Abstract
FGF21 is a multifunctional metabolic regulator. The co-factor βKlotho (KLB) allows FGF21 to signal via FGF receptors. Given the widespread nature of FGFR expression and KLB presence in several organs, it remains unclear which tissue/FGFR isoform determine FGF21 action. Here we show that deletion of FGFR1 in fat (FR1KO) leads to a complete ablation of FGF21 stimulated transcriptional activity in this tissue. Furthermore, FR1KO mice showed no FGF21-mediated lowering of plasma glucose, insulin and triglycerides, altered serum levels of adipokines, no increase in energy expenditure, but preserved reductions in serum/liver FFAs as compared to wild type mice. Of importance, the anti-glycaemic actions of FGF19 were fully evident in FR1KO mice implying that FGF19 functions in a FGFR1/adipose independent manner. Taken together, our findings reveal the existence of an adipose FGFR1 driven axis of cross-tissue communication which defines several aspects of FGF21 biology and delineates mechanistic distinctions between FGF21 and FGF19.
Abbreviations: BAT, Brown adipose tissue; βHB, β-HydroxyButyrate; KLB, βKlotho; DIO, Diet induced obese; EGR1, Early growth response protein 1; FGF19, Fibroblast growth factor 19; FGF21, Fibroblast growth factor 21; FGFR, Fibroblast growth factor receptor; FFA, Free fatty acids; ACADL, Long chain acetyl-CoA dehydrogenase; PGC1α, PPARγ coactivator 1α; SCD1, Stearoyl-Coenzyme A desaturase 1; TG, Triglycerides; WAT, White adipose tissue; UCP1, Uncoupling protein 1; ACADVL, Very long chain acetyl-CoA dehydrogenase.
Keywords: FGF21, Adipose tissue, FGFR1, FGF19
1. Introduction
FGF21, FGF19, and FGF23 comprise an “endocrine” FGF sub-family separating them from the canonical FGFs [1]. This classification was based on a high degree of structural homology and the ability of FGF19/21/23 to escape into the general circulation due to a lack of the conventional heparin-binding domain common to the classical FGFs [1,2]. The FGF21 receptor complex is comprised of an activity competent fibroblast growth factor receptor (FGFR) and the co-factor βKlotho (KLB) [3]. In vitro, the presence of KLB allows FGF21 to bind to and activate a variety of FGFRs [4]. In animals, KLB and FGFRs are co-expressed in several tissues in which FGF21 was shown to signal and thus induce the gamut of its metabolic actions including regulation of glucose and lipid metabolism, and correction of obesity [5]. To date, the identity of specific FGFR isoforms within FGF21 receptor complex, the tissues through which FGF21 exerts its metabolic effects, and how tissue-specific actions of FGF21 couple to the individual aspects of its versatile in vivo physiology, remain poorly understood. Furthermore, though FGF19 and FGF21 utilize the same co-receptor, KLB [4], and show similar metabolic effects in animals, these two factors still differ in their actions in vivo [4], and understanding of the molecular and tissue-specific nature of this distinction at the whole animal level is presently lacking.
We initially reported bioactivity of FGF21 in 3T3-L1 adipocytes which primarily express FGFR1c, the main FGF receptor isoform present in WAT [2]. We later went on to demonstrate in vitro that this FGFR in the context of KLB expression is sufficient to facilitate both FGF21 and FGF19 signaling [4]. Here we evaluate the requirement of FGFR1 in adipose tissue for mediating both signaling and metabolic actions of FGF21 and FGF19 action in vivo. We utilized FGFR1lox/lox AP2CRE mice with a conditional deletion of FGFR1 in fat tissue (FR1KO) which exhibit a significant reduction in FGFR1 expression in adipose tissue ([6] and Supplemental Figure 2A), and subjected these animals to a panel of acute and chronic administration with recombinant human FGF21 and FGF19.
2. Materials and methods
2.1. Proteins
For all in vivo studies FGF19 and FGF21 were generated as previously described [2].
2.2. Animals
All animals were individually housed in a temperature-controlled (24 °C) facility with a 12 h/12 h light/dark cycle. Animal protocols in this study were approved by the IACUC of the Institute of Biosciences and Technology, Texas A&M Health Science Center, and by the Eli Lilly and Co. Animal Use and Care Committee (Protocol no. 09012).
