. doi: 10.1016/s0009-9120(01)00196-5. [DOI] [PubMed] [Google Scholar]
- 41.Vogeser M., Parhofer K.G. Limited preanalytical requirements for insulin measurement. Clin Biochem. 2005;38:572–575. doi: 10.1016/j.clinbiochem.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 42.O'Keane M.P., Cunningham S.K. Evaluation of three different specimen types (serum, plasma lithium heparin and serum gel separator) for analysis of certain analytes: clinical significance of differences in results and efficiency in use. Clin Chem Lab Med. 2006;44:662–668. doi: 10.1515/CCLM.2006.099. [DOI] [PubMed] [Google Scholar]
- 43.Heijboer A.C., Frans A., Lomecky M., Blankenstein M.A. Analysis of glucagon-like peptide 1: what to measure? Clin Chim Acta. 2011;412:1191–1194. doi: 10.1016/j.cca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Thornberry N.A., Gallwitz B. Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4) Best Pract Res Clin Endocrinol Metab. 2009;23:479–486. doi: 10.1016/j.beem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Prudom C., Liu J., Patrie J., Gaylinn B.D., Foster-Schubert K.E., Cummings D.E. Comparison of competitive radioimmunoassays and two-site sandwich assays for the measurement and interpretation of plasma ghrelin levels. J Clin Endocrinol Metab. 2010;95:2351–2358. doi: 10.1210/jc.2009-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stengel A., Keire D., Goebel M., Evilevitch L., Wiggins B., Tache Y. The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–5118. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippi G., Montagnana M., Giavarina D. National survey on the pre-analytical variability in a representative cohort of Italian laboratories. Clin Chem Lab Med. 2006;44:1491–1494. doi: 10.1515/CCLM.2006.274. [DOI] [PubMed] [Google Scholar]
- 48.Watt A.D., Perez K.A., Rembach A.R., Masters C.L., Villemagne V.L., Barnham K.J. Variability in blood-based amyloid-beta assays: the need for consensus on pre-analytical processing. J Alzheimers Dis. 2012;30:323–336. doi: 10.3233/JAD-2012-120058. [DOI] [PubMed] [Google Scholar]
- 49.Vanderstichele H., Bibl M., Engelborghs S., Le Bastard N., Lewczuk P., Molinuevo J.L. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Alsina M.J., Alvarez V., Barba N., Bullich S., Cortes M., Escoda I. Preanalytical quality control program—an overview of results (2001–2005 summary) Clin Chem Lab Med. 2008;46:849–854. doi: 10.1515/CCLM.2008.168. [DOI] [PubMed] [Google Scholar]
- 51.Wallin O., Soderberg J., Van Guelpen B., Stenlund H., Grankvist K., Brulin C. Blood sample collection and patient identification demand improvement: a questionnaire study of preanalytical practices in hospital wards and laboratories. Scand J Caring Sci. 2010;24:581–591. doi: 10.1111/j.1471-6712.2009.00753.x. [DOI] [PubMed] [Google Scholar]
- 52.Simundic A.M., Nikolac N., Vukasovic I., Vrkic N. The prevalence of preanalytical errors in a Croatian ISO 15189 accredited laboratory. Clin Chem Lab Med. 2010;48:1009–1014. doi: 10.1515/CCLM.2010.221. [DOI] [PubMed] [Google Scholar]
- 53.Tuck M.K., Chan D.W., Chia D., Godwin A.K., Grizzle W.E., Krueger K.E. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8:113–117. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vacata V, Jahns-Streubel G, Baldus M, Wood WG. Practical solution for control of the pre-analytical phase in decentralized clinical laboratories for meeting the requirements of the medical laboratory accreditation standard DIN EN ISO 15189. Clin Lab. 2007;53:211–215. [PubMed] [Google Scholar]
- 55.Diver M.J., Hughes J.G., Hutton J.L., West C.R., Hipkin L.J. The long-term stability in whole blood of 14 commonly-requested hormone analytes. Ann Clin Biochem. 1994;31(Part 6):561–565. doi: 10.1177/000456329403100606. [DOI] [PubMed] [Google Scholar]
- 56.Bernini P., Bertini I., Luchinat C., Nincheri P., Staderini S., Turano P. Standard operating procedures for pre-analytical handling of blood and urine for metabolomic studies and biobanks. J Biomol NMR. 2011;49:231–243. doi: 10.1007/s10858-011-9489-1. [DOI] [PubMed] [Google Scholar]
- 57.Zielhuis G.A. Biobanking for epidemiology. Public Health. 2012;126:214–216. doi: 10.1016/j.puhe.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Tworoger S.S., Hankinson S.E. Collection, processing, and storage of biological samples in epidemiologic studies: sex hormones, carotenoids, inflammatory markers, and proteomics as examples. Cancer Epidemiol Biomarkers Prev. 2006;15:1578–1581. doi: 10.1158/1055-9965.EPI-06-0629. [DOI] [PubMed] [Google Scholar]
- 59.Arnold M., Langhans W. Effects of anesthesia and blood sampling techniques on plasma metabolites and corticosterone in the rat. Physiol Behav. 2010;99:592–598. doi: 10.1016/j.physbeh.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 60.Ceron J.J., Martinez-Subiela S., Hennemann C., Tecles F. The effects of different anticoagulants on routine canine plasma biochemistry. Vet J. 2004;167:294–301. doi: 10.1016/j.tvjl.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Olsen A.K., Bladbjerg E.M., Jensen A.L., Hansen A.K. Effect of pre-analytical handling on haematological variables in minipigs. Lab Anim. 2001;35:147–152. doi: 10.1258/0023677011911516. [DOI] [PubMed] [Google Scholar]
- 62.Stokol T., Nydam D.V. Effect of anticoagulant and storage conditions on bovine nonesterified fatty acid and beta-hydroxybutyrate concentrations in blood. J Dairy Sci. 2005;88:3139–3144. doi: 10.3168/jds.S0022-0302(05)72996-9. [DOI] [PubMed] [Google Scholar]
- 63.Mohri M., Rezapoor H. Effects of heparin, citrate, and EDTA on plasma biochemistry of sheep: comparison with serum. Res Vet Sci. 2009;86:111–114. doi: 10.1016/j.rvsc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Holobaugh P.A., McChesney D.C. Effect of anticoagulants and heat on the detection of tumor necrosis factor in murine blood. J Immunol Methods. 1990;135:95–99. doi: 10.1016/0022-1759(90)90261-s. [DOI] [PubMed] [Google Scholar]
- 65.Liu J., Prudom C.E., Nass R., Pezzoli S.S., Oliveri M.C., Johnson M.L. Novel ghrelin assays provide evidence for independent regulation of ghrelin acylation and secretion in healthy young men. J Clin Endocrinol Metab. 2008;93:1980–1987. doi: 10.1210/jc.2007-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]