2.3. Acute FGF signaling
Male 20 week old WT (FGFR1lox/lox) or FGFR1lox/loxAP2Cre (referred to hence as FR1KO) mice which had been fed a high fat diet (Harlan Teklad, 6414) for 12 weeks were injected intraperitoneally with either vehicle or recombinant human FGF19 or FGF21 (1 mg/kg). Following injection mice were sacrificed at predetermined time points as indicated and tissues were then collected and snap frozen in liquid nitrogen. Prior to sacrifice glucose levels were determined using Precision G Blood Glucose Testing System (Abbott Laboratories, Abbott Park, IL). A minimum of 5 mice were used for each time-point.
2.4. Chronic FGF treatment
Male WT (n=10 per group) or FR1KO mice (n=10 per group) fed a high fat diet (Harlan Teklad, 6414) were implanted with Alzet mini-osmotic pumps loaded with either vehicle or specific doses of recombinant human FGF19 or FGF21 (0.1 mg/kg/day, 0.3 mg/kg/day or 1 mg/kg/day) for a period of 8 days. On day 8 mice were sacrificed and tissues were then collected and snap frozen in liquid nitrogen. Prior to sacrifice glucose levels were determined using Precision G Blood Glucose Testing System (Abbott Laboratories, Abbott Park, IL).
2.5. Metabolites and hormones
Triglycerides, cholesterol and free fatty acids were measured using a Hitachi 912 Clinical Chemistry analyzer (Roche Diagnostics, Indianapolis, IN). Tissue levels were extracted and measured as previously described [2]. Serum β-hydroxybutyrate was measured using a colorimetric assay (Stanbio Laboratories). ELISA assays were run to determine circulating levels of leptin (Crystal Chem, Chicago, IL), insulin (Crystal Chem, Chicago, IL) and total adiponectin (BioVendor Inc).
2.6. Energy homeostasis measurements
Indirect calorimeter chambers (OXYMAX; Columbus Instruments, Columbus, OH) were used to monitor various parameters of energy expenditure in WT and AKO animals over a 24 h period starting a day 5 of treatment.
2.7. RNA isolation, RT and real-time quantitative PCR
RNA was isolated from tissues using TRIzol reagent (Invitrogen, Carlsbad, CA) or by homogenization of frozen samples in Lysing Matrix D shaker tubes (MP Biomedicals, Santa Ana, CA) and was reverse transcribed into cDNA using a High-Capacity cDNA Reverse Transcription Kit (PE Applied Biosystems, Foster City, CA). Reactions were performed in triplicate on an ABI Prism 7900HT (PE Applied Biosystems) and were normalized to either 36B4 mRNA or 18 S rRNA. Assays-on-Demand Gene Expression Products (PE Applied Biosystems).
2.8. Statistical analysis
Data are presented as mean±SEM. Statistical analysis was performed using one-way ANOVA, followed by Dunnett's multiple comparisons test where appropriate. Differences were considered significant when P≤0.05.
3. Results
3.1. Acute response to exogenous FGF21
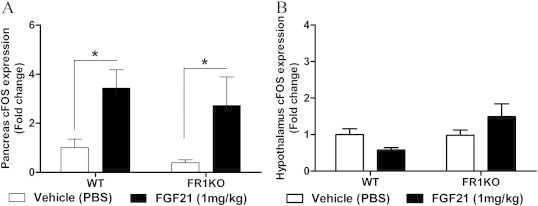
We first examined the acute transcriptional response to a single bolus injection of FGF21 in adipose, liver, pancreas and hypothalamus, putative tissues for FGF21 action [5]. Consistent with previously published data, a robust induction of immediate early gene expression was observed in WAT (Figure 1A), liver (Figure 1B) and pancreas (Supplemental Figure 1A), but not in the hypothalamus (Supplemental Figure 1B). In WAT of FR1KO mice, the FGF21 induced EGR-1 signal was drastically reduced (Figure 1A), while induction of transcription in pancreas and its absence in hypothalamus remained unaltered (Supplemental Figure 1). Unexpectedly, the acute EGR-1 response in liver was also significantly blunted in the FR1KO mice at 45 min (Figure 1B) even though the ablation of FGFR1 occurred in a distant tissue and in spite of elevated hepatic FGFR expression in the FR1KO mice (Supplemental Figure 2B). This finding suggests that part of FGF21's induction of hepatic transcription at 45 min may be indirect and mediated via a novel FGF21-induced adipose-to-liver axis of communication while the relatively larger EGR-1 response at 15 min (Figure 1B) is likely due to an accentuation of direct signaling FGF21 action on the liver given the increased hepatic FGFR expression in the FR1KO mice (Supplemental Figure 2B).
Figure 1.
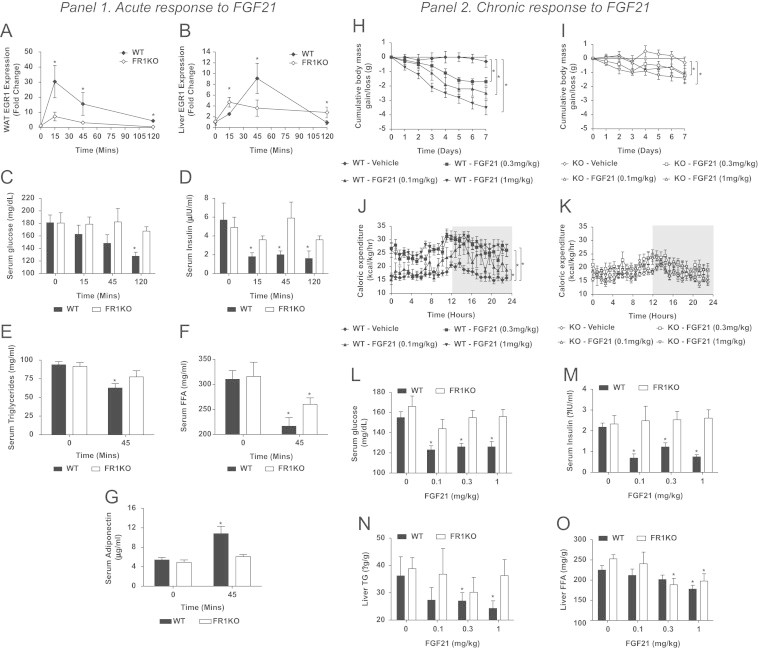
Panel 1. Immediate early gene (EGR-1) expression was examined in WAT (A) and liver (B) of WT and FR1KO animals following acute FGF21 treatment. Additionally we examined the effects of a single dose of FGF21 on serum glucose (C), insulin (D), triglycerides (E), free fatty acids (F) and adiponectin (G). Panel 2. Following chronic FGF21 treatment we assessed weight loss (H and I), energy expenditure (J and K), glucose (L) and insulin (M) in addition to hepatic TG (N) and FFA (O) levels. Statistical analysis was performed using one-way ANOVA, followed by Dunnett's multiple comparisons test where appropriate. Differences were considered significant when P≤0.05.
We next determined whether acute dosing with FGF21 impacts metabolically relevant outcomes using diet induced obese (DIO) WT and FR1KO mice. Following administration of a single bolus of FGF21 (1 mg/kg, IP), we were able to recapitulate the known spectrum of FGF21 acute in vivo activity in WT animals including lowering of plasma glucose, insulin, triglycerides, and free fatty acids (FFAs) [7] (Figure 1C–F). Strikingly, with the exception of FFA lowering, these effects were absent in FR1KO animals suggesting that FGF21 induced modulation of glucose/lipid metabolism is mediated by FGFR1 in adipose tissue. Of further importance, a significant and rapid elevation of adiponectin was observed in WT mice but was completely absent in FR1KO mice linking the biology of this known sensitizer of insulin action [8] with the glucose lowering mechanism of FGF21 (Figure 1G).
3.2. Metabolic responses to chronic FGF21 administration
Given these striking results in the acute setting we went on to examine the metabolic effects of chronic (8 day) FGF21 treatment in WT and FR1KO DIO mice. It is important to mention that following 12 weeks HFD feeding there were no differences noted in body weight, energy expenditure, ambulatory activity, plasma metabolites or hormones between the WT and FR1KO animals suggesting FGFR1/FGF21 action in adipose does not play a role in basal energy homeostasis. Consistent with the anti-obesity action of FGF21 [7], FGF21 treatment of WT animals caused significant weight loss amounting to an approximate 4 g reduction at the highest dose over the course of the study (Figure 1H). While the magnitude of this effect was attenuated by around 70% we still observed lowering of body weight in FR1KO mice suggesting that FGFR1 and adipose tissue are not the only receptor and organ responsible for mediating FGF21 induced weight loss (Figure 1I).
As our group and others have previously reported that FGF21 corrects obesity by shifting total body energy balance [7,9] we compared caloric expenditure in the WT and FR1KO mice. There was no difference between the genotypes at baseline suggesting FGFR1 in adipose is not required for maintenance of energy expenditure in the basal state. Nevertheless, these two strains of mice differed significantly in their response to FGF21 treatment. WT mice exhibited a massive dose dependent increase in caloric expenditure at all FGF21 doses tested (Figure 1J) while FR1KO mice did not respond at all to FGF21 stimulation (Figure 1K). These data suggest that FGFR1 activation in adipose tissue is required to facilitate FGF21's effect on energy expenditure. Consistent with previous reports [4,7], chronic FGF21 administration for 8 days in WT mice robustly impacted a variety of serum analytes (Table 1). At all doses tested, glucose (Figure 1L) and insulin (M) levels were reduced in WT mice but no such effect was observed in FR1KO animals. This result strongly indicates that FGFR1 activation in adipose tissue alone fully accounts for the propagation of FGF21's glycemic and insulin sensitization signal.
Table 1.
Serum metabolites and hormone levels post chronic FGF21 treatment.
|
Wild type |
FR1KO |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | Vehicle | 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | |
| Metabolites | ||||||||
| FFA | 367.3 (32.4) | 283.4 (50.67) | 244.6 (21.6)⁎ | 254 (26.5)⁎ | 348.6 (23.6) | 364 (46.4) | 300.9 (19.77)⁎ | 279.3 (16.8)⁎ |
| Cholesterol | 80.6 (4.7) | 65.2 (4.8)⁎ | 59.4 (2.2)⁎ | 59.5 (2.9)⁎ | 74.6 (3.4) | 79.8 (2.6) | 65.9 (4.2) | 71.7 (2.7) |
| Triglycerides | 65.8 (4.1) | 48.6 (7.7)⁎ | 46.8 (6.5)⁎ | 41.6 (3.5)⁎ | 54.4 (2.6) | 51.7 (4.6) | 52.2 (2.1) | 58.6 (3.6) |
| βHB | 1.01 (0.3) | 1.51 (0.6) | 1.51 (0.4) | 2.36 (0.7)⁎ | 0.92 (0.3) | 0.76 (0.6) | 1.05 (0.4) | 1.14 (0.5) |
| Hormones | ||||||||
| IGF-1 | 447.0 (71.9) | 455.9 (63.4) | 306.9 (50.2) | 282.4 (23.4)⁎ | 344.9 (39.6) | 483.9 (61.3) | 412.9 (44.1) | 350.2 (54.1) |
| Leptin | 8.3 (1.6) | 5.1 (0.7)⁎ | 5.5 (1.5)⁎ | 4.3 (1.3)⁎ | 9.4 (3.3) | 7.1 (3.4) | 8.6 (2.8) | 10.6 (0.5) |
Serum metabolites and hormones were measured in WT and FR1KO mice following chronic treatment with either vehicle or FGF21 at the doses stated. Data are presented as mean±SEM in brackets. Statistical analysis was performed using one-way ANOVA, followed by Dunnett's multiple comparisons test where appropriate. Differences were considered significant when P≤0.05 and are denoted by *.
As anticipated, plasma cholesterol, serum and liver triglycerides (TGs), leptin and IGF-1 were significantly reduced, while βHB rose in WT mice, and all of these effects were absent in FR1KO animals (Figure 1N; Table 1). While FGF21-dependent leptin [7] and IGF-1 [10] lowering indicative of improved sensitivity to these factors has been reported previously, our data link this modulation to the activation of the FGFR1-mediated pathway in adipose tissue.
Similar to our acute results, FGF21 induced lowering of plasma FFA remained significant in both genotypes and was only slightly attenuated in FR1KO mice (Table 1). This indicates that FGF21 lowers serum FFAs independent of FGFR1 in adipose. Reduced plasma FFAs were coincident with decreased hepatic FFA which was observed in both WT and FR1KO mice (Figure 1O) further implying the regulation of hepatic FFA metabolism is dependent on direct FGF21 action in the liver.
3.3. Acute response to exogenous FGF19
In an attempt to dissect commonalities and differences between FGF19 and FGF21 mechanisms [4] we also treated FR1KO animals both acutely and chronically with FGF19 (1 mg/kg, IP). In contrast to our data with FGF21, acute treatment with FGF19 had a profound effect on liver EGR-1 expression (Figure 2A) which was significantly greater in magnitude than the respective FGF19 response in WAT (Figure 2B). These data represent a complete reversal of the effects we observed with FGF21 in these tissues (compare Figure 1A–B vs. Figure 2A–B). Critically, there was no attenuation in FGF19-mediated signaling in either the liver or the WAT of the FR1KO mice. Unaltered signaling was accompanied by a significant acute reduction in glucose (Figure 2C) and insulin levels (Figure 2D), an effect observed in both genotypes indicative that in contrast to FGF21, FGFR1 in WAT is not required for the acute anti-glycemic and insulin lowering effects of FGF19.
Figure 2.
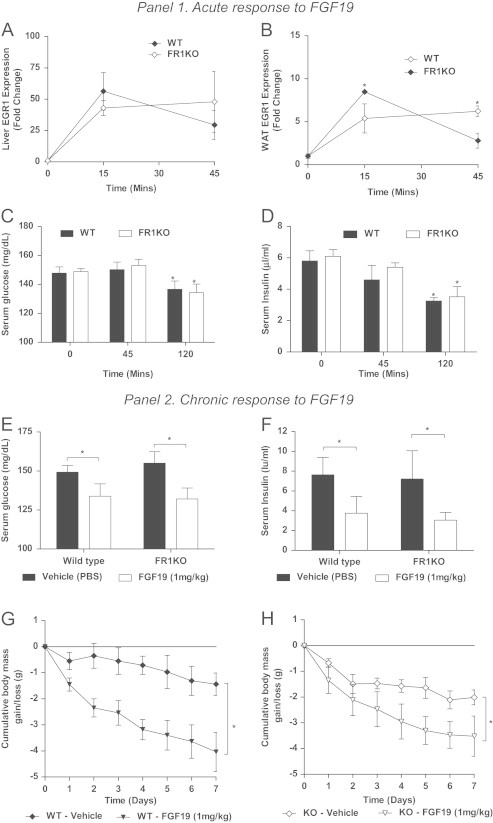
Panel 1. Following acute administration of FGF19 we examined EGR-1 in liver (A) and WAT (B). We then measured both serum glucose (C) and insulin (D) in the acute dosing paradigm. Panel 2. Following chronic administration we assessed effects of FGF19 on glucose (E), insulin (F) and body mass (G and H). Statistical analysis was performed using one-way ANOVA, followed by Dunnett's multiple comparisons test where appropriate. Differences were considered significant when P≤0.05.
3.4. Metabolic responses to chronic FGF19 administration
Given these striking acute differences we went on to treat WT and FR1KO mice chronically with FGF19. Following 8 days of treatment we found no attenuation of FGF19's effects on glucose (Figure 2E) and insulin levels (Figure 2F) in the FR1KO mice. Together, these data demonstrate that even though both FGF19 and FGF21 modulate glucose homeostasis and insulin lowering to a similar extent, FGF21 acts via a novel FGFR1/adipose-mediated pathway, whereas FGF19's effects are likely propagated by its direct hepatic action. The latter is further supported by earlier reports in which the liver has been considered the primary, if not sole target of FGF19 in vivo [11]. Finally, and similar to our results with FGF21, we found that weight loss following FGF19 treatment was also profoundly evident in the FGFR1 WKO mice (Figure 2G and H) with no effect on food intake (data not shown).
3.5. Transcriptional response to chronic FGF21 administration
To study the molecular determinants of FGF21 physiology we next examined FGF induced gene expression changes in WAT, BAT and the liver. In WAT and liver FGF21 treatment caused a significant elevation in leptin receptor expression further supporting the role of FGF21 in hormonal sensitization. Concomitant with this increase was the elevation of thermogenesis associated genes, such as uncoupling protein 1 (UCP1) and PPARγ coactivator 1α (PGC1α) (Table 2), which was recently linked to the “browning” of WAT [9]. However, when examined in the FR1KO mice FGF21 effects on these genes were entirely absent, coupling activation of FGFR1 in fat to the FGF21 gene expression signature in this tissue. It has been previously demonstrated that knockdown of FGF21 leads to a reduction in expression of genes involved in β-oxidation, including long (ACADL) and very long (ACADVL) chain acetyl-CoA dehydrogenase [12] (Table 2). In support of this contention this contention we show that in the WAT of WT mice FGF21 treatment leads to increased expression of these genes which correlates with increased production of ketone bodies (Table 1). Conversely, no regulation of genes related to β-oxidation and complete lack of FGF21 stimulated ketone body synthesis was found in FR1KO mice.
Table 2.
Gene expression following chronic FGF21 treatment.
|
Wild type |
FR1KO |
|||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | Vehicle | 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | |
| White adipose tissue | ||||||||
| ACADL | 0.53 (0.06) | 0.72 (0.11) | 0.59 (0.06) | 0.74 (0.11)⁎ | 0.75 (0.05) | 0.57 (0.06) | 0.80 (0.04) | 0.69 (0.04) |
| ACADVL | 0.25 (0.04) | 0.41 (0.06)⁎⁎ | 0.32 (0.02) | 0.55 (0.12)⁎ | 0.34 (0.04) | 0.28 (0.04) | 0.48 (0.04) | 0.30 (0.04) |
| PGC1α | 0.08 (0.01) | 0.11 (0.02) | 0.12 (0.01) | 0.14 (0.02)⁎ | 0.07 (0.01) | 0.07 (0.01) | 0.10 (0.01) | 0.08 (0.01) |
| UCP1 | 0.23 (0.03) | 0.42 (0.15) | 1.16 (0.32)⁎ | 1.87 (0.49)⁎ | 0.31 (0.09) | 0.41 (0.09) | 0.68 (0.46) | 0.83 (0.45) |
| Brown adipose tissue | ||||||||
| CPT1a | 0.16 (0.02) | 0.20 (0.03) | 0.22 (0.03)⁎ | 0.022 (0.03)⁎ | 0.17 (0.01) | 0.13 (0.03) | 0.16 (0.02) | 0.19 (0.05) |
| DIO2 | 0.08 (0.02) | 0.20 (0.06)⁎ | 0.13 (0.03) | 0.13 (0.02)⁎ | 0.09 (0.01) | 0.04 (0.01) | 0.10 (0.01) | 0.09 (0.02) |
| UCP1 | 47.81 (4.51) | 92.18 (41.57) | 66.62 (9.18) | 110.14 (21.53)⁎ | 53.29 (20.85) | 34.36 (6.07) | 44.02 (11.74) | 58.17 (17.12) |
| Liver | ||||||||
| CYP7a1 | 1.08 (0.20) | 1.16 (0.32) | 1.42 (0.33) | 1.74 (0.32) | 0.89 (0.12) | 0.83 (0.27) | 1.03 (0.15) | 1.03 (0.14) |
| CYP8b1 | 3.83 (0.28) | 4.89 (0.54) | 4.61 (0.65) | 4.42 (0.60) | 3.51 (0.27) | 2.99 (0.44) | 3.59 (0.40) | 3.59 (0.46) |
| LEPR | 0.03 (0.01) | 0.03 (0.01) | 0.11 (0.02)⁎ | 0.15 (0.03)⁎ | 0.03 (0.01) | 0.04 (0.01) | 0.03 (0.01) | 0.03 (0.01) |
| SCD1 | 29.94 (3.3) | 18.73 (4.67) | 15.90 (2.66)⁎ | 9.70 (3.51)⁎ | 35.69 (4.89) | 38.11 (7.48) | 26.82 (4.26) | 26.82 (3.21)⁎ |
To determine if the transcriptional events downstream of FGFR1 activation were altered we examined gene expression in metabolically relevant tissues. Data are presented as mean ±SEM in brackets. Statistical analysis was performed using one-way ANOVA, followed by Dunnett's multiple comparisons test where appropriate. Differences were considered significant when P≤0.05 and are denoted by *.
Previous studies have reported that treatment with exogenous FGF21 leads to activation of brown fat [2] which was confirmed in this report in WT animals as evidenced by increases in carnitine palmitoyltransferase 1A (CPT1a), type 2 diodinase (DIO2) and UCP1 mRNA [13] (Table 2). Indeed, we have previously postulated that the energy expenditure effect of FGF21 may be rooted in an increase in futile energy cycling in adipose tissues [7]. This effect was absent in the FR1KO animals indicative of FGFR1 in adipose tissue as a necessary component for FGF21-induced thermogenesis.
Given that FGF21 has previously been shown to have direct hepatic signaling through FGFRs in the liver [14] we anticipated that the hepatic gene expression profile in response to chronic FGF21 treatment may be largely preserved in the FR1KO mice. Surprisingly, almost the entirety of FGF21 induced hepatic gene expression signature was lost in the FR1KO mice (Table 2). An exception was stearoyl-Coenzyme A desaturase 1 (SCD1) mRNA, lowering of which in FR1KO animals was in part attenuated but still significant. This finding implies that SCD1 in the liver is regulated by FGF21 a direct and FGFR1/adipose independent manner. Given the role of this enzyme in FFA metabolism [15,16], it may also explain why lowering of both liver and serum FFA was still evident in the FR1KO animals. Finally, while the precise mechanism underlying the partial attenuation of FGF21's effect on SCD1 expression and systemic/hepatic FFA in FR1KO mice remains unknown, it may be linked to a defect in the interplay between FGF21 and leptin signaling in FR1KO animals. Indeed, in contrast to FR1KO mice, the suppression of SCD1, a known leptin target gene [17], was concomitant in WT animals with improved total body leptin sensitivity as evidenced by increased leptin receptor expression (Table 2) and reduced circulating leptin levels (Table 1).
Of special interest is the nature of FGF21-induced lowering of systemic insulin in WT animals. We showed previously that FGF21 is able to regulate insulin production in isolated rat islets [18] suggesting that FGF21 may regulate plasma insulin levels via a direct effect on pancreas. However, FGF21 signaling was largely unaltered in the pancreas of both WT and FR1KO animals which was nevertheless accompanied by a complete lack of FGF21-induced insulin lowering effect in the FR1KO mice. This data strongly indicates that the ability of FGF21 to reduce systemic insulin is not due to its direct action on the pancreas, but rather occurs via an indirect and as yet unknown mechanism. Thus, FGF21's direct effects in the pancreas may be limited to protection of acinar and β-cells from stress conditions and glucagon lowering as reported previously reported previously [2,18,19].
FGF19 has previously been shown to reduce expression of both hepatic cholesterol 7 alpha-hydroxylase (CYP7a1) and sterol 12-alpha-hydroxylase (CYP8b1) [20]. Importantly, reduction in both CYP mRNAs in FGF19 treated animals was evident regardless of genotype (Supplemental Table 1). While others have previously communicated an FGF21-induced CYP lowering [20], we did not observe the effect in this study (Table 2). This data further differentiates FGF21 from FGF19 indicating that direct signaling responses to FGF21 in liver are not linked to the regulation of hepatic bile acid synthesis, and the regulation of metabolism and liver function in particular is driven in large part via systemic signals initiated by FGF21 in adipocytes. Together, these findings raise the intriguing possibility that FGF19 and FGF21 at the total body/tissue level engage different variants of FGF receptors which are coupled to diverse downstream functional outputs. This hypothesis is consistent with preferential ability of FGF19 to act in vitro via FGFR4 as opposed to FGF21 which primarily functions via FGFR1 [6], and will be of ultimate interest to evaluate in future studies.
4. Conclusions
The evidence presented here defines FGFR1 in adipose tissue as the key receptor/organ for the initiation of the majority of the metabolic effects of FGF21 in vivo. Supporting this hypothesis are recent reports in which lipodystrophic mice failed to respond to FGF21 treatment [21], and mice lacking adipose FGF1 present with marked metabolic deficiencies [22] together indicative of signaling via FGFRs in adipose being critical for normal glucose homeostasis. Furthermore, our findings confirm earlier communications [6,23] that FGFR1 is an attractive drug discovery target and significantly extends this idea by identifying the precise tissue location in which it should be targeted. While it is important to note that there is some hypothalamic and macrophage expression of CRE in this line, however the AP2 CRE mouse remains a widely used tool for generation of adipose tissue conditional deletions for use in metabolic studies [22]. Nevertheless, in an effort to mitigate this potentially confounding factor we also tested FGFR1/Nestin CRE mice and found no differences from WT in either the acute or chronic responses to FGF21 treatment (Adams AC, Personal communication).
We go on to show that secretion of adipokines, such as adiponectin and leptin, represent the next critical step in FGF21's in vivo mechanism of action, downstream of FGFR1 activation in adipose tissue. Indeed, the absence of FGF21 induced glucose lowering in adiponectin null mice has been demonstrated [24] while FGF21's weight loss effect is significantly attenuated in both ob/ob and db/db mice which lack functional leptin signaling [25]. Moreover, a robust attenuation of FGF21 signaling/gene expression effects in the liver of FR1KO mice provides evidence of the existence of a novel adipose-centric axis of cross-tissue communication that underlies FGF21's mechanism of action. Thus, we propose that adipose tissue acts as a metabolic rheostat upon which FGF21 acts to control the production and secretion of endocrine factors which in turn fine tune metabolic outputs. Finally, our data contribute to a better understanding of the in vivo physiology of FGF19, deconvoluting its in vivo mechanism of action at the tissue/FGFR isoform level and highlighting the commonalities and differences to FGF21 physiology. Taken as a whole our data represent a significant advance in understanding of the in vivo mode of action of the “hormone-like” FGF's, which spans a variety of mechanistic levels from initial receptor activation events to the integration of their metabolic outcomes.
Acknowledgments
We thank Prof W McKeehan (Texas A&M Health Science Center, Houston, TX) for contributions to conceptual aspects of the study and provision of the FGFR1lox/loxAP2CRE mice. CY and YL were supported by grants from Eli Lilly, the Susan Komen Foundation and the John S. Dunn Foundation.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.molmet.2012.08.007.
Appendix A. Supplementary materials
Supplementary data
References
- 1.Itoh N. Hormone-like (endocrine) Fgfs: their evolutionary history and roles in development, metabolism, and disease. Cell and Tissue Research. 2010;342:1–11. doi: 10.1007/s00441-010-1024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharitonenkov A., Shiyanova T.L., Koester A., Ford A.M., Micanovic R. FGF-21 as a novel metabolic regulator. Journal of Clinical Investigation. 2005;115:1627–1635. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharitonenkov A., Dunbar J.D., Bina H.A., Bright S., Moyers J.S. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. Journal of Cellular Physiology. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 4.Adams A.C., Coskun T., Irizarry Rovira A.R., Schneider M.A., Raches D.W. Fundamentals of FGF19 and FGF21 action in vitro and in vivo. PLoS One. 2012;7:e38438. doi: 10.1371/journal.pone.0038438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kharitonenkov A., Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends in Endocrinology and Metabolism. 2011;22:81–86. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Yang C., Jin C., Li X., Wang F., McKeehan W.L. Differential specificity of endocrine FGF19 and FGF21 to FGFR1 and FGFR4 in complex with KLB. PLoS One. 2012;7:e33870. doi: 10.1371/journal.pone.0033870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coskun T., Bina H.A., Schneider M.A., Dunbar J.D., Hu C.C. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–6027. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 8.Turer A.T., Scherer P.E. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;26:271–281. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inagaki T., Lin V.Y., Goetz R., Mohammadi M., Mangelsdorf D.J. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell Metabolism. 2008;8:77–83. doi: 10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kir S., Beddow S.A., Samuel V.T., Miller P., Previs S.F. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Hondares E., Rosell M., Gonzalez F.J., Giralt M., Iglesias R. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metabolism. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher F.M., Estall J.L., Adams A.C., Antonellis P.J., Bina H.A. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology. 2011;152:2996–3004. doi: 10.1210/en.2011-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karpe F., Hodson L. Caution on the interpretation of plasma fatty acid composition as a proxy marker for SCD1 activity: particular implications for using the 16:1/16:0 ratio in QTL studies involving hyperlipidemic patients. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28:e152–e153. doi: 10.1161/ATVBAHA.108.167718. (author reply) [DOI] [PubMed] [Google Scholar]
- 16.Binczek E., Jenke B., Holz B., Gunter R.H., Thevis M. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1-/-) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biological Chemistry. 2007;388:405–418. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W., Della-Fera M.A., Hartzell D.L., Hausman D., Baile C.A. Adipose tissue gene expression profiles in ob/ob mice treated with leptin. Life Sciences. 2008;83:35–42. doi: 10.1016/j.lfs.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Wente W., Efanov A.M., Brenner M., Kharitonenkov A., Koster A. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 19.Johnson C.L., Weston J.Y., Chadi S.A., Fazio E.N., Huff M.W. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology. 2009;137:1795–1804. doi: 10.1053/j.gastro.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 20.Wu A.L., Coulter S., Liddle C., Wong A., Eastham-Anderson J. FGF19 regulates cell proliferation, glucose and bile acid metabolism via FGFR4-dependent and independent pathways. PLoS One. 2011;6:e17868. doi: 10.1371/journal.pone.0017868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veniant M.M., Hale C., Helmering J., Chen M.M., Stanislaus S. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS One. 2012;7:e40164. doi: 10.1371/journal.pone.0040164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonker J.W., Suh J.M., Atkins A.R., Ahmadian M., Li P. A PPARgamma-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X., Weiszmann J., Ge H., Baribault H., Stevens J. A unique FGF23 with the ability to activate FGFR signaling through both alphaKlotho and betaKlotho. Journal of Molecular Biology. 2012;418:82–89. doi: 10.1016/j.jmb.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Holland W, Adams AC, Bauer S, Brozinick J, et al. 2012 Adiponectin is critical for acute insulin sensitizing effects of FGF-21. NPG—International Symposium on Adiponectin Biology and Medicine. Tomakomai, Japan.
- 25.Coskun T, et al., in preparation
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


